Dugesia afromontana Stocchino & Sluys
|
publication ID |
https://doi.org/ 10.5281/zenodo.282847 |
|
DOI |
https://doi.org/10.5281/zenodo.6176098 |
|
persistent identifier |
https://treatment.plazi.org/id/03F587C2-FFD5-FFE2-4487-F1D2FB31FDF1 |
|
treatment provided by |
Plazi |
|
scientific name |
Dugesia afromontana Stocchino & Sluys |
| status |
sp. nov. |
Dugesia afromontana Stocchino & Sluys sp. nov.
( Figs 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 ; Tables 1–3)
Material examined. Holotype: ZMA V.Pl. 7108.1, one set of sagittal sections on 15 slides, rivulet along a mountain stream (station 2), Hogsback State Forest (32°36’390”S, 26°57’920”E), Amatola Mountains, Eastern Cape Province, South Africa, April 2006, coll. R. Manconi.
Paratypes: ZMA V.Pl. 7108.2, ibid., sagittal sections on 13 slides; ZMA V.Pl. 7108.3, ibid., sagittal sections on 13 slides; ZMA V.Pl. 7108.4, ibid., sagittal sections on 10 slides; ZMA V.Pl. 7108.5, ibid., sagittal sections on 13 slides; CGAS Pla 5.1, ibid., sagittal sections on 19 slides, CGAS Pla 5.2, ibid., transverse sections on 31 slides, CGAS Pla 5.3 ibid., sagittal sections on 24 slides.
Other material: ZMA V.Pl. 7109.1, sagittal sections on 12 slides, ZMA V.Pl. 7109.2, sagittal sections on 12 slides, ZMA V.Pl. 7109.3, sagittal sections on 54 slides, ZMA V.Pl. 7109.4, horizontal sections on 25 slides CGAS Pla 5.4 sagittal sections on 49 slides, CGAS Pla 5.5 sagittal sections on 55 slides, CGAS Pla 5.6 sagittal sections on 53 slides, CGAS Pla 5.7 transverse sections on 65 slides, main mountain stream (station 1), Hogsback State Forest, Amatola Mountains, Eastern Cape Province, South Africa, April 2006, coll. R. Manconi.
Etymology. The specific epithet refers to the Afromontane forest in South Africa.
Habitat. Planarians were found in a main stream (station 1) and a rivulet (station 2) along the southernmost patches of the Afromontane forest in the Amatola Mountains of South Africa ( Fig. 1 View FIGURE 1 ). The animals were collected from under boulders and pebbles in shallow, clear, running water (30 cm maximum depth), at an altitude ca. 1150 m asl. In the Amatola forest rain falls throughout the year, with maxima in early and late summer, and with maximum and minimum air temperatures ranging from 19.7 to 8.9°C, respectively.
Geographical distribution. Known only from the type locality.
Diagnosis. Dugesia afromontana is characterized by the presence of the following features: central course of the ejaculatory duct; terminal opening of the ejaculatory duct; elongated seminal vesicle; asymmetrical openings of the oviducts into the bursal canal; openings of vasa deferentia at halfway along the seminal vesicle; barrel-shaped penis papilla; ejaculatory duct with irregularly shaped lumen with several folds.
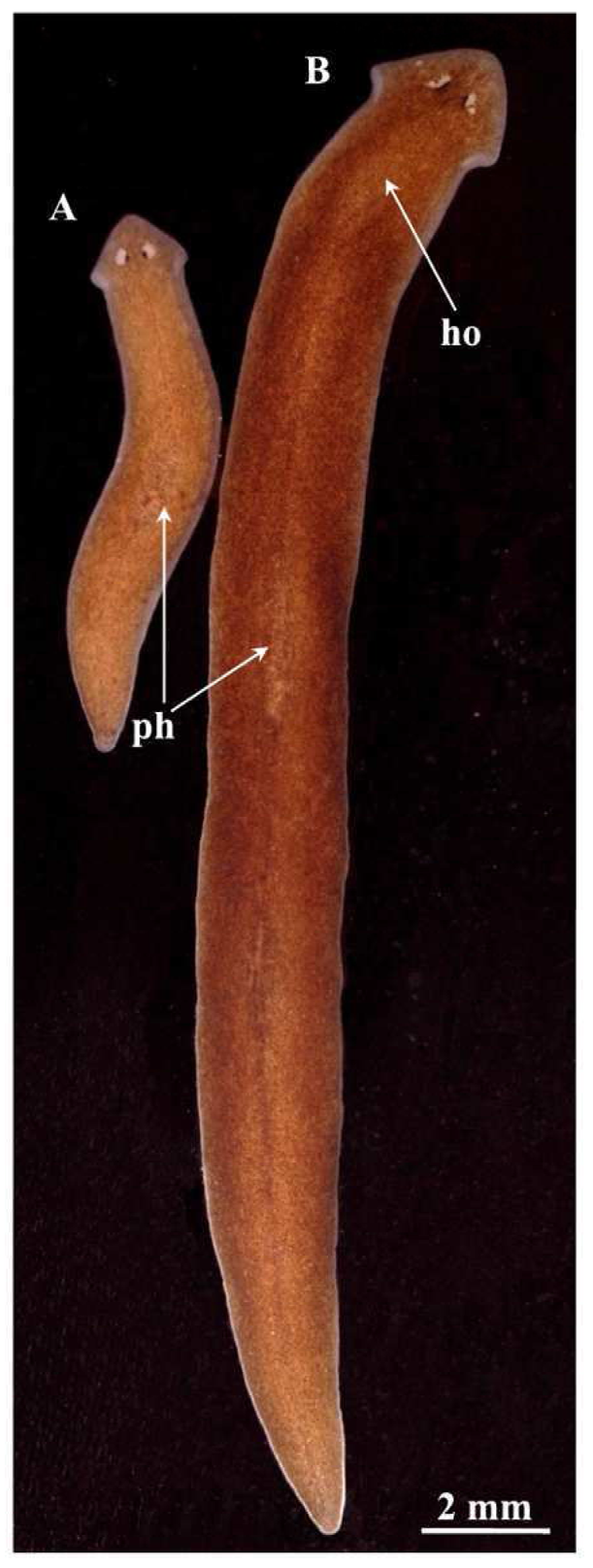
Description. Body size of living sexualized specimens ranged from 18 to 20 mm in length and 2–2.5 mm in width. Fissiparous specimens are notably smaller, measuring less than half the size of ex-fissiparous animals ( Fig. 2 View FIGURE 2 ). Two eyes are present in the middle of the head, and unpigmented auricular grooves are marginally placed just posteriorly to the eyes. The colour is uniformly brown dorsally, and pale ventrally. However, in the fissiparous specimens the pigmentation is always lighter than the ex-fissiparous animals.
Inner and outer pharyngeal musculature is bilayered, i.e. without an extra, third, outer longitudinal muscle layer. The ovaries are hyperplasic, with several scattered masses distributed in the body region directly posterior to the brain, filling up the entire dorso-ventral space. These masses of ovarian tissue are already visible in living specimens through the dorsal body wall as small spot-like depigmented areas ( Fig. 2 View FIGURE 2 ). A degenerative condition is clearly evident in the ovaries. The oocytes develop by a regular mechanism up to the beginning of diplotene stage, then they show progressive cytoplasm vacuolation, followed by collapse of the entire cell content and by cell necrosis. The anterior portion of the infranucleate oviducts is expanded into a seminal receptacle that arises from the ovarian masses at a variable position, dependent upon the hyperplasic condition of the ovaries. The oviducts run ventrally in a caudal direction up to the vaginal area and open asymmetrically into the proximal section of the bursal canal. The right oviduct opens directly dorsally to the openings of the shell glands into the bursal canal, whereas the left oviduct opens more ventrally, very close to the point where the canal communicates with the common atrium ( Fig. 3 View FIGURE 3 A).
The testes are situated dorsally and extend from some distance posterior to the ovaries in station 2 specimens and from the level of the ovaries in station 1 animals to the posterior end of the body. The majority of the follicles are under-developed in station 2 specimens. Spermatogonia (ca. 70% of all germinal cells) are prevalent in the testes, while spermatocytes and spermatids are also evident. In only some specimens from the station 2 population mature sperms are present in a few follicles. In contrast, in the majority of the examined specimens of the station 1 population there are well developed testes with mature spermatozoa. However, in both cases anomalies were observed, such as an irregular shape of the spermatids and spermatozoa. Vitellaria are located, as usual, between the testes and the intestinal branches.
The large sac-shaped copulatory bursa is lined by a columnar, glandular epithelium bearing basal nuclei and it is surrounded by a layer of longitudinal muscles. In some specimens empty spermatophores are present in the lumen of the bursa. From the dorso-posterior wall of the bursa the bursal canal runs in a caudal direction, to the left of the copulatory apparatus, subsequently communicating with the common atrium. The bursal canal is lined by cylindrical, infranucleate, ciliated cells and is surrounded by a thin subepithelial layer of longitudinal muscles, followed by a very thick layer of circular muscle. The latter is thicker on the ventral side than on the dorsal side of the canal ( Fig. 3 View FIGURE 3 B). The moderately developed penis bulb, rich in secreting glands, consists of intermingled longitudinal and circular muscle fibres. It houses an elongated seminal vesicle, lined by a nucleate epithelium, the cells of which irregularly protrude into the lumen.
The vasa deferentia penetrate the proximal anterior section of the penis bulb and open separately and symmetrically into the seminal vesicle at a position about halfway along the vesicle. The seminal vesicle opens into the ejaculatory duct via a small diaphragm. The seminal vesicle is surrounded by loosely interwoven layers of circular and longitudinal muscle fibres ( Fig. 3 View FIGURE 3 A). In the majority of the station 1 specimens the sperm ducts form well-developed spermiducal vesicles, packed with sperm. The diaphragm, located approximately at the base of the penis papilla, receives the openings of penis glands. The barrel-shaped penis papilla is lined by a nucleated epithelium that is underlain with a thin subepithelial layer of circular muscles fibres, followed by a layer of longitudinal muscles. The ejaculatory duct follows a basically central course through the penis papilla and opens terminally at the tip of the papilla. The ejaculatory duct has an irregularly shaped lumen with several folds; it is lined by a cuboidal, infranucleate epithelium. The ejaculatory duct receives the abundant erythrophil secretion of penis glands; in the majority of specimens examined the duct contained an empty spermatophore.
The genital atrium is divided into a common atrium and a male atrium and is lined by a nucleated epithelium that is underlain by a subepithelial layer of circular muscle, followed by a layer of longitudinal muscle fibres. The common atrium opens ventrally through the gonopore, forming a small enlargement characterized by a columnar epithelium, which receives the opening of very abundant cement glands ( Fig. 3 View FIGURE 3 A).
Karyology. Metaphasic plates obtained by the squashing method revealed that each of the 10 studied specimens constantly showed a set of 27+1–2 B-chromosomes, with a basic number of n = 9. Chromosomes from six metaphasic plates were arranged, according to their length, in nine groups of three chromosomes. Analysis within each group of three chromosomes revealed a distinct variation in centromere position and sometimes also in chromosome length. The karyometric values were calculated referring to a haploid set obtained from the single triplets by choosing the chromosome showing at least a second homologue. The first four chromosomes and the 9th element, the latter being the smallest, were clearly identifiable. Instead, in the 5th to 8th elements the differences in length were so slight that contiguous chromosomes overlapped ( Fig. 4 View FIGURE 4 ).
Chromosomal length decreases gradually, with low standard deviation values. In contrast, centromeric indices showed great variations, with the standard deviation being high for some elements. The karyometric data indicate that the triploid complement is characterized by metacentric heterobrachial chromosomes, except the third element, which is submetacentric ( Table 1).
chromosome
1 2 3 4 5 6 7 8 9 r.l. 15.24±0.40 13.68±0.35 12.25±0.58 11.50±0.32 10.62±0.23 10.16±0.26 9.74±0.17 8.99±0.34 8.11±0.41 c.i. 47.60±2.08 45.95±3.50 35.83±2.56 44.21±2.30 46.63±2.95 42.82±3.10 45.96±1.83 46.75±2.89 46.63±2.95 Life cycle. The life cycle of the two populations, one from station 1 and the other from station 2, was monitored under laboratory conditions for five years. All individuals (n = 25) from the station 1 population were asexual at collection. After having been kept in the laboratory for about one year, during which the strain notably increased in numbers due to asexual reproduction by fission, approximately 30% of the specimens shifted from the fissiparous reproductive mode towards a tendency to sexualize, i.e. to develop reproductive organs. These sexualized animals displayed the characteristic features of ex-fissiparous individuals: large body size, development of the copulatory apparatus, hyperplasic ovaries. After more than two years a strain of two ex-fissiparous individuals produced four sterile cocoons. During the following two years other individuals also displayed a sexualization process but produced only sterile cocoons.
All individuals (n = 6) from the station 2 population were also asexual at collection. After having been kept in the laboratory for about 2 years, during which the strain notably increased in numbers due to fissioning processes, approximately 30% of the specimens attained the ex-fissiparous, sexual state. About one year later, a strain of five ex-fissiparous individuals produced eight cocoons. From the only three fertile cocoons five young planarians hatched after 2–3 weeks of development. In the following two months another 22 sterile cocoons were laid. Half a year later the five young planarians displayed fissioning processes. At more or less the same time the five exfissiparous individuals laid 11 cocoons from which one young planarian hatched after 3 weeks of development from the only three cocoons from which hatchlings arose. During the following two years other individuals also displayed a sexualization process but produced only sterile cocoons.
Overview of African freshwater triclads. The genus Dugesia is widely distributed in the Palaearctic, Afrotropical, Oriental, and Australasian regions, with about 75 nominal species (cf. Sluys et al., 1998; Pala et al., 2000; Stocchino et al., 2002, 2009; Sluys, 2007; Harrath et al., 2012b). From the entire African continent only 19 species have been described ( Fig. 1 View FIGURE 1 ; Table 2): four for North-Africa ( D. sicula Lepori, 1948 , D. subtentaculata ( Draparnaud, 1801) , D. maghrebiana Stocchino et al., 2009 , D. tubqalis Harrath & Sluys, 2012 ), 12 for Central Africa ( D. sudanica Dahm, 1971 , D. lamottei de Beauchamp, 1952, D. colapha Dahm, 1967 , D. neumanni ( Neppi, 1904) , D. lanzai Banchetti & Del Papa, 1971 , D. congolensis De Beauchamp, 1951 , D. ectophysa Marcus, 1953 , D. astrocheta Marcus, 1953 , D. didiaphragma De Vries, 1988 , D. machadoi De Beauchamp, 1952 , D. mirabilis De Vries, 1988 , D. aethiopica Stocchino et al., 2002 ), and three species for South-Africa ( D. monomyoda , D. capensis , D. afromontana , this paper) (cf. Marcus, 1953; De Vries, 1988a; Stocchino et al., 2002, 2009; Sluys, 2007; Harrath et al., 2012b; this paper).
This rather low number of records for Africa contrasts with the high values of taxonomic richness reported for other areas, such as the circum-Mediterranean subregion harbouring more than 20 species of Dugesia from a world total of about 75 nominal species (cf. De Vries, 1984, 1986, 1988b; Pala et al., 2000; Stocchino et al., 2005). Besides the genus Dugesia , three other genera ( Schmidtea , Cura and Neppia ), comprising 7 species, have been reported for the Dugesiidae on the African continent. For the Dendrocoelidae and Planariidae , which are exclusively present in the Palaearctic section of the continent, only 7 species belonging to 3 genera ( Dendrocoelum , Acromyadenium and Polycelis ) have been reported up to now, out of a total of 33 species (Table 2).
| ZMA |
Universiteit van Amsterdam, Zoologisch Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.