Pennaria wilsoni (Bale, 1913)
|
publication ID |
https://doi.org/ 10.24199/j.mmv.2015.73.03 |
|
DOI |
https://doi.org/10.5281/zenodo.10886670 |
|
persistent identifier |
https://treatment.plazi.org/id/03DA87E6-5C40-6A51-F6F3-29F7FD85D93F |
|
treatment provided by |
Felipe |
|
scientific name |
Pennaria wilsoni (Bale, 1913) |
| status |
|
Pennaria wilsoni (Bale, 1913) View in CoL
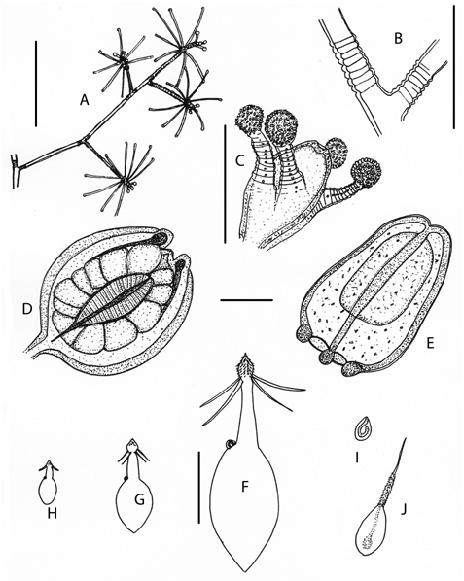
Figures 5 View Figure 5 , 6 View Figure 6 A−J
Pennaria wilsoni Bale, 1913: 116 View in CoL .− Blackburn, 1937: 176, figs 8, 9.− Hirohito, 1988: 30, fig. 9e.
Halocordyle australis Bale, 1894: 94 View in CoL .− Pennycuik, 1959: 160, pl. 1, fig. 8.− Watson 1982: 88, fig. 4.6 g, h, pl. 10.2.
(?) Pennaria wilsoni Gibbons and Ryland, 1989: 388 View in CoL , fig. 6.− Kirkendale and Calder, 2003: 166.− Bouillon et al. 2006: 247.− Calder, 2010: 65.
Material examined. NMV F202880 View Materials , Popes Eye reef, southern Port Phillip, on rubble, depth 8 m, coll: J. Watson, 26/08/2013. Material examined alive, later fixed in 4% formalin, then transferred to 70% ethanol .
Description (from live material). Mature colonies comprising one to many branched stems to 20 cm long arising from a ramified hydrorhiza, stolons tubular, almost smooth in young colonies to rugose and gnarled in older colonies.
Stems erect, monosiphonic, flexuous, cylindrical, perisarc smooth and shining, straight to weakly sympodial, a slight change of direction at origin of each primary branch; branches widely separated along stem in indefinite whorls of three, directed upwards at 30−40º to stem. Stem widest at base, narrowing slightly distally, ringed above base with up to 50 deep annulations and with up to 20 deep annulations above origin of older branches; sometimes groups of annulations along stem not associated with branching.
Hydranth pedicels variable in length (depending on age of colony), widely separated, given off more or less triserially, up to 15 on weakly annulated pedicels, sometimes with a smooth mid-section, branch always with a terminal hydranth. Hydranth long, cylindrical to spindle-shaped, hypostome flattened dome-shaped. Four to five, rarely six short capitate oral tentacles clustered around hypostome, tentacles transversely segmented, each segment with a small central reddish spot, capitala with batteries of nematocysts. Aboral tentacles long, slender, semicapitate, in one whorl of 7-8, arched in life, a thick fringe of nematocysts along the outer side of tentacle, inner side transversely segmented with large transparent cells with reddish inclusions.
Gonophore eumedusoid, ovoid to oblong, one to four in various stages of development on short peduncles just above aboral tentacles. At release umbrella thick, evenly covered in nematocysts, radial canals straight, gonads large, brown, tentacles reduced to knobs, velum closed, female with large ova. Medusa pulsates feebly before and after release.
Perisarc of stem and branches thick, shining brown, hydranth pedicels paler brown; hydranth and tentacles translucent white, stomach brown to red. Medusa colourless, manubrium red, radial canals reddish before release, becoming brown after release.
Cnidome comprising four categories of nematocysts; stenoteles present in a wide range of shapes, sizes and abundances, even between hydranths on the same stem.
(i) stenoteles, capsule large, elongate ovoid, 51−56 x 27−31 µm, shaft 50 µm long, cylindrical, head 15 µm long with 2−4 long basal spines, distal part of head with many small bristles, spinous thread at least 30 µm long, in capitate tentacles and at base of medusa; easily discharged.
(ii) stenoteles, similar to but smaller than stenotele (i), capsule elongate ovoid 44−45 x 20−22 µm, shaft cylindrical, 40 µm long, base of head with 2−4 long spines, head distally with bristles, thread with many small spines; in capitate tentacles, easily discharged.
(iii) stenoteles, capsule ovoid, 19−20 x 14 µm, shaft stout, 19 µm long, head with several spines; in oral tentacles; easily discharged.
(iv) stenoteles, capsule ovoid, 11−13 x 8−10 µm, shaft 10 µm long, abundant in aboral tentacles; difficult to discharge.
(v) stenoteles, capsule inflated ovoid, 25 x 23 µm, in aboral tentacles; undischarged.
(vi) isorhizas, capsule loaf−shaped 15 x 5−7 µm, thread long, abundant in aboral tentacles; difficult to discharge.
(vii) microbasic mastigophores, capsule elongate pyriform, 15−19 x 6 µm, shaft 12 µm, spinous, thread coiled in a circle at base of capsule, abundant in aboral tentacles and on medusa; difficult to discharge.
(viii) desmonemes, capsule almond shaped, 7 x 4.5−5 µm, rare in aboral tentacles and around hypostome; undischarged.
Remarks. Pennaria is a genus with five accepted species ( Bouillon et al. 2006, Schuchert 2006). Pennaria disticha is the best known of the group with cosmopolitan distribution in tropical and temperate seas; it is present around Australia except in cooler Victorian waters (author’s pers. obsv.).
P. disticha View in CoL . Bale (1894) described Halocordyle australis View in CoL from Capel Sound, Port Phillip. The hand written label on the presumed holotype microslide ( NMV F58747) is not Bale’s and may be that of the collector, John Bracebridge Wilson. Ralph (1966) reported Pennaria disticha View in CoL from a benthic material from southern Port Phillip collected by the National Museum of Victoria Port Phillip (1957). Examination of this material ( NMV F150168, F150169) shows it to be Pennaria wilsoni View in CoL , not Pennaria disticha View in CoL . The flexuous spirally branching habit of P. wilsoni View in CoL easily distinguishes it from the pinnate stems of P. disticha View in CoL . Bale (1913) renamed the species Pennaria wilsoni View in CoL and Blackburn (1937) described and figured the gonophores of material from Western Port, Victoria.
Gibbons and Ryland (1989) reported fertile material as Pennaria wilsoni from Suva Barrier Reef, Fiji and Kirkendale and Calder (2003) also referred infertile tropical material from Guam to P. wilsoni . Gibbons and Ryland’s microslide mount (GL 10177) loaned by the Queensland Museum contains several mature balloon–shaped gonophores approximately 1.5 mm long and 1.2 mm wide, but the contents are too degraded for description. I have examined four preserved samples of infertile material from the Pennycuik collection ( QM 5513- 5516 inclusive) loaned by the Queensland Museum. The material was collected from under coral reef shelves, 21−24 th August, 1954, at a depth of 2 m at the Low Isles on the Great Barrier Reef, Queensland. The Fiji and Great Barrier Reef material are clearly the same species.
The only sure means of determining whether there are two or one geographically wide-ranging species is based on morphology of the cnidome. For comparison with P. wilsoni a small crushed hydranth of QM 5515 was examined under high magnification. The scarcely distinguishable nematocysts in the capitate tentacles comprised some undischarged?stenoteles of three sizes: i) 28−36 x 20−24 µm, ii) 25 x 17 µm and iii) 11−12 x 8−9 µm. While allowing for approximately 10% shrinkage in preservation, the largest capsules are much smaller than those of fresh P.wilsoni . Therefore, until the cnidome and gonophores of fresh tropical material are available for examination it the tropical material is best regarded as a different species.
The extensive and highly variable cnidome with nematocysts of four categories is similar to that described for Pennaria disticha (see Schuchert 1996), but in P. wilsoni the largest stenoteles are bigger than those of P. disticha . In P. wilsoni the relative abundance of the larger sizes of stenoteles varies between hydranths, sometimes from the same stem. The smaller stenoteles, microbasic mastigophores and isorhizas are present in all hydranths while desmonemes are sometimes rare. Uneven distribution of the large stenoteles may be related to maturity of the hydranth, the larger ones usually occurring on the older hydranths.
Pennaria wilsoni is known from several Victorian localities and is likely to be more widespread than is presently known. Colonies are most luxuriant in strong current flow on open reef at 6−10 m depth while in less rigorous environments they usually comprise only a few stems. Colonies persist throughout the year with major growth during winter months at water temperatures of 10−14ºC, becoming moribund over summer. Mature colonies are host to many epizooites including other small hydroids, anemones and ascidians; as the water temperature increases in late winter they are often overgrown by small filamentous red algae. Hydranths and gonophores are heavily preyed upon by the pycnogonid Tanystylum and several species of the nudibranch Trinchesia .
| NMV |
Museum Victoria |
| QM |
Queensland Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Pennaria wilsoni (Bale, 1913)
| Watson, Jeanette E. 2015 |
Pennaria wilsoni
| Hirohito & Showa Emperor of Japan 1988: 30 |
| Blackburn, M. 1937: 176 |
Halocordyle australis
| Watson, J. E. 1982: 88 |
| Pennycuik, P. R. 1959: 160 |
| Bale, W. M. 1894: 94 |