Thraulodes, Ulmer, 1920Thraulodes, Ulmer, 1920Thraulodes, Ulmer, 1920
|
publication ID |
https://doi.org/10.11646/zootaxa.4756.1.1 |
|
publication LSID |
urn:lsid:zoobank.org:pub:9FF62616-A7FA-4331-AC51-0F534400631D |
|
DOI |
https://doi.org/10.5281/zenodo.3811761 |
|
persistent identifier |
https://treatment.plazi.org/id/039787A6-FF99-8470-8CFB-FD77DBFAF85A |
|
treatment provided by |
Carolina |
|
scientific name |
Thraulodes Thraulodes Thraulodes |
| status |
|
Species composition of Thraulodes View in CoL View at ENA
All species of Thraulodes are distributed in new New World only, being limited by the southern part of North America, Central America (except Antilles) and South America (except Patagonia). Traver & Edmunds (1967) reported a larva from southern Chile, which they determined as belonging to Thraulodes .
The species named Thraulodes marhieus Dubey 1970 was described as female imago from Himalayas; judging by the words «The new species differs from the other Palaearctic species ...» the author confused the New World genus Thraulodes with something other; judging by confusion of vestigial paracercus with ovipositor, he was not familiar with insect morphology. Hubbard and Peters (1978: 25) wrote that «this species is in all probability misplaced at the generic level»; Kluge (2004: 208–209) placed it to Radulapalpata, i.e. Heptageniidae s. str.
The species vitripennis Blanchard in Gay 1851 [ Ephemera ], colombiae Walker 1853 [ Ephemera ], nervosa Eaton 1892 [ Choroterpes ], subfasciata Navás 1934 [ Thraulodes ], humeralis Navás 1935 [ Thraulodes ], and limbatus Navás 1936 [ Thraulodes ] have unclear systematic position within Leptophlebiidae and should be treated as Atalophlebolinguata INCERTAE SEDIS.
The species originally described as Ephemera vitripennis Blanchard in Gay 1851 from Chile was attributed to Thraulodes by Ulmer (1920). This species is know as poorly preserved male subimago, which cannot be reliably determined, and its placement to Thraulodes is probably wrong ( Traver & Edmunds 1967, Dominguez et al. 2006). Most probably, vitripennis [ Ephemera ] belongs to one of Chilean leptophlebiid genera, while Thraulodes is not ordinary for Chile (if occurs there at all).
The species originally described as Ephemera colombiae Walker 1853 was attributed to Thraulodes by Ulmer (1920) and is recently placed to this genus ( Traver & Edmunds 1967, Dominguez et al. 2006). This species is known as female subimago only and has no characters of Thraulode s: in contrast to all known Thraulodes , costal angulation of hind wing is located «about 2/3 of distance from base to apex»; in contrast to nearly all species of Thraulodes , femora are not banded.
The species originally described as Choroterpes nervosa Eaton 1892 is known as female only. Its MP of hind wing is not forked (Eaton 1982: fig. 6a), so it is unclear, why Ulmer (1920) placed it to Thraulodes . Traver and Edmunds (1967) assumed that «it comes closer to Traverella Edmunds », i.e. belongs to Hermanellognatha.
Two species originally named Thraulodes subfasciata Navás 1934 and Th. limbatus Navás 1936 were described as males, but without illustrations of genitalia. Traver & Edmunds (1967) assumed that they «are more likely to belong to the genus Ulmeritus Traver than to Thraulodes »; however, some authors place them to Thraulodes without argumentation (e.g., Dominguez et al. 2006). Genitalia of subfasciata [ Thraulodes ] were described as «forcipe longa, pallida, primo articulo interne basi et apice dilatato» ( Navás 1934: 165), that is not characteristic for Thraulodes .
The species originally named Thraulodes humeralis Navás 1935 also was described as male imago without illustration of genitalia. Recently it is placed to Thraulodes (e.g. Traver & Edmunds 1967). However, according to the original description, its hind wing has no triad of RS ( Navás 1935: fig. 119), in contrast to all known Thraulodes ( Fig. 41 View FIGURES 35–41 ). Figures made by Navás are often inaccurate, but in his other figures (e.g. fig. 120, in the same paper) triad RA of the hind wing is adequately drawn.
The species originally named Thraulodes irretitus Navás 1924 is recently regarded to belong to Thraulodes , but with uncertain position within this genus, because its genital structure is unknown ( Traver & Edmunds 1967).
All known species of Thraulodes can be distinguished by characters of male imagines, which often have species-specific coloration of body, legs, wings and caudalii, as well as species-specific structure of genitalia. In contrast to this, larvae of Thraulodes have fewer species-specific characters. For most species of Thraulodes larvae remain to be undescribed; in those cases when larvae are described, the descriptions do not allow us to distinguish them from other species. In this situation formal species described as larvae only, appear to be non-comparable with others, and can be qualified as NOMINA DUBIA. These are Thraulodes salinus Kilgore & Allen 1973 , Th. eccentricus Lugo-Ortiz & McCafferty 1996 , Th. grandis Lugo-Ortiz & McCafferty 1996 , Th. tenulineus Lugo-Ortiz & McCafferty 1996 and Th. pacaya McCafferty, Baumbgardner & Guenter 2004 (see below).
The single complete key to male imagines of Thraulodes was published by Traver and Edmunds (1967). It begins with characters of coloration of abdomen, which are peculiar only for males, being different in females. Below, a new key is given, which includes also species described after 1967 and new species described in this paper. In or- der to make this key available for determination not only males, but also some females, it begins with the characters common for males and females; such characters are coloration of legs and wings. Species, whose leg coloration or genital structure is unknown, are not included in this key; these are Thraulodes mexicanus ( Eaton 1884 [ Thaulus]) (legs coloration unknown), Th. bomplandi ( Esben-Petersen 1912 [ Thraulus ]) (fore and middle legs missing), Th. irretitus Navás 1924 (genitalia unknown), Th. pedregoso Traver 1946 (legs presumably associated), Th. hilaroides Traver 1946 (legs missing), Th. brunneus Koss 1966 (fore and hind legs missing), Th. lunatus Traver & Edmunds 1967 (fore and hind legs missing), Th. osiris Traver & Edmunds 1967 (legs missing).
This is a nested key, in which the number of antithesis is given in parentheses after the number of thesis, and the next thesis is given just after the correct thesis. Species described in this paper are numbered and marked by bold face.
Key to male imagines of Thraulodes View in CoL View at ENA
1 (8) All tibiae either entirely dark ( Figs 467–469 View FIGURES 464–473 , 491–492 View FIGURES 491–497 , 526–527 View FIGURES 522–531 ), or dark in proximal half ( Fig. 437 View FIGURES 427–438 ).
2 (7) All femora uniformly dark brown ( Figs 436–437 View FIGURES 427–438 , 467–469 View FIGURES 464–473 , 491–492 View FIGURES 491–497 ).
3 (4) Fore wing with costal and subcostal fields contrastingly yellowish ( Figs 427, 435 View FIGURES 427–438 )..................... 13. Th. niger sp. n.
4 (3) Fore wing with costal and subcostal fields not colored ( Figs 465 View FIGURES 464–473 , 493 View FIGURES 491–497 ).
5 (6) Fore wing with dark macula on costal brace ( Fig. 493 View FIGURES 491–497 ). ♂: Abdominal terga II–VI light ocher, terga VII–X reddish ( Fig. 495 View FIGURES 491–497 ).................................................................................... 15. Th. nigripes sp. n.
6 (5) Fore wing without dark macula on costal brace ( Fig. 465 View FIGURES 464–473 ). ♂: Abdominal terga unicolor ( Fig. 466 View FIGURES 464–473 )................................................................................................... 14. Th. nigrabdominalis sp. n.
7 (2) All femora twice banded, i.e. with small light area at middle of femur between bands ( Figs 526–527 View FIGURES 522–531 )...................................................................................................... 6. Th. nigrotibialis sp. n.
8 (1) At least middle and hind tibiae either entirely light ( Fig. 29–30 View FIGURES 25–34 ), or light with dark apex ( Figs 306–307 View FIGURES 297–308 , 659–660 View FIGURES 654–665 )
9 (14) Fore tibia entirely dark brown ( Fig. 597 View FIGURES 591–602 ).
10 (11) At least in male, proximal half of fore wing brown, distal half colorless (female unknown)......................................................................................... Th. basimaculatus Giordano & Dominguez 2005 View in CoL
11 (10) No such difference between proximal and distal halves of fore wing.
12 (13) All femora uniformly dark brown ( Figs 597–598 View FIGURES 591–602 )..................................... 18. Th. lepidus ( Eaton 1884) View in CoL
13 (12) Middle and hind femora yellowish with black bands in distal half......................... Th. venezuelana Ulmer 1943 View in CoL
14 (9) Fore tibia at least partly light ( Figs 28 View FIGURES 25–34 , 305 View FIGURES 297–308 , 339 View FIGURES 337–345 , 365 View FIGURES 365–370 , 574 View FIGURES 569–577 ) (as well as middle and hind ones).
15 (16) Fore tibia with brown band at middle, apex whitish; hind femur whitish with two narrow black bands close to apex ( Figs 574–575 View FIGURES 569–577 ).......................................................................... 17. Th. alboniger sp. n.
16 (15) Fore tibia with dark band adjacent to apex ( Figs 28 View FIGURES 25–34 , 305 View FIGURES 297–308 , 339 View FIGURES 337–345 , 365 View FIGURES 365–370 ). Hind femur not as above.
17 (18) Femora of middle and hind legs yellowish, without dark maculae; only femur of fore leg with dark maculae apically............................................................................ Th. papilionis Traver & Edmunds 1967
1 (17) Femora of middle and hind legs with dark maculae at least in distal half ( Figs 29–30 View FIGURES 25–34 , 97 View FIGURES 94–100 , 306–307 View FIGURES 297–308 , 367 View FIGURES 365–370 ).
19 (22) Fore femur evenly blackish brown nearly all over its length (paler at extreme base and/or apex).
20 (21) ♂: Penis without ears, medio-ventral ridge forms lapel projected laterad of lateral margin ( Traver & Edmunds 1967: fig. 67)................................................................................... Th. hilaris Eaton 1892
21 (20) ♂: Penis with ears, no lapel projected laterad of lateral margin ( Traver & Edmunds 1967: fig. 51)............................................................................................... Th. arizonicus McDunnough 1942
22 (19) Fore femur with darker maculae at least in distal half ( Figs 28 View FIGURES 25–34 , 305 View FIGURES 297–308 , 339 View FIGURES 337–345 , 365 View FIGURES 365–370 , 398 View FIGURES 396–402 ).
23 (66) Middle and/or hind femora, besides dark band and/or maculae in distal half, with dark band or spot in proximal half ( Figs 307 View FIGURES 297–308 , 341 View FIGURES 337–345 , 365–367 View FIGURES 365–370 , 398–401 View FIGURES 396–402 , 660 View FIGURES 654–665 )
24 (57) Proximal half of middle and hind femora with band or wide spot ( Figs 307 View FIGURES 297–308 , 341 View FIGURES 337–345 , 365–367 View FIGURES 365–370 , 398–401 View FIGURES 396–402 , 660 View FIGURES 654–665 ).
25 (26) Subcostal field of fore wing yellow, apically brown ( Figs 365–367 View FIGURES 365–370 )................................. Th. flavus sp. n.
26 (25) Subcostal field of fore wing, if colored, with apex not darker than remainder part.
27 (28) Fore wing with dark macula on furcation of MA larger than other maculae. Th. alapictus Lima, Mariano & Pinheiro 2013
28 (27) If dark maculae on fore wing present, macula on furcation of MA not larger than others ( Fig. 398–401 View FIGURES 396–402 ).
29 (30) Contrasting dark brown spot on whitish background on median furcasternal concavity of mesothorax ( Fig. 400 View FIGURES 396–402 )........................................................................... 12. Th. spangleri Traver & Edmunds 1967 View in CoL
30 (29) No such unpaired spot on median furcasternal concavity.
31 (32, 33, 34) ♂: Penis with pair of apico-lateral projections directed proximally ( Kimmins 1934: fig. 8).............................................................................................. Th. valens ( Eaton 1892 [ Thraulus View in CoL ])
32 (31, 33, 34) ♂: Penis with pair of lateral projections directed proximally ( Campos & Mariano 2019: figs 10–12)............................................................................... Thraulodes catoles Campos & Mariano 2019
33 (31, 32, 34) ♂: Penis with pair of lateral projections directed distally ( Traver 1946: fig. 30; Traver & Edmunds 1967: fig. 65)................................................................................... Th. furficulus Traver 1946 View in CoL
34 (31, 32, 33) ♂: Penis without additional lateral projections.
35 (40) ♂: Penis in distal half parallel-sided, not widened apically ( Fig. 310 View FIGURES 209–314 or similar to Fig. 440 View FIGURES 439–447 ).
36 (37) ♂: Penis without ears ( Traver & Edmunds 1967: fig. 37)...................... Th. ephippiatus Traver & Edmunds 1967 View in CoL
37 (36) ♂: Penis with ears directed caudally ( Fig. 310 View FIGURES 209–314 ; Traver & Edmunds 1967: fig. 41).
38 (39) ♂: Abdominal terga II–IX equally dark ( Fig. 343 View FIGURES 337–345 ).......................... 10. Th. zonalis Traver & Edmunds 1967 View in CoL
39 (38) ♂: Abdominal terga II–V contrastingly lighter than terga VII–X ( Figs 298–301 View FIGURES 297–308 )................ 9. Th. fascipennis sp. n.
40 (35) ♂: Penis widened apically.
41 (42) ♂: Penis hidden under very wide dorsal projection of styliger (Neito & Dominguez 2001: figs 6–8)....................................................................................... Th. cryptodrilus Neito & Dominguez 2001
42 (41) ♂: Penis not hidden, dorsal projection of styliger smaller.
43 (50) ♂: Penis without lateral pouches.
44 (45) ♂: Dorsal margin of styliger rounded, without median projection ( Traver & Edmunds 1967: fig. 63)............................................................................................ Th. laetus ( Eaton 1884 [ Thraulus View in CoL ])
45 (44) ♂: Dorsal margin of styliger either pointed, or with median projection ( Traver & Edmunds 1967: fig. 44; Dominguez 1987: figs 6, 8).
46 (47) ♂: Abdominal terga IV–VI with composite dark markings ( Dominguez 1987: figs 19–20). h. liminaris Dominguez 1987 View in CoL
47 (46) ♂: Abdominal terga IV–VI with sublateral dark spots ( Dominguez 1987: figs 15–16).
48 (49) Distributed in South America; genitalia as figured by Dominguez (1987: fig. 6)......... 21. Th. consortis Dominguez 1987 View in CoL
49 (48) Distributed in North America; genitalia as figured by Traver & Edmunds (1967: fig. 44)......... Th. speciosus Traver 1934
50 (43) ♂: Penis with lateral pouches.
51 (52, 53, 54, 55, 56) ♂: Abdominal coloration and genitalia as described by Traver & Edmunds (1967: figs 8–9, 31)................................................................................... Th. packeri Traver & Edmunds 1967
52 (51, 53, 54, 55, 56) ♂: Abdominal coloration and genitalia as described by Traver & Edmunds 1967: fig. 26)................................................................................................. Th. daidaleus Thew 1960
53 (51, 52, 54, 55, 56) ♂: Abdominal coloration and genitalia as described by Traver & Edmunds (1967: figs 4–5, 46)........................................................................................ Th. paysandensis Traver 1964
54 (51, 52, 53, 55, 56) ♂: Abdominal coloration and genitalia as described by Traver & Edmunds (1967: figs 2–3, 66)............................................................................................. Th. traverae Thew 1960
55 (51, 52, 53, 54, 56) ♂: Abdominal coloration and genitalia as described by Lima et al. (2013: figs 10–11, 18–20).......................................................................... Th. luizgonzagai Lima, Mariano & Pinheiro 2013
56 (51, 52, 53, 54, 55) ♂: Abdominal coloration and genitalia as described by Lima et al. (2013: figs 16–17, 31–32)................................................................................ Th. pinhoi Lima, Mariano & Pinheiro 2013
57 (24) Proximal half of middle and hind femora with small spot (besides band in distal half).
58 (61) ♂: Abdominal terga II–IX equally dark.
59 (60) ♂: Penis with lateral pouches wider than apex ( Mariano et al. 2011: figs 9–10)............................................................................. Th. amanda Mariano & Froehlich (in Mariano, Flowers & Froehlich) 2011
60 (59) ♂: Penis with lateral pouches narrower than apex ( Mariano et al. 2011: figs 4–3)........................................................................ Th. xavantinensis Mariano & Froehlich (in Mariano, Flowers & Froehlich) 2011
61 (58) ♂: Abdominal terga II–V contrastingly lighter than terga VII–X.
62 (63) ♂: Dorsal margin of styliger rounded, without median projection ( Lima et al. 2013: fig. 12)..................................................................................... Th. sternimaculatus Lima, Mariano & Pinheiro 2013
63 (62) ♂: Dorsal margin of styliger with median projection.
64 (65) ♂: Abdominal terga II–VI with a pair of dark submedian spots (besides pair of midway spots) ( Souto et al. 2014: figs 1–4)......................... Th. luisae Souto, Da-Silva & Nessimian 2014 and Th. pinga Souto, Da-Silva & Nessimian 2014
65 (64) ♂: Abdominal terga without submedian spots................................................................ Th. insular Domínguez, Molineri & Zúñiga (in Zúñiga, Molineri, Domínguez & Cardona) 2015 and Th. eduardorum Medina & Perez 2010
66 (23) Middle and hind femora light, with dark macula(e) only in distal part ( Figs 30 View FIGURES 25–34 , 97 View FIGURES 94–100 ).
67 (68) Hind femur without distinct bands, darker toward apex. ♂: penis unusually sharply widened, with lateral denticles ( Traver 1946: fig. 27; Traver & Edmunds 1967: fig. 49)....................................... Th. prolongatus Traver 1946
68 (67) Hind femur with brown or reddish apical band usually bordered by darker brown on proximal edge ( Figs 30 View FIGURES 25–34 , 97 View FIGURES 94–100 ). ♂: Penis not as above.
69 (70) ♂: All abdominal terga I–X dark brown. Dorsal margin of styliger rounded, without median projection; spear-like rolls very long and thick, directed caudally ( Traver & Edmunds 1967: fig. 42)................. Th. regulus Traver & Edmunds 1967
70 (69) ♂: At least abdominal terga II–V light.
71 (72) ♂: Abdominal terga II–VI with grayish brown dome-shaped patches ( Traver & Edmunds 1967: figs 10–11)................................................................................... Th. gonzalesi Traver & Edmunds 1967
72 (71) ♂: Abdominal coloration not as above.
73 (80) ♂: Abdominal terga II–V mostly light, VI–VII mostly colored ( Figs 198 View FIGURES 198–202 , 232 View FIGURES 232–239 , 264 View FIGURES 260–266 ).
74 (75) ♂: Penis long and parallel-sided ( Dominguez 1987: fig. 10)........................ Th. cochunaensis Dominguez 1987
75 (74) ♂: Penis widened apically ( Figs 204 View FIGURES 203–206 , 242 View FIGURES 240–245 , 268–269 View FIGURES 267–273 ).
76 (77) ♂: Spear-like rolls directed dorsally ( Fig. 267 View FIGURES 267–273 )................................... 8. Th. quevedoensis Flowers 2009 View in CoL
77 (76) ♂: Spear-like rolls directed ventrally ( Figs 203 View FIGURES 203–206 , 240 View FIGURES 240–245 ).
78 (79) ♂: Apices of penis lobes as wide as gonostylar cavity of styliger ( Fig. 242 View FIGURES 240–245 ). Distribution: from Panama to Venezuela............................................................... 7. Th. marreroi Chacon, Segnini & Dominguez 1999 View in CoL
79 (78) ♂: Apices of penis lobes wider than gonostylar cavity of styliger ( Fig. 204 View FIGURES 203–206 ). Distribution: Peru and Brazil................................................................................. 6. Th. schlingeri Traver & Edmunds 1967 View in CoL
80 (73) ♂: Abdominal terga II–VI mostly light, VII mostly colored ( Figs 25 View FIGURES 25–34 , 52 View FIGURES 52–59 , 99 View FIGURES 94–100 , 136 View FIGURES 133–142 , 166 View FIGURES 156–168 ).
81 (84) ♂: Spear-like rolls directed ventrally-proximally, with groove widely opened ( Figs 38 View FIGURES 35–41 , 60 View FIGURES 60–63 ).
82 (83) ♂: Spear-like rolls S-shaped; dorsal extension of styliger wide and not separated from styliger ( Fig. 60 View FIGURES 60–63 )....................................................... 2. Th. sinuosus Mariano & Flowers (in Mariano, Flowers & Froehlich 2011) View in CoL
83 (82) ♂: Spear-like rolls very short, lanceolate; dorsal extension of styliger finger-like and separated from styliger ( Figs 38, 40 View FIGURES 35–41 )...................................................................................... 1. Th. ludmilae sp. n.
84 (81) ♂: Spear-like rolls directed medially (medially, medially-ventrally, or medially-dorsally) ( Figs 112–120 View FIGURES 112–120 , 145 View FIGURES 143–148 , 169 View FIGURES 169–173 ).
85 (86) ♂: Penis lobes narrowed apically (Gonçalves et al. 2010: fig. 5)......... Th. jones Gonçalves, Da-Silva & Nessimian 2010
86 (85) ♂: Penis lobes not narrowed apically ( Figs 112–120 View FIGURES 112–120 , 145 View FIGURES 143–148 , 169 View FIGURES 169–173 ).
87 (90) ♂: Abdominal sterna II–VII with 2 or 3 pairs of dark dots.
88 (89) ♂: Penis lobes long, equally narrow all over length ( Traver & Edmunds 1967: fig. 43)........................................................................................ Th. itatiajanus Traver & Edmunds 1967 [ Thraulodes View in CoL ]
89 (88) ♂: Penis lobes short and greatly expanded laterally at apex ( Dominguez 1987: fig. 7)........... Th. flinti Dominguez 1987 View in CoL
90 (87) ♂: Abdominal sterna II–VII with no more than one pair of dots.
91 (94) ♂: Penis without lateral pouches ( Figs 112–120 View FIGURES 112–120 ).
92 (93) ♂: Penis lobes short and sharply widened apically ( Boldrini et al. 2018: figs 6–7). Abdominal terga II–VI with reddish brown band adjacent to posterior margin (ibid: figs 2–3).......................... Th. rodrigoi Boldrini, Dantas & Lima 2018
93 (92) ♂: Penis lobes longer and slightly widened apically ( Figs 112–120 View FIGURES 112–120 ).Abdominal terga II–VI without contrasting dark band ( Figs 97, 99 View FIGURES 94–100 )........................................................ 3. Th. telegraphicus Needham & Murphy 1924 View in CoL
94 (91) ♂: Penis with lateral pouches ( Figs 144 View FIGURES 143–148 , 171 View FIGURES 169–173 ).
95 (101) ♂: Lateral pouches large, so that at midlength penis as wide or wider than at apex.
96 (97) ♂: Dorsal margin of styliger rounded, without median projection ( Chacon et al. 1999: fig. 5)........................................................................................ Th. mucuy Chacon, Segnini & Dominguez 1999 View in CoL
97 (96) ♂: Dorsal margin of styliger with median projection.
98 (99, 100) ♂: Abdominal coloration and genitalia as described by Traver & Edmunds (1967: fig. 33).... Th. ulmeri Edmunds 1950 View in CoL
99 (98, 100) ♂: Abdominal coloration and genitalia as described by Mariano et al. (2011: figs 14–15)................................................................ Th. pelicanus Mariano & Froehlich (in Mariano, Flowers & Froehlich) 2011 View in CoL
100 (98, 99) ♂:Abdominal coloration and genitalia as described by Gonçalves et al. (2013: figs 4–6)..................................................................................... Th. bonito Gonçalves, Da-Silva & Nessimian 2013
101 (95) ♂: Lateral pouches small, so that at midlength penis much narrower than at apex ( Figs 144 View FIGURES 143–148 , 171 View FIGURES 169–173 ).
102 (103) ♂: Abdominal terga VIII–X dark reddish brown, tergum VII lighter........................ Th. centralis Traver 1946 View in CoL
103 (102) ♂: Abdominal tergum VII not lighter than tergum VIII ( Figs 136 View FIGURES 133–142 , 166 View FIGURES 156–168 ).
104 (105, 110) Fore femur, besides dark band in distal half, with dark band in proximal half................................................................................... Th. trijuncta Banks 1918 and Th. bolivianus Dominguez 1986
105 (104, 110) Fore femur, besides dark band in distal half, with small dark spot in proximal half ( Figs 134 View FIGURES 133–142 , 160 View FIGURES 156–168 ).
106 (107) ♂: Pronotum grayish-brown with white spots...................... Th. guanare Chacon, Segnini & Dominguez 1999 View in CoL
107 (106) ♂: Pronotum white ( Figs 134, 136 View FIGURES 133–142 , 157 View FIGURES 156–168 ).
108 (109) Macula on costal brace not continuous posteriorly ( Figs 134–136 View FIGURES 133–142 ). Scutellum white ( Fig. 136 View FIGURES 133–142 )... 4. Th. panamensis sp. n.
109 (108) Macula on costal brace continuous posteriorly ( Figs 157–159 View FIGURES 156–168 ). Scutellum brown ( Fig. 159 View FIGURES 156–168 )....... 5. Th. viviparus sp. n.
110 (104, 105) Fore femur without dark marking in proximal half......................... Th. calori Campos & Mariano 2019
Key to examined larvae of Thraulodes View in CoL View at ENA
1 (8) Labrum with sharp median emargination (in anterior-dorsal view), anterior transverse row of setae in median part irregular, with many setae in section ( Figs 190–197 View FIGURES 190–197 , 209 View FIGURES 208–231 , 389–395 View FIGURES 389–395 , 650–653 View FIGURES 650–653 )
2 (3) Cuticular coloration: tibiae banded ( Figs 642–644 View FIGURES 637–649 )........................................ 20. Thraulodes View in CoL sp. «Itaya»
3 (2) Cuticular coloration: tibiae unicolor.
4 (7) Cuticular coloration: pronotum with blanks of characteristic composite form ( Figs 175, 185 View FIGURES 174–189 , 214, 218, 227 View FIGURES 208–231 ); abdominal terga IV–V, or at least tergum V contrastingly light medially ( Figs 177, 185 View FIGURES 174–189 , 215, 219, 227 View FIGURES 208–231 ).
5 (6) Tergalii with tracheal branches by sides of main trachea ( Figs 178–184 View FIGURES 174–189 )................................ 6. Th. schlingeri View in CoL
6 (5) Tergalii without tracheal branches by sides of main trachea ( Figs 220–226 View FIGURES 208–231 )....................... 7. Thraulodes marreroi View in CoL
7 (4) Cuticular coloration: pronotum without contrasting blanks ( Fig. 382 View FIGURES 379–388 ); abdominal terga without large blanks mediad of tergalii bases ( Fig. 383 View FIGURES 379–388 )......................................................................... 12. Th. spangleri View in CoL
8 (1) Labrum without median emargination, anterior transverse row of setae regular, with one seta in section ( Figs 86–88 View FIGURES 86–93 , 563 View FIGURES 563–568 ).
9 (10) Cuticular coloration: tibiae contrastingly banded ( Figs 548–550 View FIGURES 543–562 ). Tergalius with main trachea arched anteriorly (i.e. repeating outline of anal margin), with branches directed toward costal margin ( Figs. 551–557 View FIGURES 543–562 , 585–588 View FIGURES 578–588 )..... 17. Th. alboniger sp. n.
10 (9) Cuticular coloration: tibiae unicolor ( Fig. 9 View FIGURES 5–13 ). Tergalius with main trachea either straight, or arched posteriorly, with branches either directed toward both margins, or absent.
11 (14) Cuticular coloration: pronotum entirely light, anterior part of mesonotum contrastingly dark ( Figs 8 View FIGURES 5–13 , 66 View FIGURES 66–69 , 70 View FIGURES 70–85 ).
12 (13) Tergalii narrow, without tracheal branches by sides of main trachea ( Fig. 11 View FIGURES 5–13 ). On hind tibia stout setae of inner-anterior row variable, long and short ( Fig. 24 View FIGURES 21–24 )......................................................... 1. Th. ludmilae sp. n.
13 (12) Tergalii wide, with tracheal branches by sides of main trachea ( Fig. 90–93 View FIGURES 86–93 ). On hind tibia all stout setae of inner-anterior row short ( Fig. 85 View FIGURES 70–85 ).................................................................... 3. Th. telegraphicus View in CoL (part)
14 (11) Cuticular coloration: pronotum not contrastingly lighter than mesonotum.
15 (16, 17, 18, 19) Cuticular coloration: abdominal terga mostly light, with pair of large brown maculae on terga VI–VII and previous ones ( Figs 595 View FIGURES 591–602 , 603 View FIGURES 603–612 )........................................................................ 18. Th. lepidus View in CoL
16 (15, 17, 18, 19) Cuticular coloration: on each abdominal tergum area adjacent to anterior margin together with antero-median and sublateral areas brown, remainder part contrastingly light ( Figs 414, 419, 421 View FIGURES 411–426 )...................... 13. Th. niger sp. n.
17 (15, 16, 18, 19) Cuticular coloration: abdominal terga I–VII contrastingly lighter than terga VIII–IX ( Figs 453, 458, 459 View FIGURES 450–463 )........................................................................ presumably 14. Th. nigrabdominalis sp. n.
18 (15, 16, 17, 19) Cuticular coloration: abdominal terga IV–V contrastingly lighter than terga VI–VII ( Figs 66 View FIGURES 66–69 , 81 View FIGURES 70–85 )............................................................................................. 3. Th. telegraphicus View in CoL (part)
19 (15, 16, 17, 18) Cuticular coloration: all abdominal terga either uniformly dark with light lateral margins ( Fig. 297 View FIGURES 297–308 ), or some terga also with blanks medially ( Figs 154–155 View FIGURES 151–155 , 350 View FIGURES 350–360 ).
20 (21) Proximal part of fore tibia with inner-anterior row of recurved hairs ( Figs 621–622 View FIGURES 613–625 )....... 19. Thraulodes View in CoL sp. «Palo Seco»
21 (20) Fore tibia without inner-anterior row of recurved hairs.
22 (23) Tergalii darkened near costal margin ( Fig. 153 View FIGURES 151–155 )................................... presumably 5. Th. viviparus sp. n.
23 (22) Tergalii either evenly colored ( Fig. 129 View FIGURES 122–132 ), or darkened near main trachea ( Figs 291 View FIGURES 276–296 , 361 View FIGURES 361–364 , 385 View FIGURES 379–388 ).
24 (35) Tergalii without visible side tracheae ( Figs 129 View FIGURES 122–132 , 361 View FIGURES 361–364 ) or with one proximal-costal trachea on ventral lamella ( Fig. 291 View FIGURES 276–296 ).
25 (28) On hind tibia stout setae of inner-anterior row variable, long and short ( Figs 51 View FIGURES 42–51 , 259 View FIGURES 250–259 ).
26 (27) On fore and middle legs claw with small denticles anteriad of main row of denticles ( Fig. 273 View FIGURES 267–273 )......... 8. Th. quevedoensis View in CoL
27 (26) On all legs claw without small denticles anteriad of main row of denticles (as in Fig. 69 View FIGURES 66–69 )................. 2. Th. sinuosus View in CoL
28 (25) On hind tibia all stout setae of inner-anterior row short ( Figs 132 View FIGURES 122–132 , 296 View FIGURES 276–296 , 336 View FIGURES 317–336 , 358 View FIGURES 350–360 ).
29 (30) On femora and hind tibia short stout setae narrowed distally ( Figs 358, 359 View FIGURES 350–360 )....................... 11. Th. flavus sp. n.
30 (29) On femora and hind tibia short stout setae parallel-sided or widened distally ( Figs 130, 132 View FIGURES 122–132 , 293, 296 View FIGURES 276–296 , 303 View FIGURES 297–308 , 336 View FIGURES 317–336 ).
31 (32) Tergalii with both lamellae narrow, without side tracheae ( Fig. 129 View FIGURES 122–132 ).......................... 4. Th. panamensis sp. n.
32 (31) Tergalii with ventral lamella widened proximally ( Figs 284–290 View FIGURES 276–296 , 321–327 View FIGURES 317–336 ), sometimes with one side trachea ( Fig. 291 View FIGURES 276–296 ).
33 (34) Last instar male larva: protogonostyli very short, separated by shallow concavity ( Fig. 313 View FIGURES 209–314 )....... 9. Th. fascipennis sp. n.
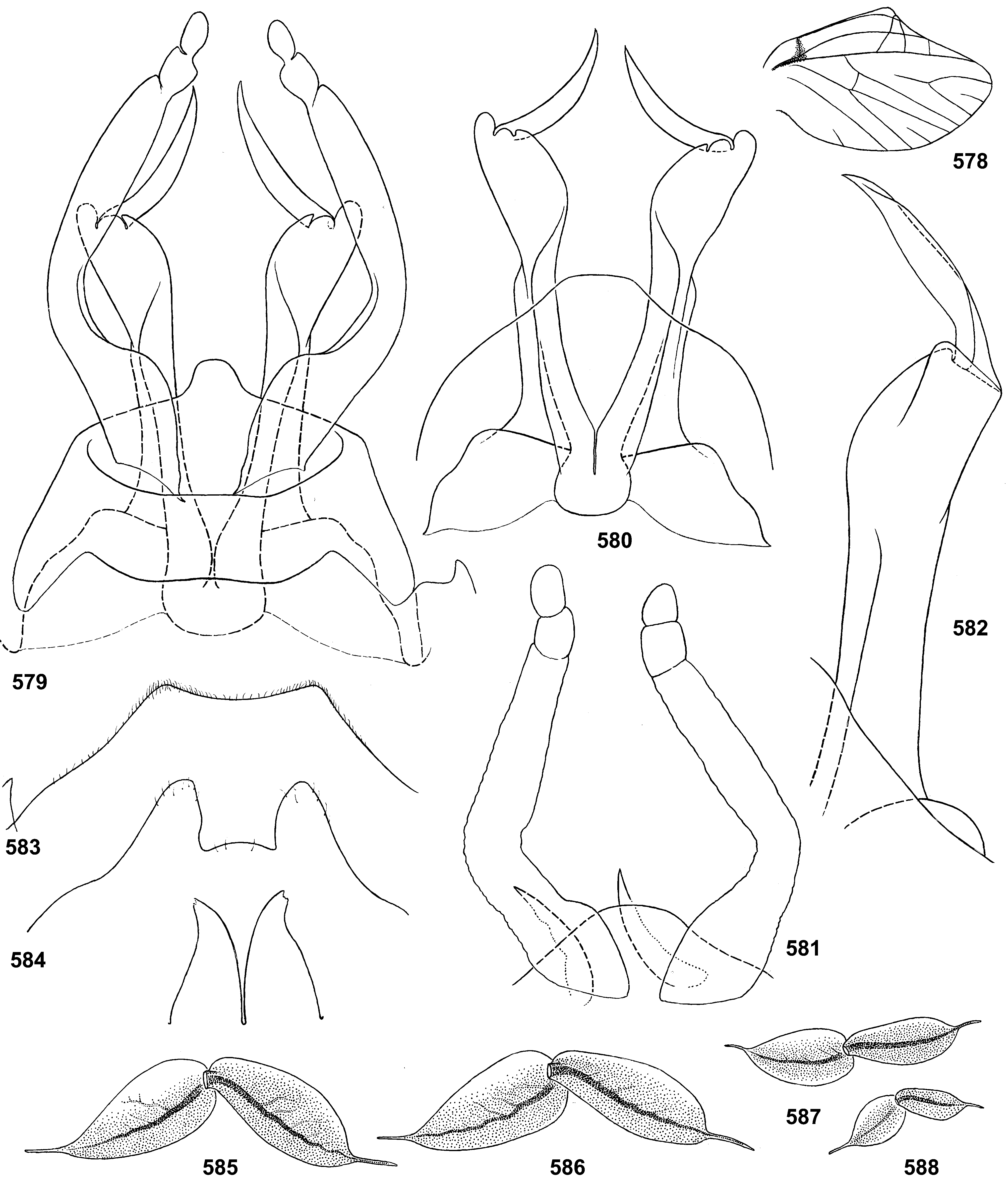
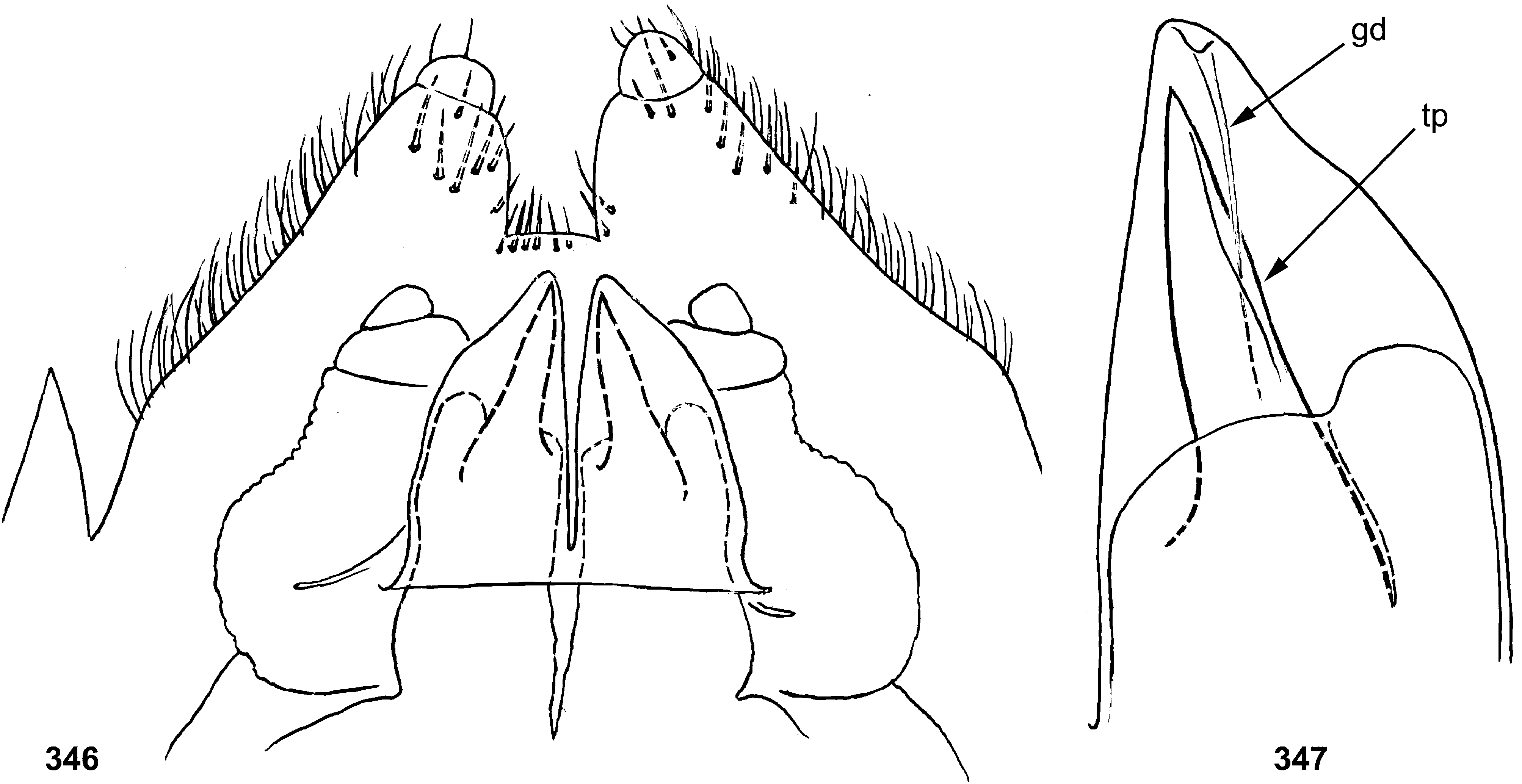
34 (33) Last instar male larva: protogonostyli long, separated by deep emargination ( Fig. 346 View FIGURES 346–347 )................... 10. Th. zonalis View in CoL
35 (24) Tergalii with well-visible side tracheae ( Figs 66–68 View FIGURES 66–69 , 90–93 View FIGURES 86–93 ).
36 (37) Tergalii very wide; dorsal lamella with costal and anal margins equally convex in proximal and distal parts, abruptly narrowed toward slender apical filament ( Figs 666–672 View FIGURES 666–672 )................................................ 21. Th.? consortis View in CoL
37 (36) Tergalii less wide; dorsal lamella widest in proximal or middle part, gradually narrowed toward slender apical filament ( Figs 66–68 View FIGURES 66–69 , 90–93 View FIGURES 86–93 , 503–506 View FIGURES 498–507 , 537–540 View FIGURES 532–540 ).
38 (39) In last instar larva hypoderm of tibiae more or less darkened with brown or blackish.......................................................................................... 1 5. Th. nigripes sp. n. and 16. Th. nigrotibialis sp. n.
39 (38) Tibia never darker than femur (in mature larva only apex of tibia with dark hypodermal coloration).................................................................................................... 3. Th. telegraphicus View in CoL (part)
Morphological notes
Traver and Edmunds (1967) gave a detailed review of imaginal, larval and egg structure of Thraulodes , noted significance of certain morphological features for diagnoses of species and discussed individual variability. The new data confirm most statements given in this publication, but some additions and clarifications should be made.
Larval habitus
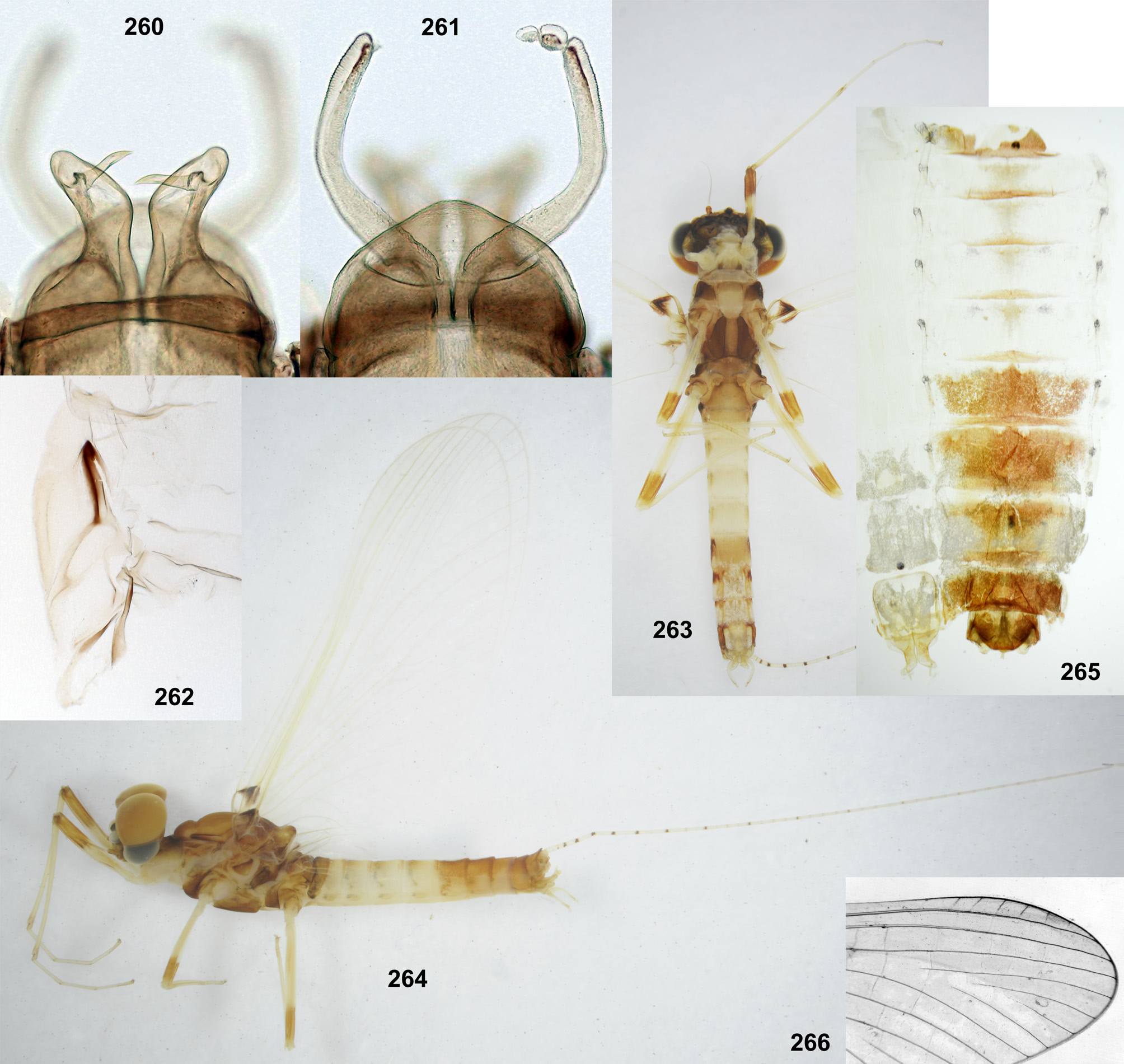
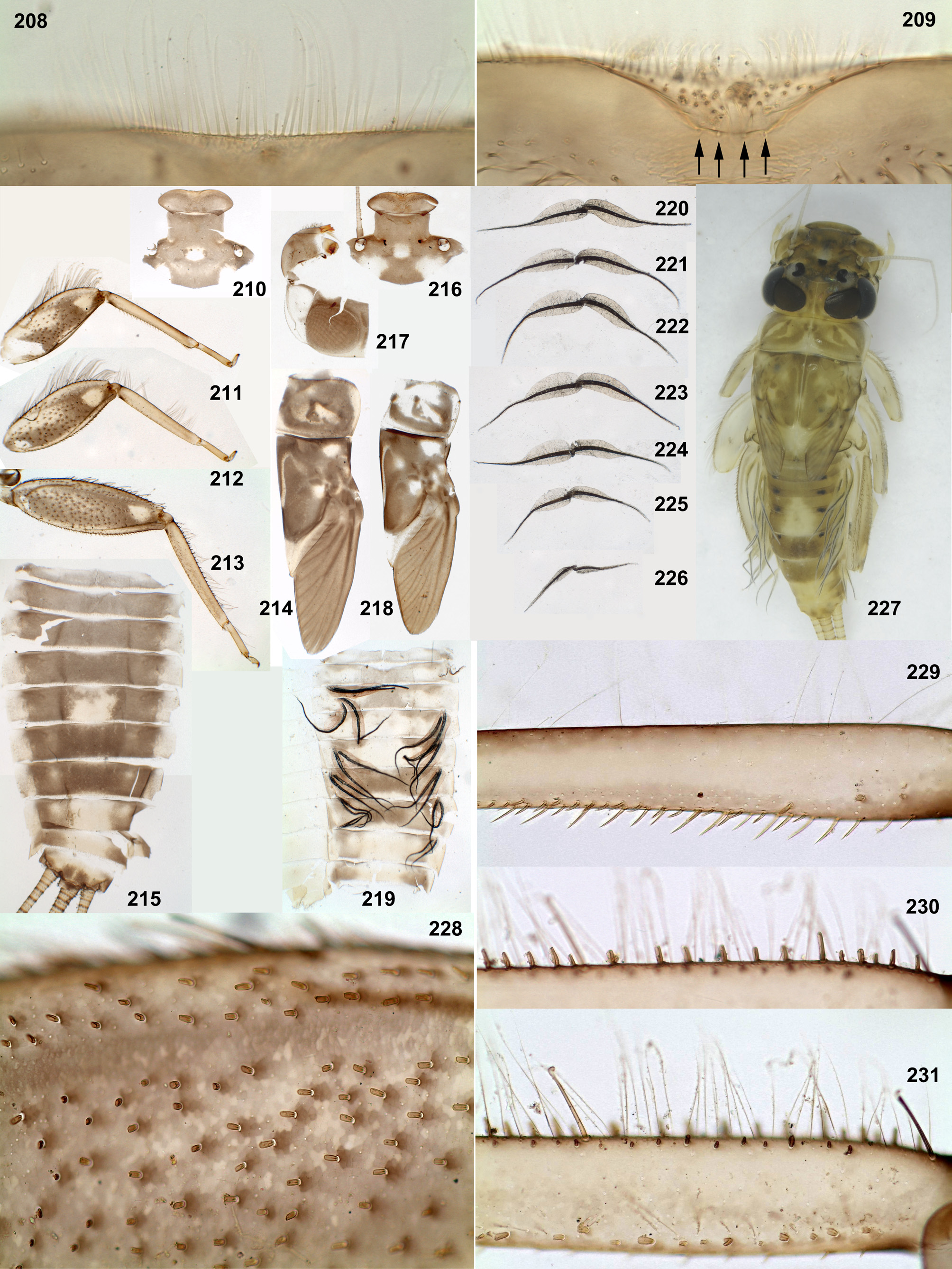
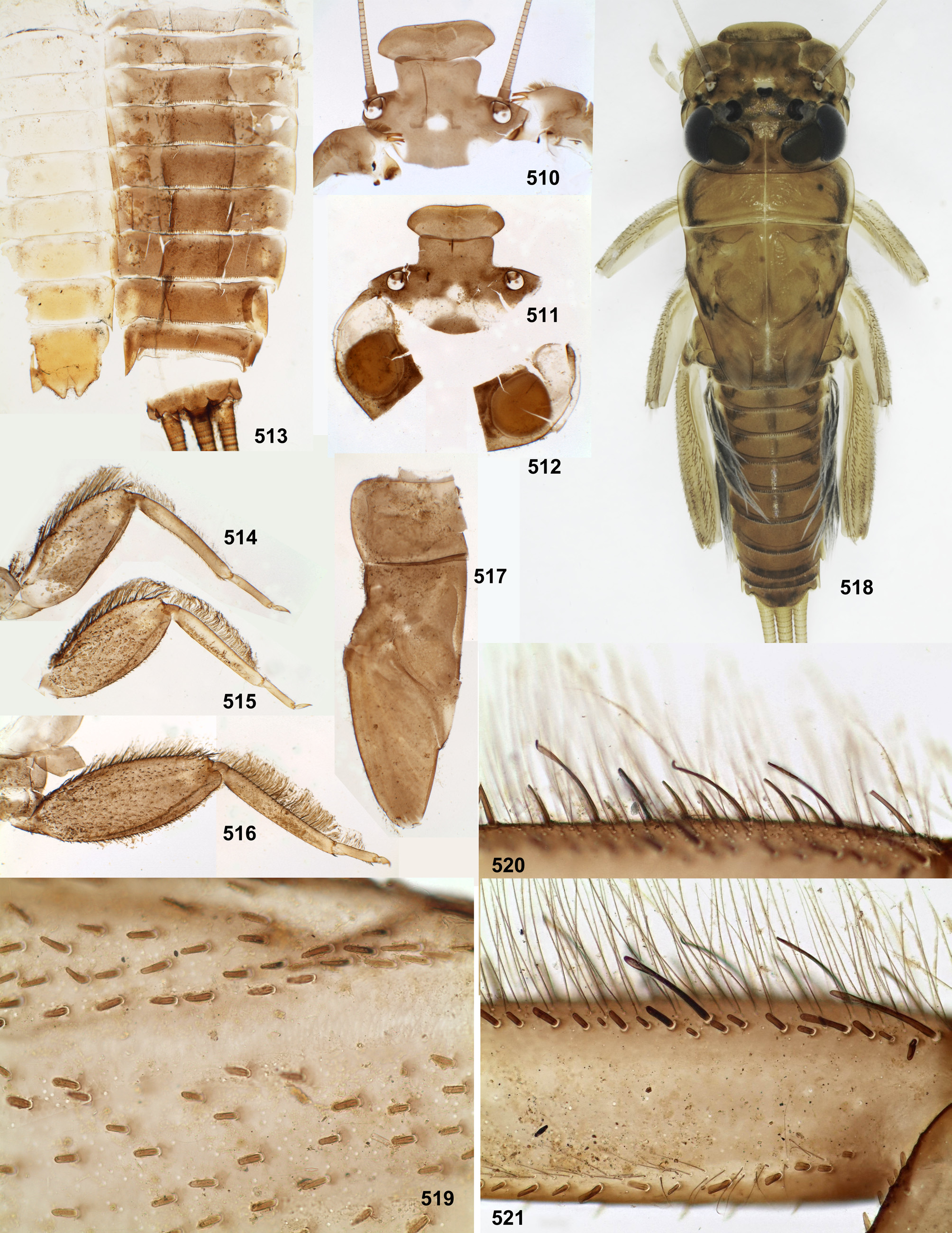
In all species of Thraulodes larvae have a similar body shape, which is widened and flattened dorsoventrally, an adaptation for inhabiting stone surfaces in rapidly running water. The head is wide, with the clypeus parallel-sided or slightly widened anteriorly, with labrum wider than clypeus. The pronotum is nearly rectangular, with rounded antero-lateral angles and a free anterior margin (i.e. transverse margin between antero-lateral angles and the neck membrane); a transverse ridge runs parallel to the free anterior margin; humeral setae (which are characteristic for Leptophlebiidae ) are stout and located between the free anterior margin and the ridge, being absent in other places; these setae are narrowed distally, either pointed or blunt, either forming a nearly regular row ( Fig. 12 View FIGURES 5–13 ), or situated rather irregularly (the same in many other Atalophlebolinguata). Legs are large, with widened femora and claws bent perpendicular to the leg flatness.
Structure of labrum
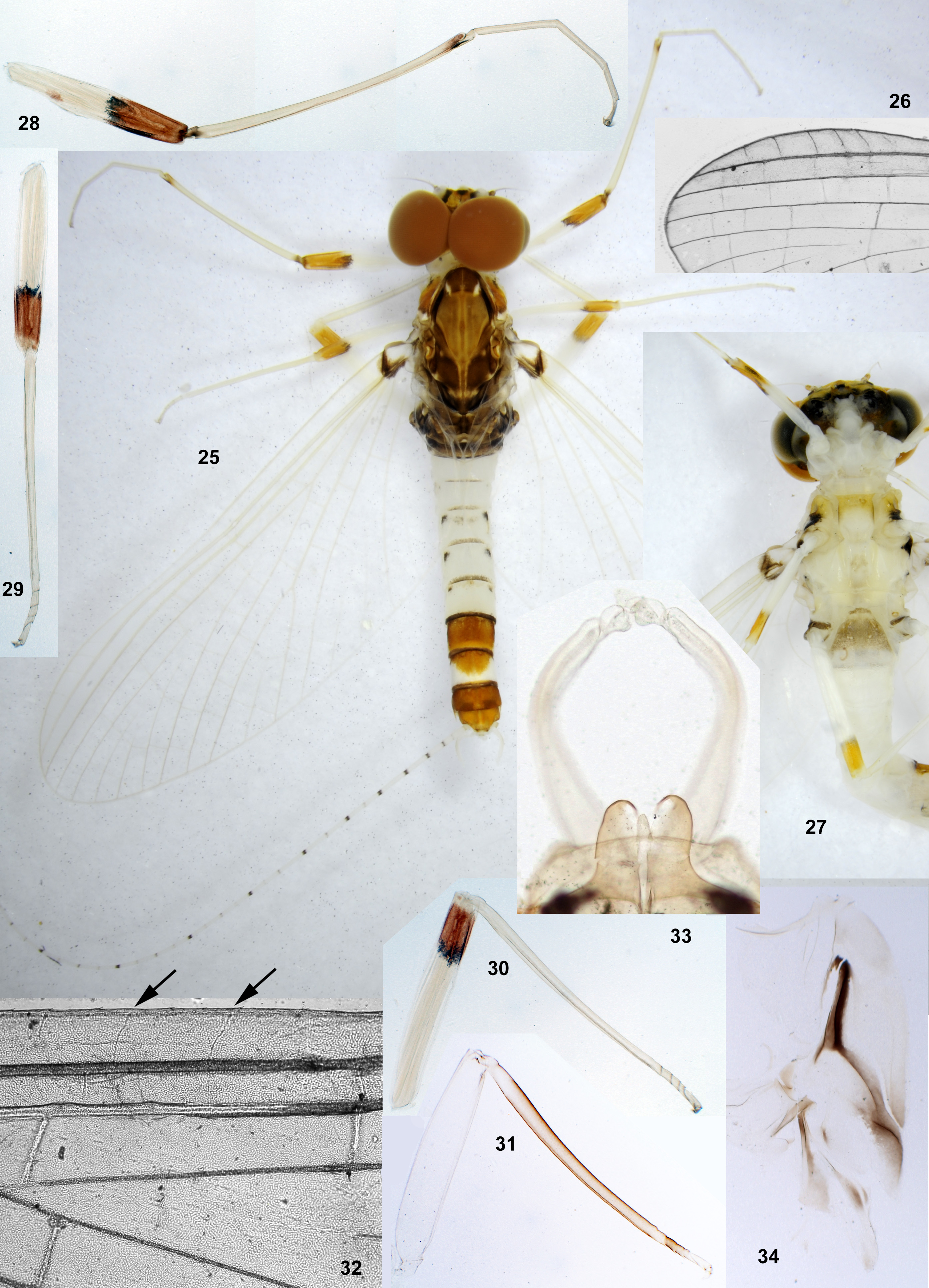
Peters (1980) divided Leptophlebiidae into two subfamilies, Leptophlebiinae and Atalophlebiinae , each of which he regarded to be holophyletic.Among autapomorphies of Atalophlebiinae , he reported the following character: «Anteromedian emargination of labrum with denticles or with acute median cleft ...», contrary to the plesiomorphy of Leptophlebiinae «Anteromedian emargination of labrum smooth, without denticles ...» ( Peters 1980: Table 1 View TABLE 1 ). According to my opinion ( Kluge 2009), his subfamily Leptophlebiinae is paraphyletic, and his subfamily Atalophlebiinae is really holophyletic and corresponds to the rank-free taxon Atalophleboculata Kluge 2009. However, the character of Atalophlebiinae cited above, seems to be not a good autapomorphy of this taxon. Denticles reported here, are convexities separated by sensilla; these sensilla are found in various taxa of Ephemeroptera , usually are 6 in number and form a regular transverse row on the distal margin of labrum ( Figs 1–3 View FIGURES 1–4 ); if surface between each two sensilla forms a convexity, labrum bears a row of 5 convexities, or denticles ( Fig. 4 View FIGURES 1–4 ). Because of this, 5 denticles in the median emargination of labrum are found not only among representatives of Atalophleboculata, but also among representatives of Leptophlebiinae sensu Peters 1980 .
Initially for Atalophlebopectinata (the taxon comprising Atalophleboculata and Habrophlebia /fg1), labrum bears two transverse rows of setae—anterior and posterior ones.
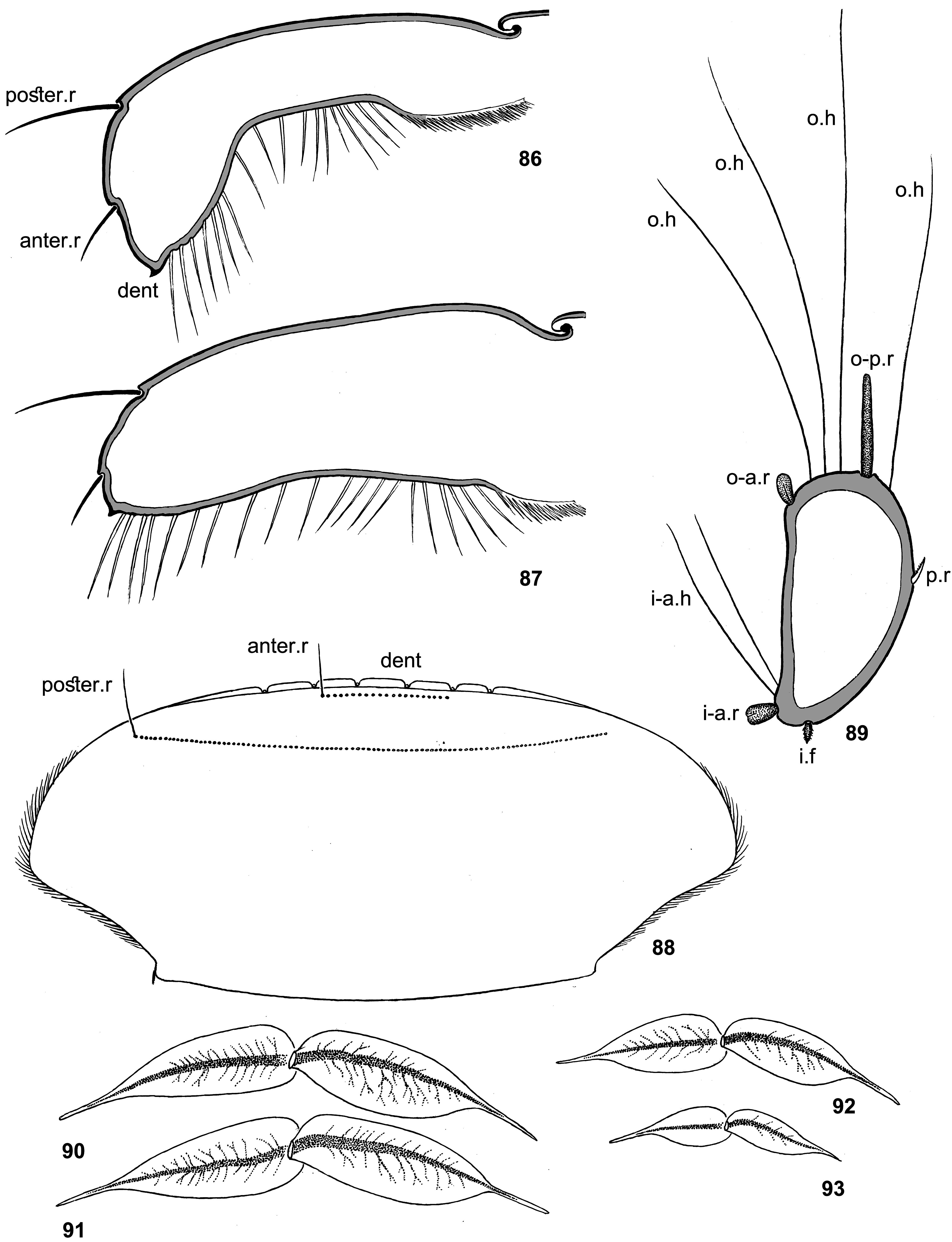
The larval head of Thraulodes is prognathous, and labrum is normally directed anteriorly. In the normal condition, the initial anterior margin of labrum (which bears denticles) is turned ventrally, so that in dorsal view the anterior transverse setal row (or even the posterior setal row) appears located on anterior margin, and the initial anterior margin with denticles is hidden beneath the labrum ( Fig. 86 View FIGURES 86–93 ). In this case shape and denticulation of the initial anterior margin can be visible in frontal view. In another condition of the same labrum, when it is swollen and straightened, its denticles can be visible in dorsal view ( Figs 87, 88 View FIGURES 86–93 ).
In all examined species of Thraulodes , the number of denticles is normally 5, but can be less in selected individuals. The posterior transverse setal row is always regular, nearly straight, located on the anterior part of labrum and occupies most of its width ( Fig. 88 View FIGURES 86–93 ). The anterior transverse setal row is often much shorter, either also regular ( Fig. 88 View FIGURES 86–93 ), or irregular.
In Th. schlingeri , Th. marreroi , Th. spangleri and Th. sp. «Itaya» the labrum has narrow and deep median emargination ( Fig. 194 View FIGURES 190–197 ), and 5 denticles are located in this emargination often in such a way that 3 of them are relatively narrow and form the bottom of the emargination, and 2 lateral denticles are wider and form lateral sides of the emargination ( Figs 197 View FIGURES 190–197 ). Since the emargination with its denticles is directed ventrally, in dorsal view the outline of the labrum appears as a shallow median concavity without denticles ( Fig. 191 View FIGURES 190–197 , 208 View FIGURES 208–231 ). In all these four species anterior transverse row of setae in its median part (i.e. just behind the median emargination) represents a field of irregularly situated setae ( Figs 193, 195 View FIGURES 190–197 ). The same median emargination is recently reported for Thraulodes sternimaculatus Lima et al. 2013 ( Nascimento et al. 2019)
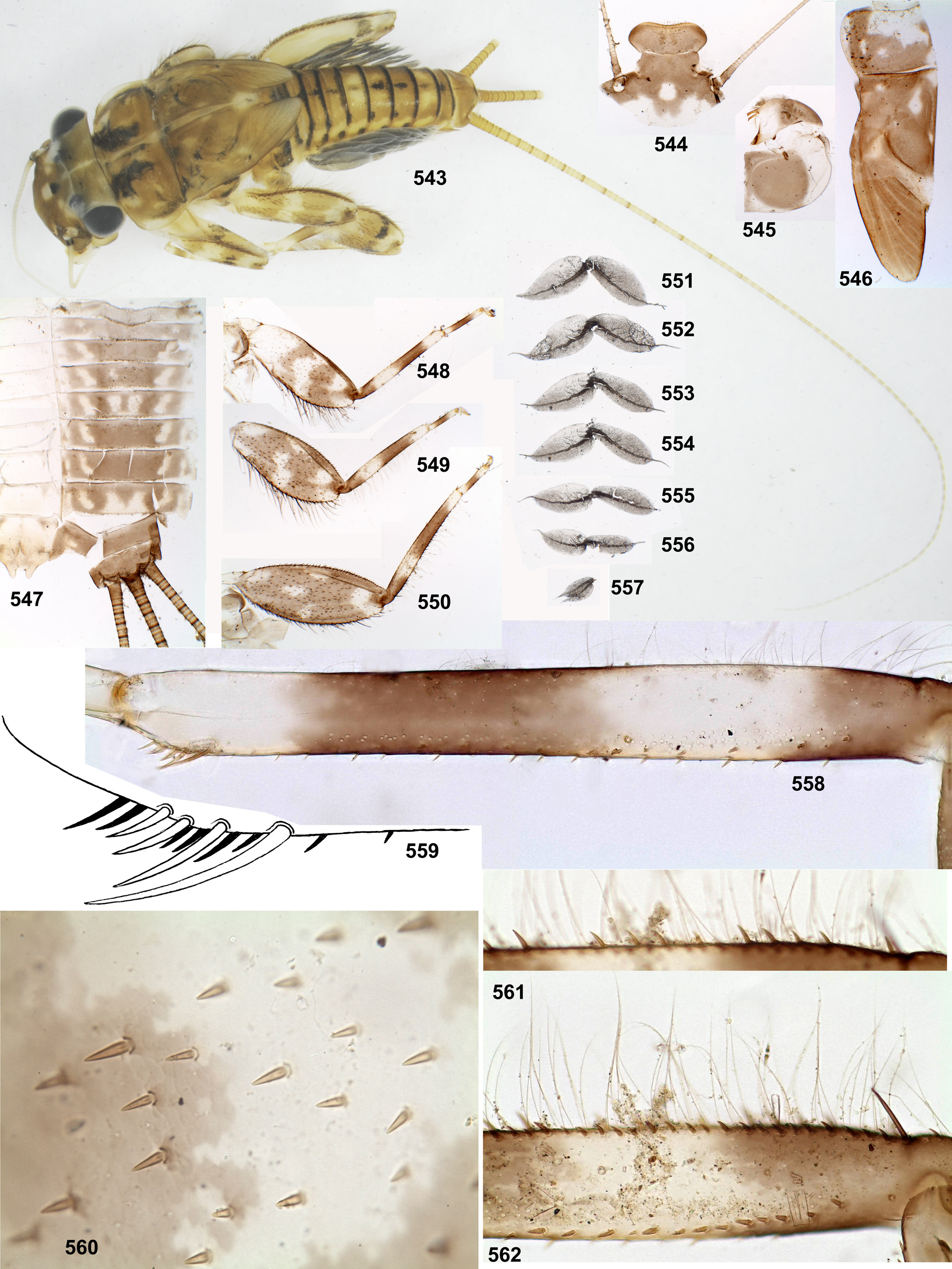
In all other examined species of Thraulodes the labrum has no median emargination, all its 5 denticles are very wide and low, and anterior transverse row of setae is regular in all its parts ( Fig. 88 View FIGURES 86–93 ); the initial anterior margin, which bears denticles, is either straight, or slightly convex ( Fig. 88 View FIGURES 86–93 ), or slightly concave ( Fig. 563 View FIGURES 563–568 ). Possibly, width of the anterior setal row is species-specific: in the examined individuals of Th. ludmilae sp. n., Th. sinuosus , Th. panamensis sp. n. and Th. quevedoensis this row is about as wide as 3 denticles (among 5 existent ones), while in examined individuals of Th. fascipennis sp. n., Th. zonalis , Th. flavus sp. n., Th. niger sp. n., Th. nigrabdominalis sp. n., Th. nigripes sp. n., Th. nigrotibialis sp. n., Th. lepidus, Th. sp. «Palo Seco» and Th. consortis (?) this row is wider, being about as wide as all 5 denticles, or wider. Among examined specimens of Th. telegraphicus , width of the anterior setal row varies from width of 3 denticles ( Fig. 88 View FIGURES 86–93 ) to width of 5 denticles. In Th. alboniger s. n., the anterior transverse setal row is wider than in all other examined species, being approximately as wide as the posterior transverse setal row ( Fig. 563 View FIGURES 563–568 ).
Structure of mandibles
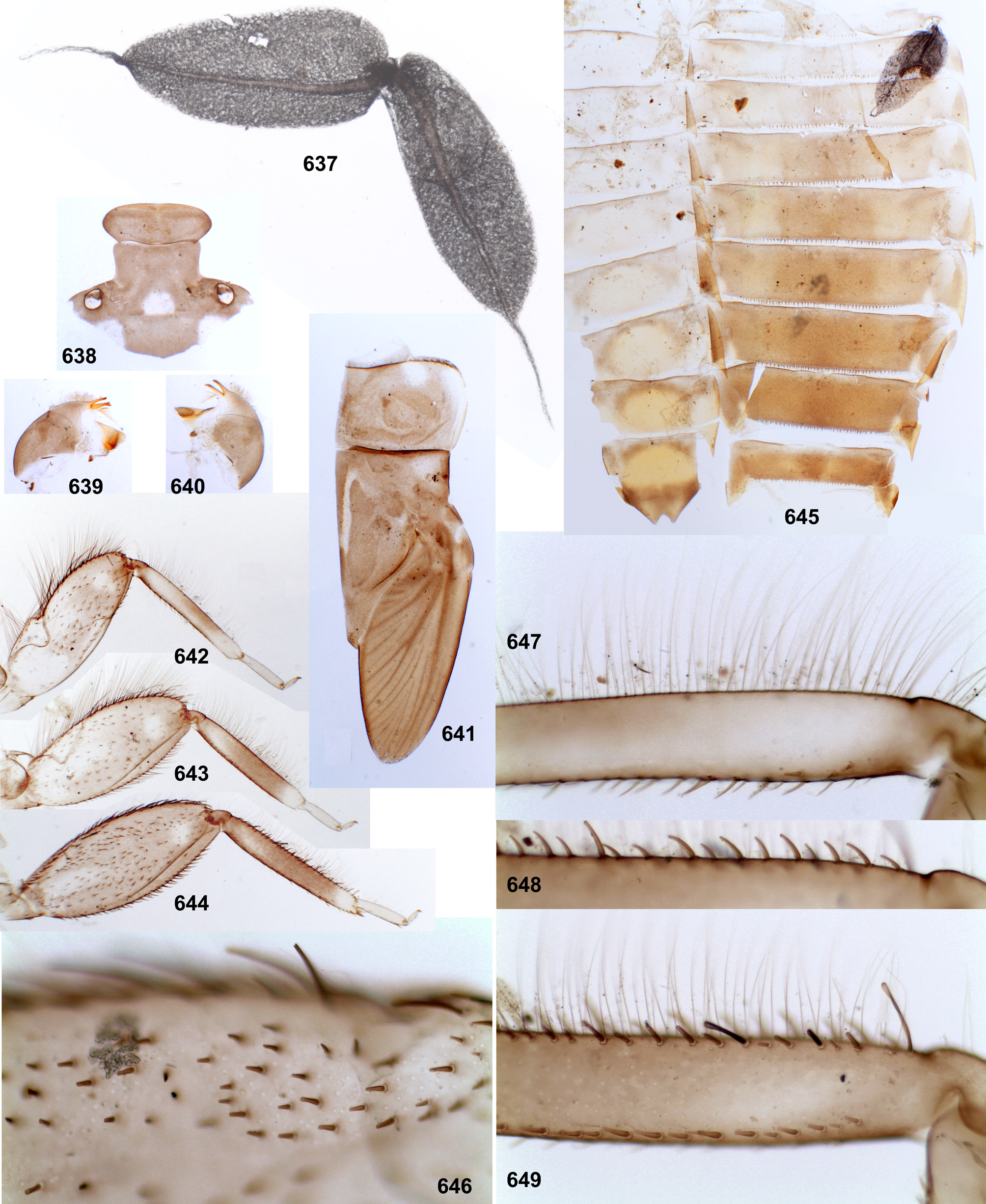
Mandibles have the outer margin either entirely convex ( Figs 17–18 View FIGURES 14–20 ), or with a shallow concavity ( Figs 250– 251 View FIGURES 250–259 ). In Thraulodes sp. «Palo Seco» the outer margin of each mandible is widened and angulate ( Figs 614–615 View FIGURES 613–625 ); the same in Thraulodes sp. from Brazil ( Traver & Edmunds 1967: fig. 76).
In all Thraulodes , outer margin of mandible bears long hairs, which are denser and longer nearer the base of incisor, i.e. form a distal tuft ( Figs 17–18 View FIGURES 14–20 ). In contrast to this, in many other Leptophlebiidae the mandibles either have no long setae on outer margin, or have most long and dense setae at the middle of outer margin. Traver and Edmunds (1967) stated, that «This series of hairs ... is so distinctive of this genus that we believe it to be of major importance in distinguishing Thraulodes nymphs from those of all other genera ...»; as genera whose mandibular setation has some similarity with that of Thraulodes , they reported only Ulmeritus Traver 1956 with allied genera, Massartellopsis Demoulin 1955a and Meridialaris Peters & Edmunds 1972 (termed «Chilean Deleatidium »). Among species examined by me, many Leptophlebiidae have more or less long and dense setae on middle part of outer side of mandible, while the area near base of incisor completely lacks setae; in many cases setae form a distinct tuft at the middle of outer side of mandible, which can be called middle tuft. In fewer cases, mandible also has setae near the base of the incisor, which either form a weak row stretched from the middle tuft toward the base of incisor (e.g., in Massartellopsis and Meridialaris ), or form a second, distal tuft, e.g. in Ulmeritus , Atopophlebia Flowers 1980 , Nathanella Demoulin 1955b , Nonnullidens Grant & Peters 1993 ( Kluge 2013: fig. 3), Kimminsula Peters & Edmunds 1970 , undescribed taxa related to Kimminsula and selected species of Thraulus Eaton 1881 . Besides Thraulodes , in a few other taxa the middle tuft is poorly expressed or absent, while the distal tuft is present. These are Ulmeritoides Traver 1959 , Neochoroterpes Allen 1974 and Petersula courtallensis Sivaramakrishnan 1984 (while an undescribed species of Petersula has both tufts). Thus, presence of the distal tuft combined with absence of the middle tuft is a rare, but not a unique character of Thraulodes ; most probably, it is an autapomorphy of Thraulodes .
Incisors, kinetodontia, prosthecae and molae of mandibles have uniform structure in all examined species of Thraulodes , being similar to that of many other Atalophlebomaxillata; both incisors and left kinetodontium are always terminated by 3 denticles each, right kinetodontium is always terminated by 2 denticles ( Figs 17–18 View FIGURES 14–20 ).
Structure of maxilla
In all species of Thraulodes the maxillae have a rather uniform structure, which is initial for the taxon Atalophlebomaxillata Kluge 2009 ( Kluge 2012) ( Fig. 19 View FIGURES 14–20 ). According to Traver and Edmunds (1967), «The slightly undulating row of large pectinate spines below the level of the crown on the ventral surface varies in number of spines from about 14 to 26 or more, a feature that may be of specific value». Among the specimens examined, number of stout pectinate setae (erroneously termed «spines») in this apical-ventral row varies from 10 to 24; in most species it varies individually as 13–18; in two examined specimens of Th. quevedoensis it is 11; in three examined specimens of Th. alboniger sp. n. it is 10; in Th. niger sp. n. and Th. nigrabdominalis sp. n. it varies as 19–21; in Th. lepidus varies as 23–24.
Traver and Edmunds (1967) paid attention to structure of the last (3rd) segment of maxillary palp, which has «a narrow clear area at base of the third segment on the outer side». Before this, some authors regarded this area to be an outgrowth of the 2nd segment: Traver (1935: 552) wrote that «The outer apical margin of the second joint prolonged into a slender process which lies appressed against the side of the short third joint»; Burks (1953: 93) wrote that «maxillary palp forceps-like at the apex». Actually, cuticle of the 3rd palpomere is pigmented on inner side and colorless on outer side of the palpomere; on dorsal side of the palpomere boundary between the pigmented and the colorless areas is sharp ( Figs 19 View FIGURES 14–20 , 363 View FIGURES 361–364 ), that makes illusion of a longitudinal line separating two lobes; on ventral side of the palpomere its pigmentation changes gradually ( Fig 362 View FIGURES 361–364 ). This coloration is present in most of examined species, except for Th. alboniger sp. n., whose colorless area of the 3rd maxillary palpomere is greatly diminished.
Structure of labium
The glossae lie in one plane with the paraglossae; long setae of their ventral side are situated densely and irregularly ( Fig. 19 View FIGURES 14–20 ); long setae on the dorsal side of glossa form irregular row or stripe of a characteristic horseshoe shape ( Fig. 20 View FIGURES 14–20 ); the same was observed in Atopophlebia sp. (in contrast to other taxa, whose dorsal setae of glossa are situated irregularly or form a transverse row). According to Traver and Edmunds (1967), «Glossae of labium ... straight on inner margins, well rounded on outer; rather long spines are present along apical margin; a curved row of short spines occurs on dorsal surface and close-set hairs on ventral surface». Actually, in all examined species setae (erroneously termed «spines») on the apical margin are either absent, or short and stout as on inner and outer margins ( Fig. 20 View FIGURES 14–20 ); the illusion of long apical setae is sometimes caused by the ventral setae (close-set hairs), which can be projected apically. Setae forming an irregular horseshoe row on dorsal side are as long and thin as setae (hairs) on the ventral side; the illusion of «short spines» appears if these setae are directed perpendicular to the surface.
In all species examined, apical (3rd) segment of labial palp is short and conic, much narrower at base than apex of 2nd segment ( Traver & Edmunds 1967: fig. 82).
Setation of larval legs
In larvae of all Leptophlebiidae , fore, middle and hind legs have significant differences in shape and setation ( Table 1 View TABLE 1 ). Most often and probably initially for Leptophlebiidae are the following differences: the fore femur is widened near the base (where the tibial adductor is attached), with outer margin convex basally ( Fig. 9 View FIGURES 5–13 ); fore tibia (which always lacks patella-tibial suture) has dense stout pointed setae on inner margin ( Fig. 21 View FIGURES 21–24 ), while other tibiae have sparser and shorter pointed setae on inner margin ( Figs 22, 24 View FIGURES 21–24 ); hind tibia has stout setae on outer margin ( Fig. 24 View FIGURES 21–24 ), while other tibiae have no stout setae on outer margin ( Figs 21, 22 View FIGURES 21–24 ). Besides stout setae, outer margins of femur, tibia and tarsus of all legs usually bear more or less dense long, thin, projected hairs. Thraulodes has the following modification of these features.
Hairs of outer margin. Long hairs form dense irregular rows or stripes not only on outer margins of femur, tibia and tarsus, but also on corresponding (hind) margin of coxa ( Fig. 77 View FIGURES 70–85 ).
Setation of femur. Outer margin of each femur bears irregular outer stripe of thin hairs, bordered by two irregular stripes of stout setae— outer-posterior and outer-anterior ones ( Fig. 83 View FIGURES 70–85 ). On all legs of all examined species of Thraulodes , stout setae of these stripes irregularly vary from short to very long, with all intermediate forms; their structure and position did not reveal species-specific characters. Thin hairs of the outer stripe are denser and longer on fore and middle legs, but sparser and/or shorter on hind leg. In most species this difference between femora is well expressed ( Fig. 9 View FIGURES 5–13 ); in some species hairs of all femora are dense and long, but in these cases hairs of hind femur are at least somewhat shorter, than hairs on fore and middle femora ( Fig. 126 View FIGURES 122–132 ). Besides the hairs on outer margin, femur can bear a row of recurved hairs on anterior surface near inner margin ( Fig. 364 View FIGURES 361–364 ); this row of recurved hairs is often present on middle and hind legs, being absent on the fore leg; in Th. flavus sp. n., Th. sp. «Palo Seco» and Th. consortis (?) the row of recurved hairs is well developed on all legs; in Th. quevedoensis and Th. alboniger the row of recurved hairs is absent on all legs. Inner margin of femur always bears an irregular inner row of relatively short, stout pointed setae ( Fig. 364 View FIGURES 361–364 ). Anterior surface of each femur bears numerous irregularly situated stout setae, which are arranged either irregularly, or form more or less distinct longitudinal stripes. The shape of these setae is species-specific: they can be either widened distally with rounded apices ( Figs 130 View FIGURES 122–132 , 293 View FIGURES 276–296 ,), or parallel-sided ( Fig. 13 View FIGURES 5–13 ), or narrowed distally with apices either truncate ( Fig. 359 View FIGURES 350–360 ) or pointed ( Fig. 560 View FIGURES 543–562 ). Shape of these stout setae is the same on femora of all leg pairs. Posterior surface of hind femur bears a few small, pointed, curved, pectinate setae, which either form an irregular row parallel to inner margin of femur, or occupy up to half of posterior surface; femora of fore and middle legs usually have no setae on posterior surface.
Setation of tibia. Tibiae of fore, middle and hind legs have significantly different structures and bear the following rows and fields of stout setae and thin hairs. Outer-anterior row of stout setae ( Figs 85 View FIGURES 70–85 , 89 View FIGURES 86–93 : o-a.r): absent on fore and middle legs, well-developed on hind leg; consists of species-specific setae, often contains long spoonlike setae ( Fig. 520 View FIGURES 510–521 ). Outer-posterior row of stout setae ( Figs 85 View FIGURES 70–85 , 89 View FIGURES 86–93 : o-p.r): absent on fore and middle legs, welldeveloped on hind leg; consists of species-specific setae, can contain long spoon-like setae ( Fig. 426 View FIGURES 411–426 ). Inner-anterior row of stout setae ( Figs 85 View FIGURES 70–85 , 89 View FIGURES 86–93 : i-a.r): on fore leg either absent (in Th. panamensis — Fig. 131 View FIGURES 122–132 ), or represented by few setae on proximal part of tibia ( Fig. 21 View FIGURES 21–24 ); on middle leg present all along tibia, but sparse and often consists of short setae ( Fig. 22 View FIGURES 21–24 ); on hind leg well-developed ( Fig. 24 View FIGURES 21–24 ); consists of setae of species-specific shape, never contains spoon-like setae. Inner field of stout pointed setae ( Fig. 89 View FIGURES 86–93 : i.f): stretched all along inner side of tibia, always consists of pointed setae, which can be either smooth, or pectinate, or heavily dentate; on middle and hind legs these setae are always very small and sparse ( Figs 22, 24 View FIGURES 21–24 ), on fore leg larger and dense, at least near apex of tibia ( Fig. 21 View FIGURES 21–24 ). Near the apex of fore tibia, the most posterior row of this field (i.e., inner-posterior row) always consists of 3–4 closely situated arched pointed setae, among which the most proximal seta is significantly larger than others ( Fig. 559 View FIGURES 543–562 ). Setae of the inner field of fore tibia are either situated irregularly, or form more or less regular longitudinal rows; if setae of these rows differ in structure, setae of the most anterior row are pectinate or dentate, while others are smooth or less heavily pectinate. In Th. spangleri setae of this field are arranged in 2 regular rows with shortened curved pectinate setae in anterior row and long thin straight setae in posterior row ( Fig. 386 View FIGURES 379–388 ). In Th. flavus sp. n. setae of this field are diminished ( Fig. 630 View FIGURES 626–636 ); in Th. alboniger sp. n., Th. lepidus and Th. sp. «Palo Seco», this field is vestigial, i.e. its setae are as small and sparse, as on middle and hind legs ( Figs 558 View FIGURES 543–562 , 599 View FIGURES 591–602 , 621 View FIGURES 613–625 ); in all cases dense setae are retained near apex of fore tibia, and the subapical inner-posterior row of arched pointed setae is retained. Posterior pointed pectinate setae ( Fig. 89 View FIGURES 86–93 : p.r): present on hind tibia only; situated either irregularly, or forming a longitudinal row; on apex of hind tibia such setae are more dense and form a transverse row ( Fig. 23 View FIGURES 21–24 ). Outer field of hairs ( Fig. 89 View FIGURES 86–93 : o.h): these hairs (i.e. long and slender setae) are directed nearly perpendicular to tibia surface; present on tibiae of all legs; on hind tibia these hairs are irregularly situated between the outer-anterior and the outerposterior rows of stout setae and form a row posteriad of the outer-posterior row of stout setae. Inner-anterior row of recurved hairs ( Fig. 89 View FIGURES 86–93 : i-a.h): located on anterior side of tibia, more distally from inner margin than the inneranterior row of stout setae; on fore leg either absent ( Fig. 21 View FIGURES 21–24 ), or present on proximal part of tibia (in Th. sp. «Palo Seco»— Fig. 622 View FIGURES 613–625 ); on middle and hind legs always present all along tibia ( Figs 22, 24 View FIGURES 21–24 ).
Larval claws
In connection with adaptation for inhabiting stone surfaces in rapidly running water, claws are bent perpendicular to the leg flatness, so that when legs are spread, and body and legs are pressed to substrate, claws are directed toward the substrate and have good adhesion with it (a similar form of body and legs evolved independently in various other rheophilous mayfly taxa). In accordance with this, in all species of Thraulodes the claw is distinctly divided into the rigid portion (main part of the claw), and the articulatory portion, located between the rigid portion and the unguitractor ( Fig. 69 View FIGURES 66–69 ). On its morphologically posterior side (functionally ventral side) the articulatory portion is membranous, and serves rotation of the claw perpendicular to the leg flatness. In various mayfly larvae, which have such or similar rotation mechanism of claw and a row of denticles on inner side of the claw, these denticles are either present on the rigid portion only, or are equally developed on both portions forming an integral row, or have significantly different shapes and sizes on these two portions. In all species of Thraulodes , one longitudinal row of stout denticles is present on inner side of the rigid portion of claw; the articulatory portion of claw often (but not always) bears a longitudinal row of minute, pointed denticles, which are significantly smaller than denticles of the rigid portion ( Figs 69 View FIGURES 66–69 , 411 View FIGURES 411–426 ). Traver and Edmunds (1967: 357) reported the denticles of rigid portion as «variable number of large tooth» and the denticles of articulatory portion as «several smaller ones nearer the base». Denticles of articulatory portion are often very small, and the most proximal of them are often fused together, so that their number cannot be adequately counted.
In majority of Thraulodes species, the number of denticles of the rigid portion varies between 5 and 10; usually (but not always) the proximal denticles are smaller and the distal ones are larger; the most distal denticle may or may not be larger than all others; it is located either on the same line as others, or offset from this line; all these characters are not species-specific, but vary individually and on different legs of the same individual.
Some species have significantly diminished number of denticles of the rigid portion: 3–5 in Th. niger sp. n. ( Fig. 411 View FIGURES 411–426 ), 2–4 in Th. nigrabdominalis sp. n. and constantly 4 in Th. alboniger sp. n.
Allen and Brusca (1978) reported the character «median denticle largest» as a species-specific character for the Mexican species Thraulodes sp. G and figured a claw which has several small denticles between the largest denticle and the claw apex ( Allen & Brusca 1978: fig. 16). Among species examined, the same is found on fore and middle legs of Th. quevedoensis ( Fig. 273 View FIGURES 267–273 ), while hind legs of the same individuals have claws of a usual structure, without small denticles between the largest denticle and the claw apex.
Larval coloration
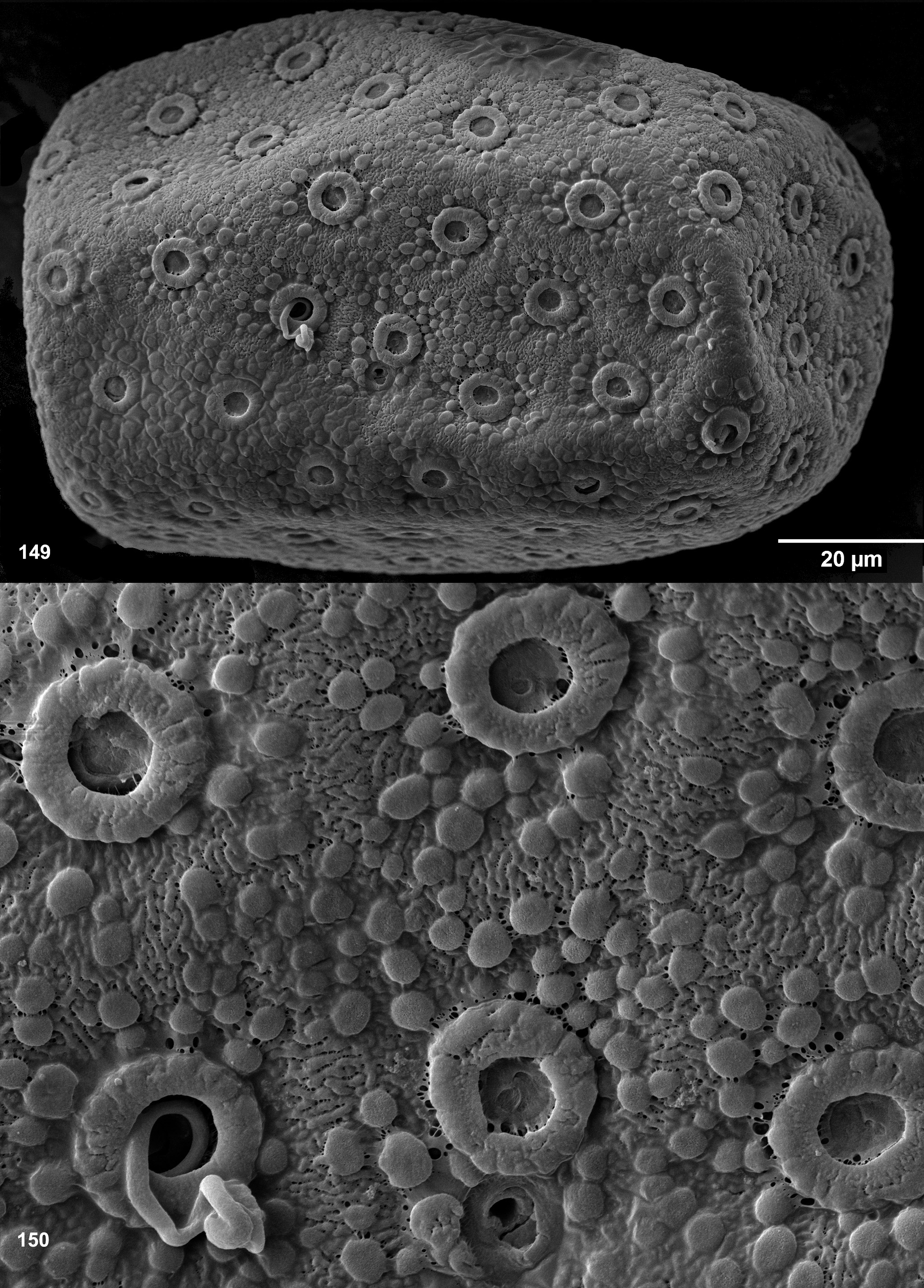
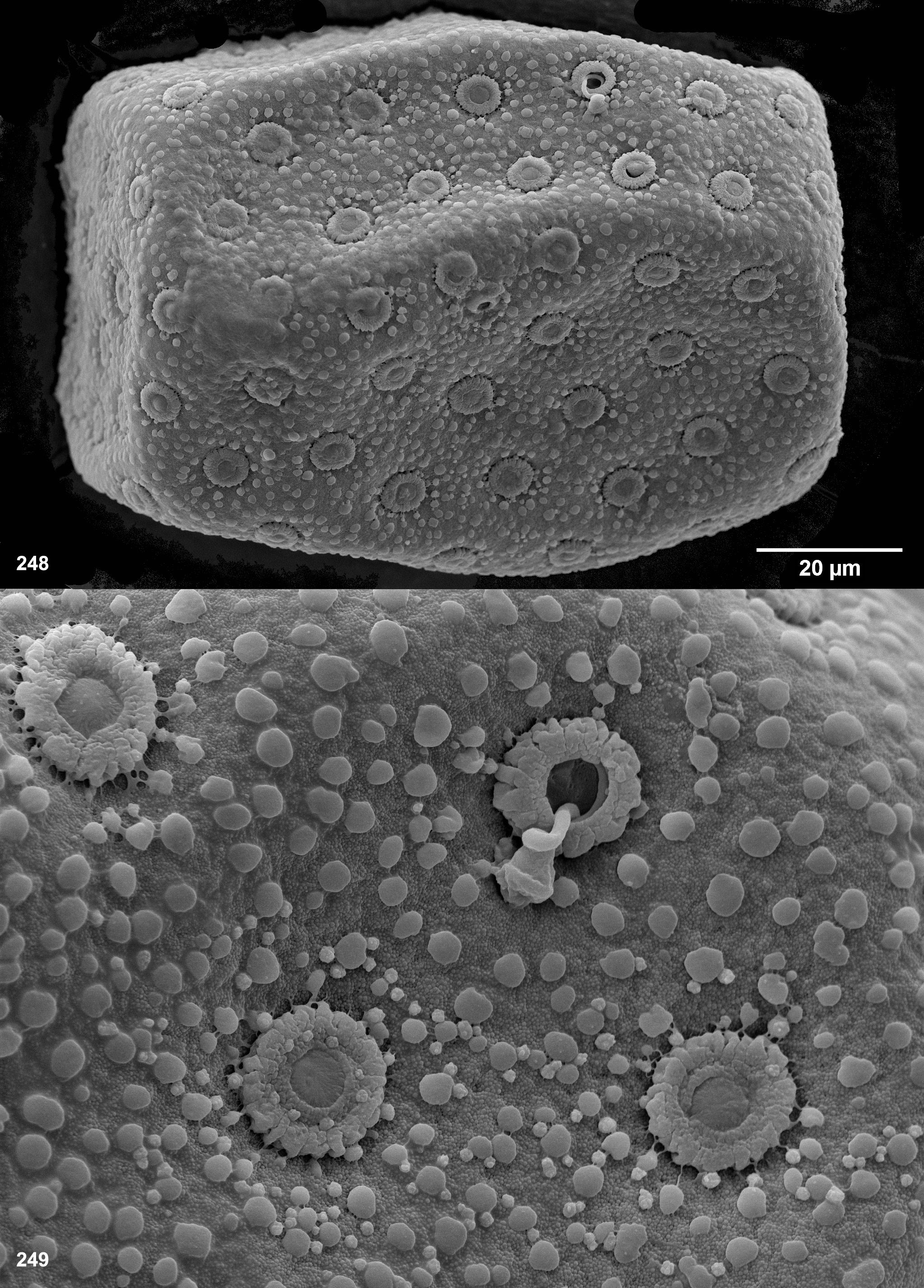
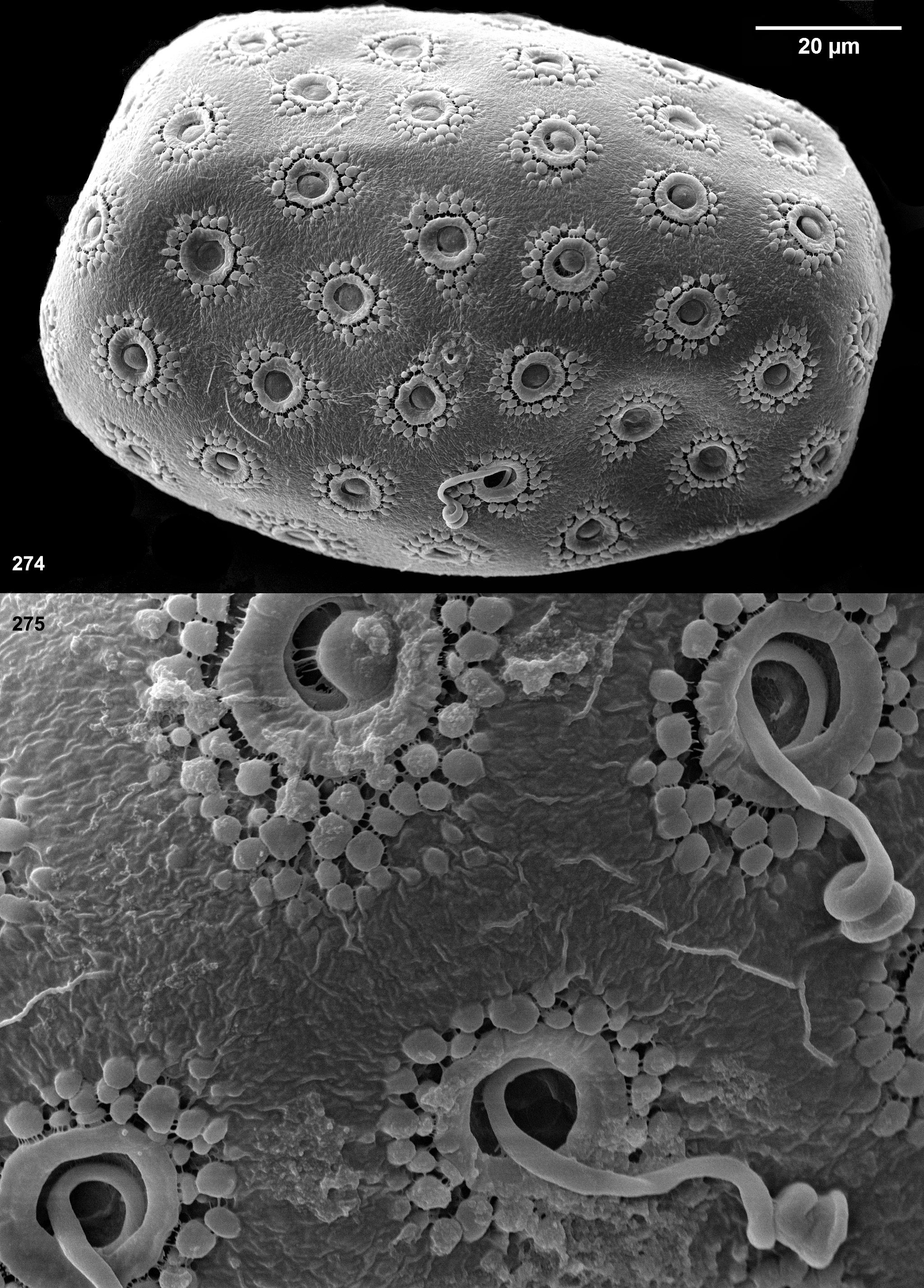
The authors, who described larval coloration, did not differentiate between cuticular and hypodermal color patterns. Such descriptions of coloration have no taxonomic significance, because during individual development cuticular coloration becomes more intensive from beginning of each instar to the end of instar, while hypodermal coloration passes from instar to instar and is gradually changed from larva to imago.
Cuticular coloration of larval thorax. In those cases when the cuticle of pronotum is intensively pigmented, its lateral margin is more or less contrastingly colorless ( Figs 75 View FIGURES 70–85 ). The cuticle of the rest surface of the pronotum and mesonotum can have darker and lighter areas, some of which are connected with muscle attachment; in various species these areas are either contrasting or not.
Among features of the cuticular coloration, Traver and Edmunds (1967) paid special attention to «the U-shaped mark on the mesonotal scutum, 1 arm of the U on each side of the mid-dorsal line, the 2 arms uniting near the posterior portion of the scutum» and noted that «in Thraulodes this U-figure ... is usually more prominent than in other related genera, but is not distinct in all specimens». This is cuticular coloration; the pair of light stripes ( Figs 66–67 View FIGURES 66–69 ) separate the area of attachment of the median longitudinal muscles from the areas of attachment of other muscles and correspond to the pair of medioparapsidal sutures of the winged stages.
In various species, cuticle of fore protoptera is either nearly uniformly pigmented, or has veins darker than background ( Fig. 175 View FIGURES 174–189 ), or has veins lighter than background ( Fig. 8 View FIGURES 5–13 ); in contrast to hypodermal coloration, this cuticular coloration does not depend upon stage of wing development.
Hypodermal coloration of larval thorax. The larval pronotum either has no hypodermal markings ( Figs 66, 68 View FIGURES 66–69 ), or has blackish stripes bordering colorless lateral margins (see above) ( Figs 332 View FIGURES 317–336 , 518 View FIGURES 510–521 , 603 View FIGURES 603–612 ), or has peculiar blackish markings corresponding to the imaginal ones (compare Figs 543 View FIGURES 543–562 and 576 View FIGURES 569–577 ). The mesonotum and thoracic pleura sometimes have irregular blackish markings. Fore and hind protoptera often have blackish hypodermal markings corresponding to certain maculae of the imaginal wing, particularly to the dark brown macula near costal brace ( Figs 620 View FIGURES 613–625 , 626 View FIGURES 626–636 ) or others ( Figs 591 View FIGURES 591–602 , 655 View FIGURES 654–665 ).
Cuticular coloration of larval legs. In all Leptophlebiidae the fore femur is more or less widened proximally; in some leptophlebiid taxa, its cuticle has a discolored area (blank) on anterior surfaces of the proximal widening; this blank is expressed if surrounding cuticle is pigmented. Presence and shape of this blank is not connected with muscle attachment: all muscle fibers which pass under anterior surface at this area belong to the femoro-tibial flexor and are attached to the proximal, outer and inners margins of the femur, but not to its dorsal surface. In all Thraulodes this proximal forefemoral blank has characteristic shape and lacks stout setae, which are irregularly spread on the anterior surface of the femur (see above). Shape of the proximal forefemoral blank somewhat resembles a triangle, which is stretched obliquely in such a way that its short side is adjacent to the convex proximal-outer margin, and its acute angle touches the inner margin more distally ( Fig. 281 View FIGURES 276–296 ). Other elements of cuticular coloration of fore, middle and hind femora vary among species often have significant individual variability. The cuticle of tibiae in most species is uniformly pigmented; only in Th. alboniger sp. n. and Th. sp. “Itaya” it has darker and lighter bands ( Figs 548–550 View FIGURES 543–562 , 642–644 View FIGURES 637–649 ).
Hypodermal coloration of larval legs. The imaginal femur often has a hypodermal multicolored distal band formed by various combinations of yellowish, reddish and blackish markings, including a blackish stripe along distal part of inner margin (e.g. Figs 28–30 View FIGURES 25–34 , 97 View FIGURES 94–100 ); in some species femur has additional middle band, or is entirely darkened (e.g., Figs 468–469 View FIGURES 464–473 , 597–598 View FIGURES 591–602 ); in these cases hypodermal coloration of larval femur is limited by blackish pigment, which often (but not always) forms a stripe along distal part of inner margin of the femur ( Figs 452 View FIGURES 450–463 , 591 View FIGURES 591–602 ). In Th. alboniger sp. n. imaginal hypodermal coloration of femur is represented by a narrow apical blackish band ( Fig. 574 View FIGURES 569–577 ), and larval hypodermal coloration of femur is similar to it ( Fig. 543 View FIGURES 543–562 ).
Hypodermal coloration of larval abdomen. Abdominal terga of imagines have species-specific color patterns. Traver and Edmunds (1967) recognized 9 elements of this coloration. Among them, a blackish or gray posterior margin and a pair of blackish or gray midway spots are present in many species. If these markings are present in imago, they are more or less expressed in larval stage ( Figs 68 View FIGURES 66–69 , 128 View FIGURES 122–132 , 155 View FIGURES 151–155 , 185 View FIGURES 174–189 , 227 View FIGURES 208–231 , 292 View FIGURES 276–296 , 332 View FIGURES 317–336 , 421 View FIGURES 411–426 ). Blackish or gray stigmatic dots, if present in imago, are also present in larva; but in contrast to the dark posterior margin and the midway spots, which are located just under cuticle and are well visible, stigmatic dots are situated on tracheae, which in larval stage are located deeply in the body and are poorly visible or invisible. Imagines of some species have paired oblique lateral streaks or darkened posterolateral triangles ( Fig. 611 View FIGURES 603–612 ); in these cases larvae can have similar blackish or gray markings ( Fig. 603 View FIGURES 603–612 ). Among species examined, only some individuals of Th. alboniger sp. n. have black median marks ( Fig. 572 View FIGURES 569–577 ), and these marks are expressed in the larval stage as well ( Fig. 543 View FIGURES 543–562 ). Some other elements of imaginal coloration, e.g. brown or reddish dorsolateral spots are absent in larval stage and appear only when larva is going to molt to subimago.
Based solely on larval characters, McCafferty, Baumgardner and Guenther (2004) established a formal species name Thraulodes pacaya for the larvae which were described by Allen and Brusca (1978) under arbitrary name Thraulodes sp. C. They report only two characters for this species, «relative narrow elongate gills with indistinct lateral tracheation» and «narrow, dark, transverse band on the posterior border of middle abdominal terga and the pair of antero-sublateral, approximately right-triangle shaped dark marks on middle abdominal terga» McCafferty et al. 2004: 214). Judging by the drawing to which they refer ( Allen & Brusca 1978: fig. 24), these dark markings represent the blackish-brown hypodermal pigmentation, which corresponds to the dark posterior margin and the midway spots on imaginal abdomen ( Traver & Edmunds 1967: fig. 29: dpm, ms). These elements of coloration are present in imagines of many species of Thraulodes and should be expressed in their larvae; most probably, « Th. pacaya » represents a mixture of larvae belonging to various species.
New terms for describing coloration of subimaginal mesonotum
The mesonotum of the mayfly subimago and imago bears the mesonotal suture, which initially represents a transverse furrow crossing the scutum behind the anteronotal transverse impression; in the stage of imago, cuticle anteriad and posteriad of the mesonotal suture usually has the same coloration (brown or ocher), but in the stage of subimago cuticle is usually colored anteriad of the mesonotal suture and colorless posteriad of it. Initially, I called this suture «transverse suture» ( Kluge 1988: figs 90–91: TS), but later suggested the term «mesonotal suture», because in some cases its direction is changed, being more longitudinal than transverse ( Kluge 1994a, 2004). In the cases, when the mesonotal suture is shifted backward, the colored zone anteriad of the mesonotal suture occupies a part of medioscutum and a part of submedioscutum ( Kluge 1994a: fig. 15; 2004: fig. 106D). In some cases, coloration of these zones represents a species-specific character, so these zones should be clearly named in species descriptions. In one of the previous papers, I used the letter «a» for two zones located anteriad of the mesonotal suture and the letter «p» for two zones located posteriad of the mesonotal suture ( Kluge 2013: figs 51–53). However, these two abbreviations are not enough to describe all variants of coloration of subimaginal mesonotum.
Here I suggest the following new terms ( Fig. 94a View FIGURES 94–100 ): the term « chromozone » for the zone anteriad of the mesonotal suture; the term « achromozone » for the zone posteriad of the mesonotal suture; and the term « chromozonal suture » as additional term for the mesonotal suture to indicate that it is a line separating the chromozone and the achromozone of subimaginal cuticle. The terms «chromozone» and «achromozone» should be used for certain zones, independently if they are actually colored or not. In Leptophlebiidae and some other taxa, the chromozonal suture is shifted backward and crosses the medioparapsidal suture, which separates medioscutum (zone of attachment of the median tergal muscle) and submedioscutum (zone of attachment of the scuto-episternal muscle) (the terms «medioscutum» and «submedioscutum» were introduced in Kluge 1994); in this case, 4 zones can be distinguished, which can be termed «chromozone of medioscutum», «chromozone of submedioscutum», «achromozone of medioscutum» and «achromozone of submedioscutum»; two branches of the chromozonal suture can be termed «medioscutal chromozonal suture» and «submedioscutal chromozonal suture» ( Fig. 94a View FIGURES 94–100 ).
In the stage of subimago, the cuticle of the chromozone(s) is usually more or less intensively colored by brown, while the cuticle of the achromozone(s) is usually colorless. In some taxa the cuticle of the chromozone(s) is also colorless. In some taxa the cuticle of achromozone(s) is colored; in this case its color is different from the color of the chromozone (e.g., Kluge 2013: fig. 53).
In the primitive condition, the chromozonal (i.e., mesonotal) suture represents a deep furrow running transversely across the anterior part of scutum shortly posteriad of the anteronotal transverse impression, being either medially stretched backward into a short point ( Kluge 1994a: fig. 1; Kluge 2004: figs 6), or entirely transverse ( Kluge 2004: fig. 63). In these cases the subimaginal chromozone is located anteriad of the chromozonal suture, being not divided into medioscutal and submedioscutal zones, and the achromozone is located posteriad of the mesonotal suture, including either both medioscutum and submedioscutum ( Kluge 1994a: fig. 12; Kluge 2004: figs 18E, 19H, 21A, D–E, 23F, 25C, 30D, 32B, 33D–E, 34A, F, 39C–E, 54I, 63A, C, 64A, D, 90D; Kluge 1996: fig. 25) or medioscutum only, while submedioscutum is occupies by another pigmented zone ( Kluge 1994a: figs 13–14; Kluge 2004: figs 17D, 21B, 40D).
In certain mayfly taxa the shape of the chromozonal (mesonotal) suture is markedly changed, or this suture is lost. Particularly, in Fimbriatotergaliae and Leptophlebiidae the chromozonal suture is initially shifted backward by the side of the median line, so that on each side of the mesonotum it crosses the medioparapsidal suture ( Kluge 1994a: fig. 15; Kluge 2004: figs 68F, 71E–F, 74A, 83F, 106D). In these cases there can be distinguished chromozones of medioscutum and submedioscutum, and achromozones of medioscutum and submedioscutum. Both chromozones (medioscutal and submedioscutal ones) can be either equally pigmented ( Fig. 337 View FIGURES 337–345 ; Kluge 2013: fig. 51), or colorless ( Fig. 368 View FIGURES 365–370 ), or differ by coloration ( Fig. 94 View FIGURES 94–100 ). Both achromozones (medioscutal and submedioscutal ones) can be either colorless, or pigmented, sometimes differently one from another ( Kluge 2013: fig. 52).
In many representatives of Leptophlebiidae , including the most primitive taxa ( Leptophlebiinae and Habrophlebiinae sensu Kluge 1994c), the chromozonal suture passes at a distance from median longitudinal suture, medioparapsidal suture and lateroparapsidal suture, so that both chromozones and both achromozones (medioscutal and submedioscutal ones) are well expressed ( Kluge 1994a: fig. 12, 1994c: fig. 47; 2004: fig. 40D; Kluge 2012: fig. 37, 92; 2013: figs 51–53; Kluge 2014: fig. 39). In some leptophlebiid taxa the chromozonal suture approaches the medioparapsidal suture, so that the chromozone is narrowed; particularly, this occurs in Terpidinae ( Kluge 2009: fig. 14) and in Hagenulus /fg2 ( Kluge 1994b: figs 20, 50, 87, 113, 201, 222). In Thraulodes the submedioscutal chro- mozonal suture approaches the lateroparapsidal and antelateroparapsidal sutures and repeats their shape, so that the submedioscutal achromozone looks as a narrow light stripe bordering dark lateroparapsidal and antelateroparapsidal sutures ( Fig. 94 View FIGURES 94–100 ). The taxon Hermanellonota Kluge 2008 is characterized by loss of the chromozonal suture, so that in subimago the whole medioscutum and submedioscutum are either entirely colorless ( Kluge 1994b: fig. 13), or entirely colored ( Kluge 2008: fig. 28).
Fore wing venation
Venation of fore wing is uniform among Thraulodes , and only a few characters are used in species diagnoses.
A generally accepted species-specific character is presence/absence of cross veins in the proximal half of costal field (i.e. proximad of bulla). However, among species examined, none lacks these veins completely; this is true for the species which were described as lacking these veins— Th. telegraphicus , Th. sinuosus , Th. schlingeri , Th. marreroi , Th. quevedoensis and Th. zonalis . In all these cases, costal cross veins proximad of bulla are present, but few in number (one, two or more), colorless and much thinner than other cross veins, and can be visible only at magnification higher than necessary for examination of other veins ( Figs 32 View FIGURES 25–34 , 142 View FIGURES 133–142 , 344–345 View FIGURES 337–345 ); in these species only selected individuals or individual wings lack these veins. Some authors use exact number of cross veins to separate species of Thraulodes (e.g. Souto et al. 2014). This is also wrong, because the number of cross veins in all fields of Leptophlebiidae and most other mayflies varies individually. Species-specific characters connected with cross veins, are only thickness and/or coloration of cross veins and their approximate number or density in certain fields.
One more character in wing venation was reported as species-specific for Thraulodes liminaris Dominguez 1987 : in this species vein ICu 2 is joined at base to vein CuP (Domínguez 1981: fig. 3; Domínguez et al. 2006: fig. 187D, in contrast to other Thraulodes , where ICu 2 is joined to ICu 1, but not to CuP. Among the species examined, the same condition, when the vein ICu 2 is joined at base to vein CuP, was found on one wing of one individual of Th. niger sp. n. ( Fig. 435 View FIGURES 427–438 ), while on another wing of this individual ICu 2 is joined only to ICu 1, ( Fig. 434 View FIGURES 427–438 ). Probably, this character is individual.
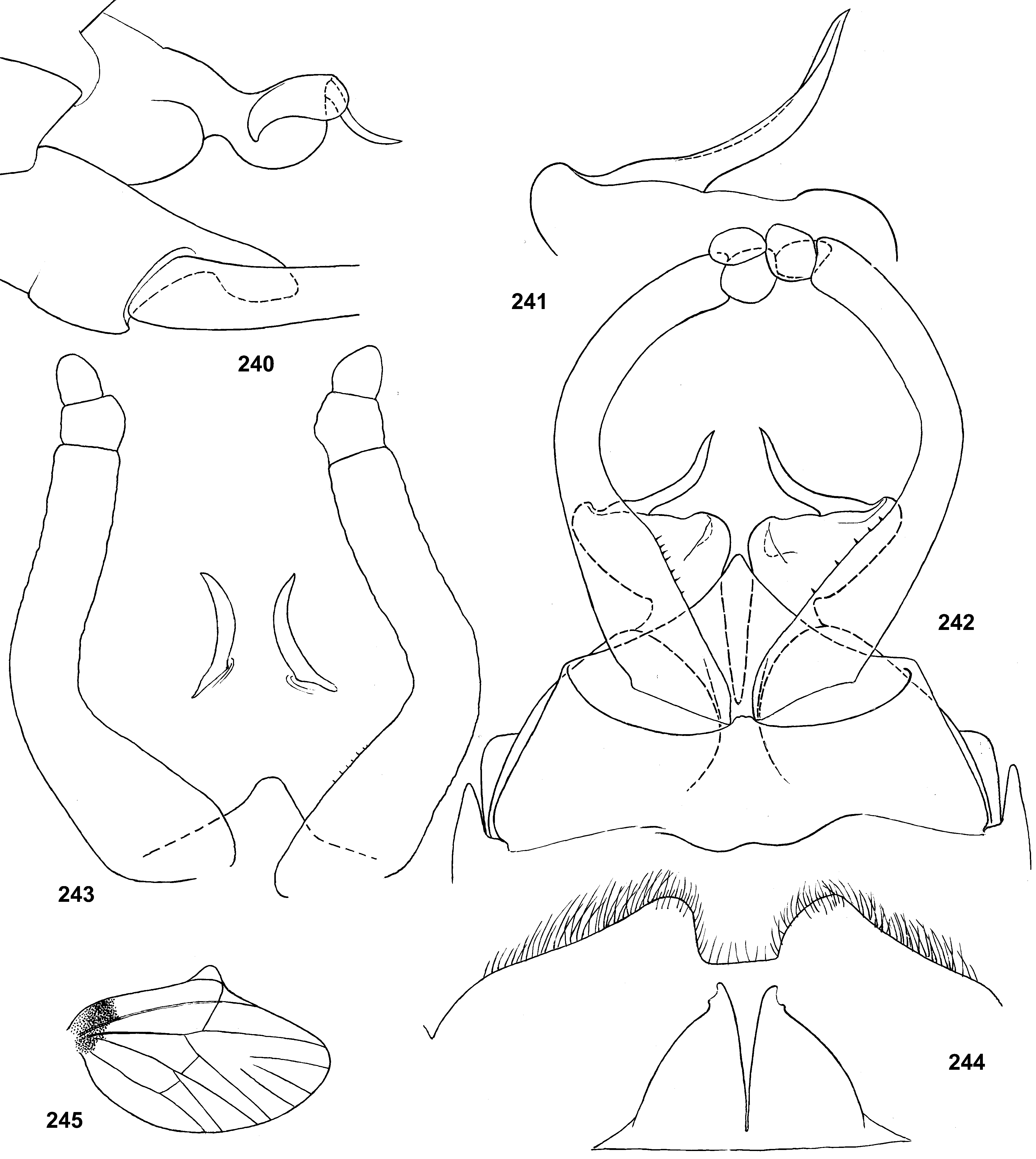
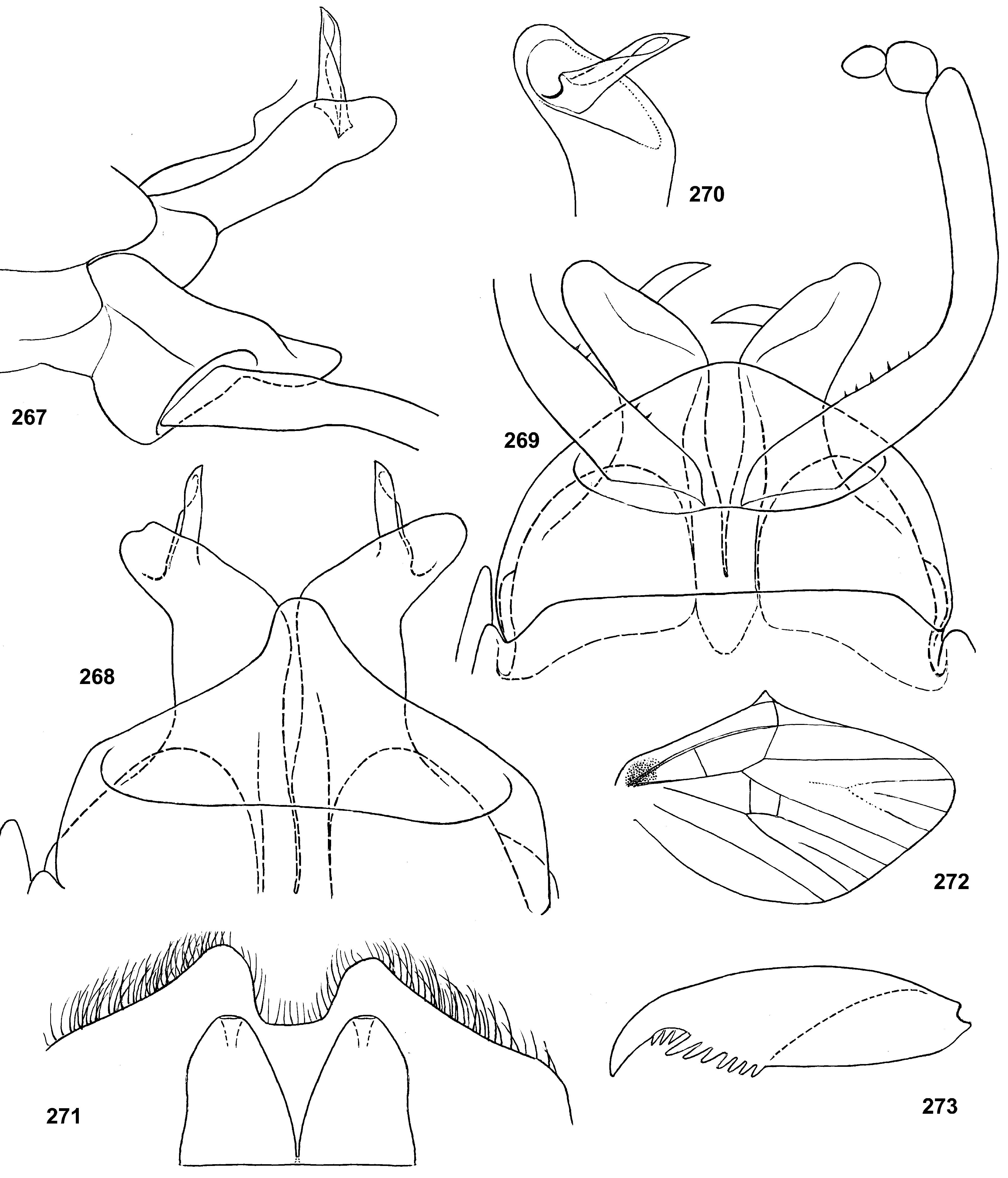
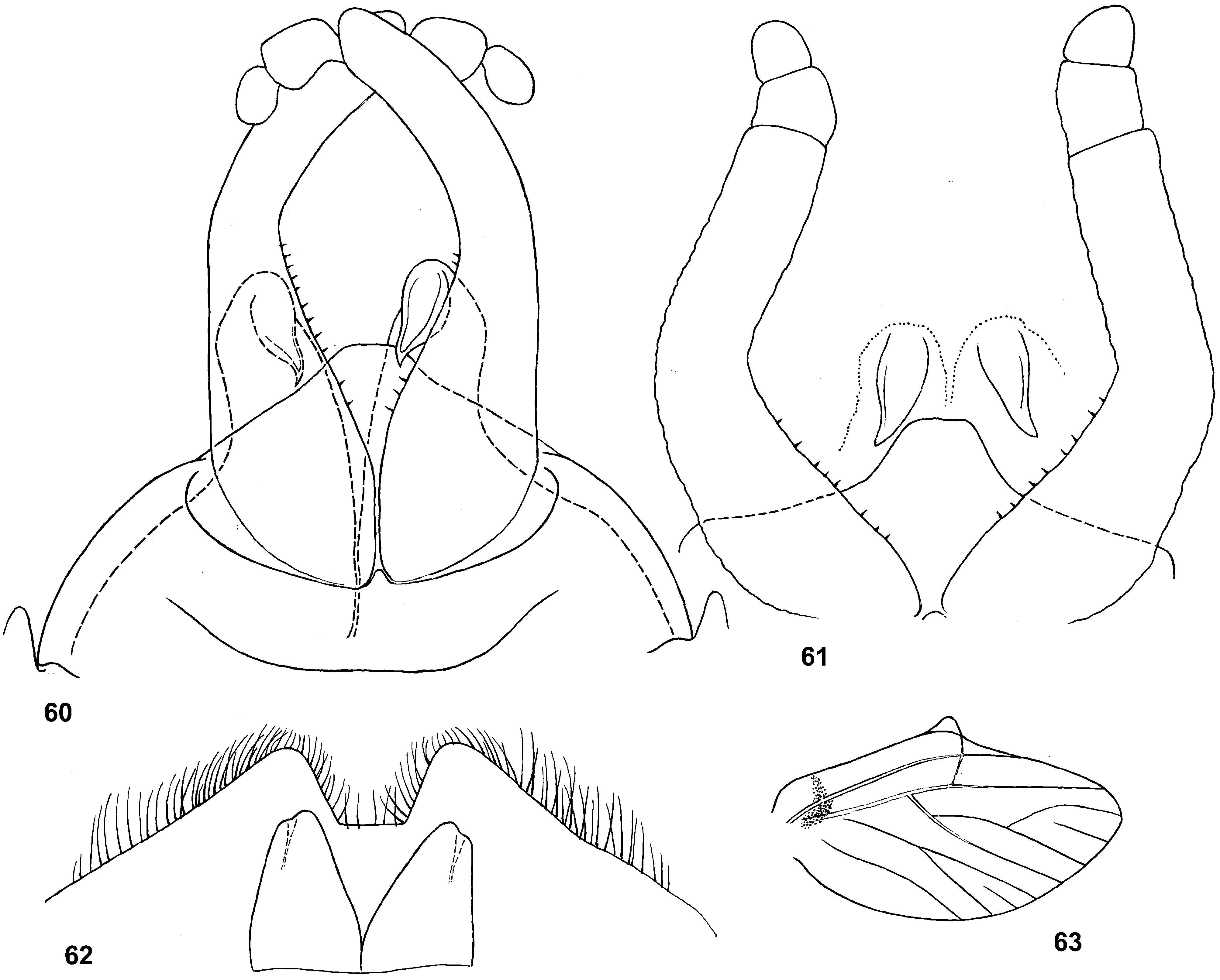
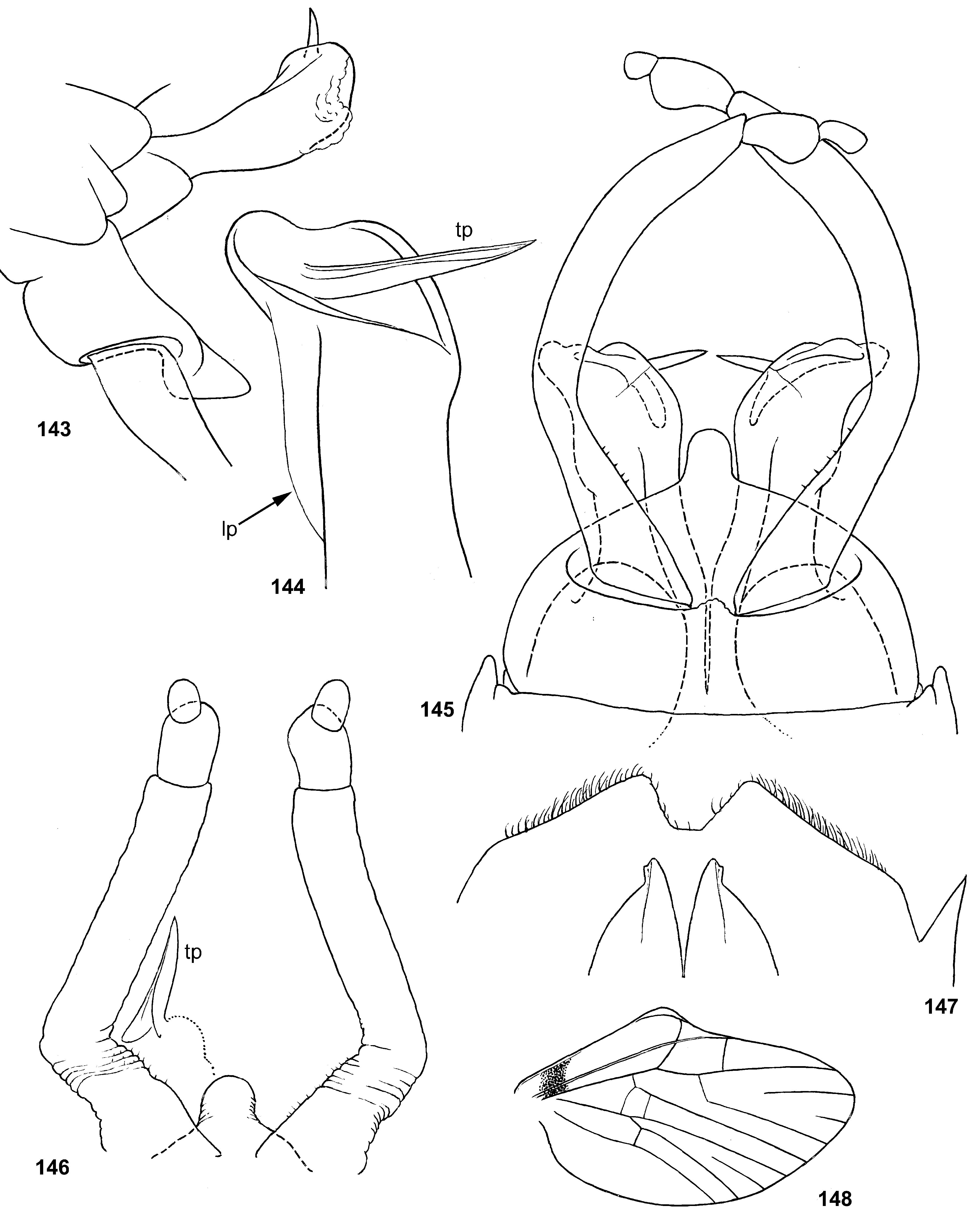
Genital structure
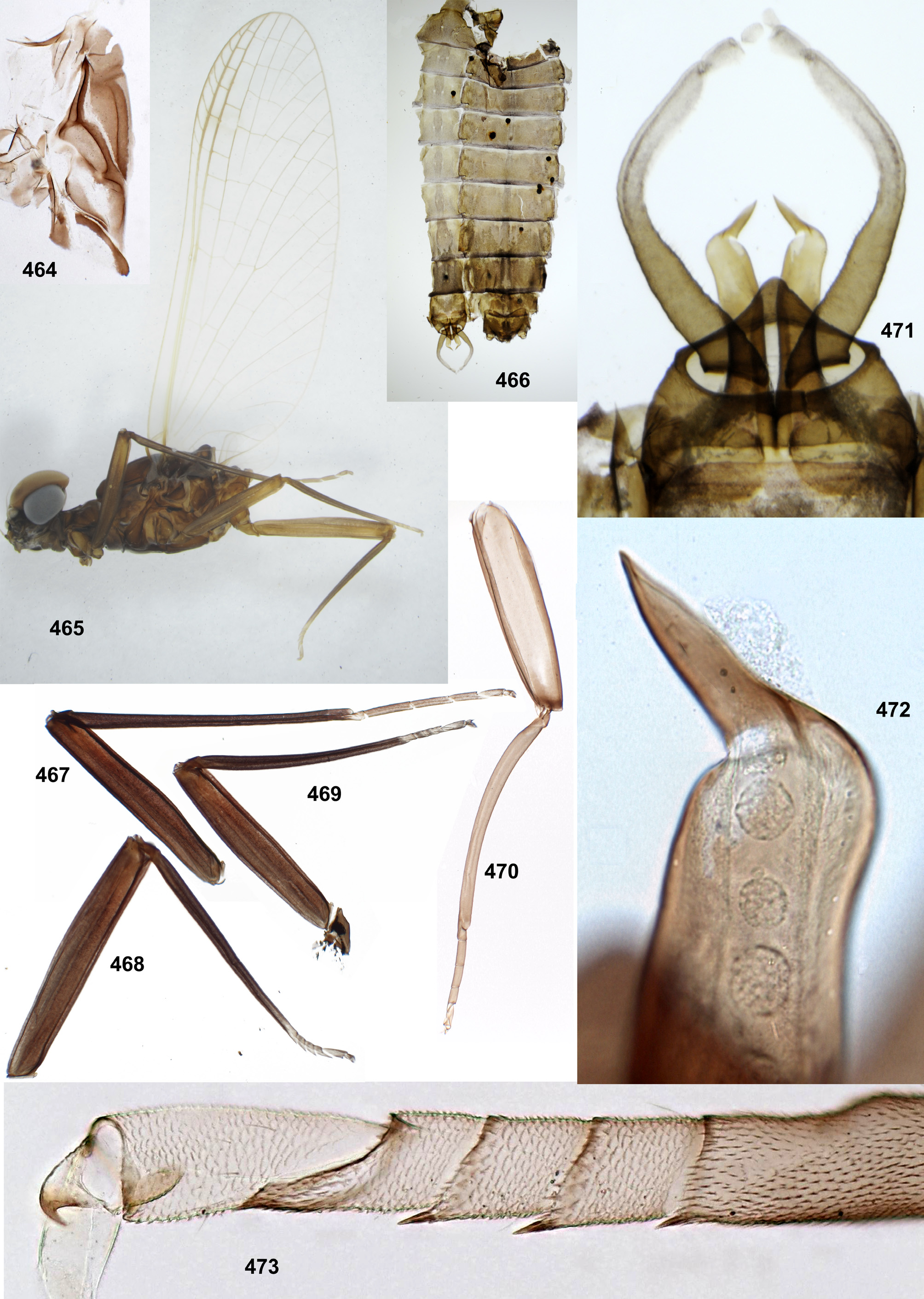
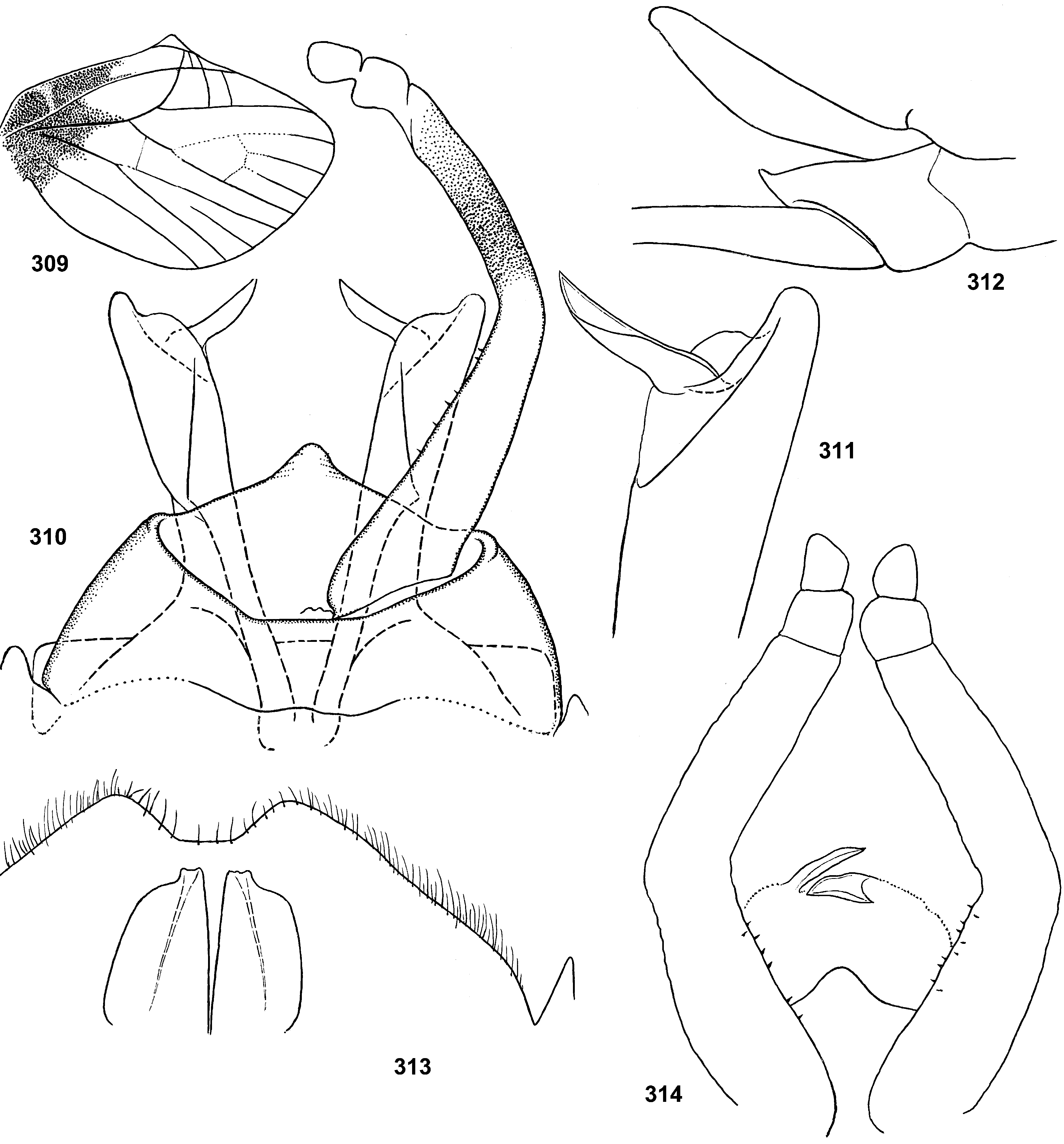
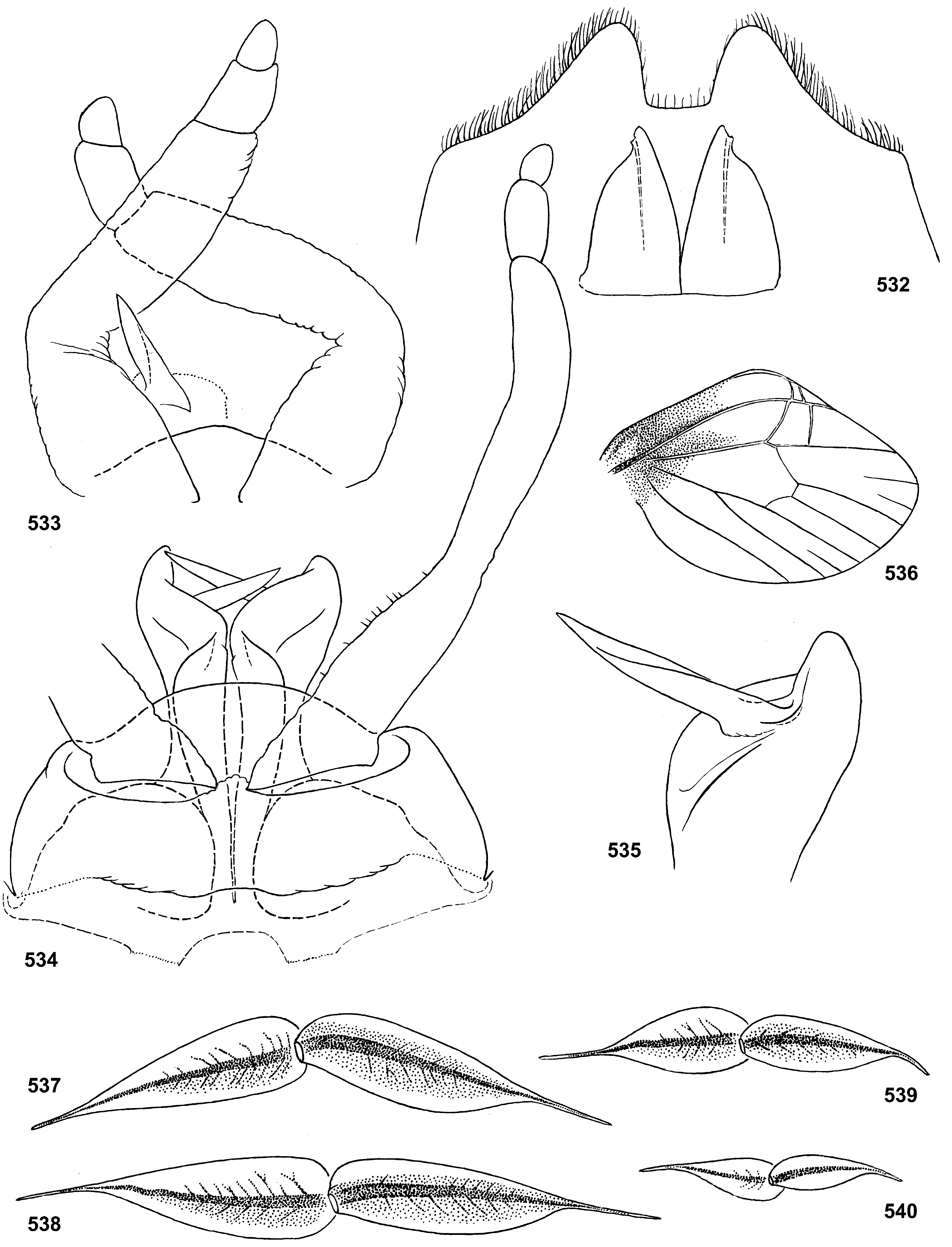
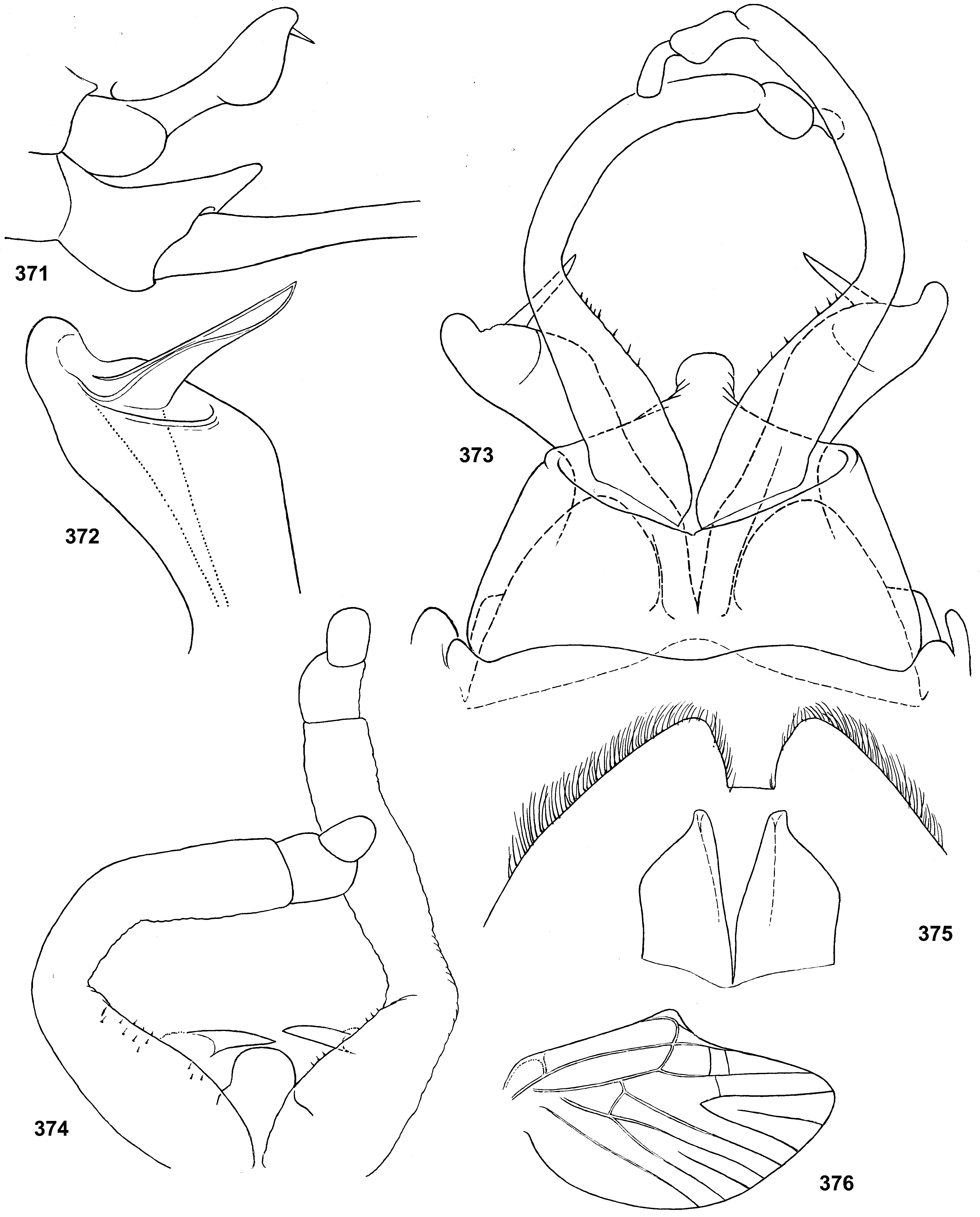
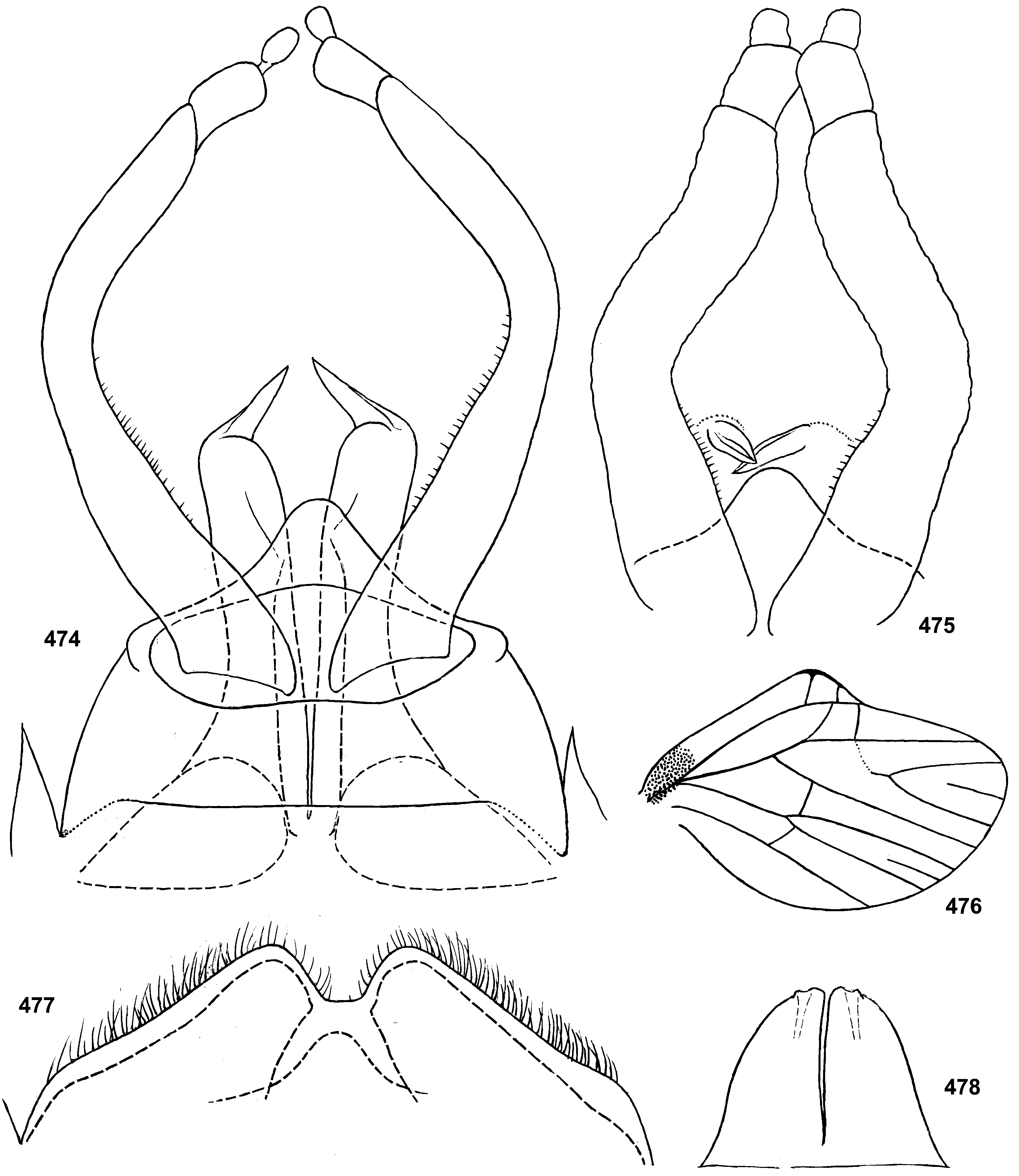
Styliger. Thraulodes differs from other taxa by the structure of the styliger, which has a common unpaired gonostylar cavity [termed by Traver and Edmunds (1967) «forceps plate»]. The gonostylar cavity is exposed ventrally-caudally ( Fig. 112 View FIGURES 112–120 ), being well visible in ventral view ( Fig. 114 View FIGURES 112–120 ), so that its dorsal margin is projected caudally. In various species this dorsal margin of styliger is either smoothly convex ( Fig. 534 View FIGURES 532–540 ), or medially stretched into an unpaired dorsal projection ( Fig. 110 View FIGURES 106–111 ), whose shape is species-specific or varies individually; in some species this projection is separated from the dorsal edge of styliger, shifted more dorsally and proximally, so that in ventral view its base is partly hidden by styliger ( Figs 38, 40 View FIGURES 35–41 ). Since the bases of gonostyli are brought together in the gonostylar cavity, the pair of gonostylar muscles, which run from the styliger to the bases of gonostyli, are convergent ( Figs 102 View FIGURES 101–105 , 110 View FIGURES 106–111 ).
Gonostyli. The gonostylus retains all 4 primary segments, but the initial 1st and 2nd segments are not separated and often have equal thickness, and so can be taken for one segment; Traver and Edmunds (1967) called them together «basal segment». The 1st and 2nd segments are often bent in an obtuse angle; both distal segments (3rd and 4th ones) are short and always well separated ( Fig. 102 View FIGURES 101–105 ). About the development of gonostyli, Traver and Edmunds (1967: 355) wrote «In male nymphs, only tips of forceps seen externally; dissection shows long basal segment to be strongly folded on itself in a U-shaped fashion, opening of U toward midline. In all other Neotropical genera of this family that we have studied, forceps relatively much longer and not folded on themselves as in Thraulodes ». Larval protogonostyli are really short ( Figs 106, 108 View FIGURES 106–111 ) and in some species reduces to low protuberances ( Fig. 313 View FIGURES 209–314 ). Subimaginal gonostyli developing inside are located mainly proximad of the protogonostyli, being folded in the junction of initial 1st and initial 2nd segments ( Fig. 108 View FIGURES 106–111 ).
Musculature of penis. As in most mayflies, in Leptophlebiidae penis has two pairs of extrinsic muscles attached to its base: the pair of large median muscles, which are attached to anterior-lateral part of ninth abdominal sternum ( Figs 101 View FIGURES 101–105 , 111 View FIGURES 106–111 : m.med), and the pair of small lateral muscles, which are attached to lateral parts of ninth tergum ( Fig. 101 View FIGURES 101–105 : m.lat) ( Grandi 1960). In Thraulodes , the median penial muscles are attached to the base of penis medially, close together. Penis lobes are separated nearly up to the base, so contraction of the median penial muscles should provide an active movement, bringing penis lobes together. In connection with this, median sides of penis lobes are often strengthened by a pair of medio-ventral ridges, or recurved folds ( Traver & Edmunds 1967); each of these ridges forms the median margin of the penis lobe and is projected on its ventral side; it stretches from the base of penis (where the median muscle is attached) in distal direction ( Figs 103 View FIGURES 101–105 , 111 View FIGURES 106–111 ). In some species the free ventral margin of this medio-ventral ridge extends laterally, so that the ridge represents a flap overlapping some part of the ventral surface of penis ( Figs 530 View FIGURES 522–531 , 534 View FIGURES 532–540 ); a certain kind of such recurved flap is termed lapel ( Traver & Edmunds 1967). The medio-ventral ridges are either developed only in basal part of penis ( Fig. 38 View FIGURES 35–41 ), or are stretched distally, sometimes up to the apex. Length and shape of the medio-ventral ridge is regarded as an important species-specific character ( Traver & Edmunds 1967); in some species it is such, but in Th. telegraphicus it demonstrates a great individual variability ( Figs 114–120 View FIGURES 112–120 ).
Spermatic pump. Leptophlebiidae have aflagellate spermatozoons ( Soldan 1979, Gaino & Mazzini 1991); in connection with this, their spermatic ducts are equipped by ring muscles, which serve as spermatic pumps ( Grimm 1985; Brito et al. 2012). In many species of Thraulodes , basal part of each penis lobe forms a ventro-lateral swelling, which Traver & Edmunds (1967) named basal lobe. Inside the basal lobe and proximad of it, the gonoduct is widened ( Traver & Edmunds 1967: fig. 30) and surrounded by more or less thick layer of ring-like muscles, which forms the spermatic pump ( Figs 101, 103 View FIGURES 101–105 , 111 View FIGURES 106–111 ). Apart of the muscles of spermatic pump located in the basal lobe, the penis of Thraulodes has no any other intrinsic muscles.
Spear-like rolls. The pair of pointed processes, or «the spears, a very characteristic feature of this genus» ( Traver & Edmunds 1967: 353), were characterized as the following: «Each penis lobe bears a slender spearlike process arising dorsally from distal margin, the points of the spears directed inward and toward one another» (ibid, 352) and «The narrow portion of [spermatic] duct can be traced apicad from the basal lobe as far as the base of the spear» (ibid, 353). Some other authors call these processes «spines» ( Dominguez 1987). In all publications these processes are described and figured as integral spines. On some figures such spine is crossed by an oblique line, but without comments (e.g. Zúñiga et al. 2015: fig. 8D).
Actually, these processes are not integral, but each represents a tube with longitudinal cleft stretched up to base. Such an incomplete tube can be interpreted as a rolled plate, whose margins are either separated ( Figs 104 View FIGURES 101–105 , 144 View FIGURES 143–148 , 171 View FIGURES 169–173 , 311 View FIGURES 209–314 , 439 View FIGURES 439–447 ,), or contiguous ( Figs 200 View FIGURES 198–202 , 372 View FIGURES 371–376 , 405 View FIGURES 403–408 , 535 View FIGURES 532–540 ,), or overlap one another ( Figs 270 View FIGURES 267–273 , 582 View FIGURES 578–588 ). Apex of this roll is always obliquely truncate, forming a sharp point. The spermatic duct is opined into the canal of this roll, so that sperm escapes from the apical foramen and the cleft of the roll ( Fig. 472 View FIGURES 464–473 ).
The spear-like processes are attached either on apical, or apical-dorsal side of the penis, and in most species are directed more or less dorsally ( Figs 143 View FIGURES 143–148 , 267 View FIGURES 267–273 ); in some species they are inclined ventrally ( Figs 203 View FIGURES 203–206 , 239 View FIGURES 232–239 , 240 View FIGURES 240–245 ); this is an important species-specific character, which is often overlooked or confused on the drawings where genitalia are shown in ventral or dorsal view.
In the overwhelming majority of species, the spear-like rolls are directed medially (i.e., inward and toward one another) and each has a narrow cleft exposed caudally-laterally-dorsally ( Figs 112–114 View FIGURES 112–120 ). Only in two closely related species, Th. sinuosus and Th. ludmilae sp. n., these processes are directed ventrally and unrolled, so that each process represents a concave plate directed by its concavity ventrally-caudally ( Fig. 60 View FIGURES 60–63 ) or ventrally-laterally ( Figs 38, 40 View FIGURES 35–41 ).
Larval protopenis and development of spear-like rolls. Traver and Edmunds (1967: 355) wrote that «In male nymphs, ... Each penis lobe bears a slender elongate projection». This is true for the protopenis (i.e., non-functional larval penis) of the majority of the species examined ( Fig. 107 View FIGURES 106–111 ), but in some species these projections are not expressed ( Fig. 271 View FIGURES 267–273 ). The shape of the protopenis is species-specific. In all cases, the apex of each protopenis lobe bears a foramen, which leads to a narrow, long, thin-walled, cuticular tube, corresponding to the imaginal spermatic duct. If the apical projection is present, the foramen is located on its top, being exposed either caudally ( Fig. 313 View FIGURES 209–314 ), or caudally-laterally ( Fig. 205 View FIGURES 203–206 ).
When the subimaginal penis lobe is developed under the larval cuticle, its spear-like roll appears as inserted into the apical projection of protopenis, and the cuticular tube of the protopenis is inserted into the canal of the spear-like roll ( Fig. 107 View FIGURES 106–111 ). During subsequent growth, the spear-like roll is elongated, being inserted by its base into the growing penis, and remains to be directed caudally with the cuticular tube of protopenis inside ( Figs 109 View FIGURES 106–111 , 347 View FIGURES 346–347 , 500–501 View FIGURES 498–507 , 607 View FIGURES 603–612 ). During the molt from larva to subimago, the spear-like rolls turn medially and get definite position when subimaginal genitalia become free from cuticle of larval protopenis, even when subimaginal abdomen is not yet taken out from the larval cuticle (observed for Th. telegraphicus ).
In subimago, the spear-like rolls have the same size and shape as in imago; their cuticle is rigid and retains its shape on subimaginal exuviae after molt from subimago to imago, while remainding exuviae of the subimaginal penis is very delicate and is crumpled during the molt ( Fig. 475 View FIGURES 474–478 ).
The slender projection of protopenis can be interpreted as a larval precursor of the spear-like roll. Length of this larval projection does not correlate with length of imaginal spear-like roll: for example, in Th. quevedoensis the projections of larval protopenis are absent, while the imaginal spear-like rolls are well developed.
Unfortunately, development of unusual penes of Th. sinuosus and Th. ludmilae sp. n. was not examined.
Judging by their structure and transformations in ontogenesis, the spear-like rolls of Thraulus are homologous to the tubular telopenes of Hermanellonota Kluge 2008. Their presence is a synapomorphy of Thraulodes and Hermanellonota, which allows uniting them to a new taxon Hermanellandria (see below).
Egg structure
In all species of Thraulodes , knob-terminated coiled threads (KTC) are evenly dispersed all over egg surface, equally on equator and pole regions, independently from shape of the egg, which varies from ellipsoid ( Fig. 589 View FIGURES 589–590 ) to barrel-shaped, i.e. with flat pole areas ( Fig. 348 View FIGURES 348–349 ). Each KTC is located in a round cavity and coiled as a regular spiral always directed anticlockwise ( Fig. 150 View FIGURES 149–150 ). The knob, which terminates this coiled thread, is located in the middle of the spiral. The coiled thread can be either located openly [in Th. zonalis — Fig. 349 View FIGURES 348–349 ; in Th. paysandensis ( Dominguez 1987: fig. V.4)], or hidden by a ring-like cover, which surrounds the knob (in other examined species). In various species, this ring-like cover can be narrower or wider, smooth or rugose.
Other chorion is either smooth ( Fig. 121 View FIGURE 121 ) or bears round protuberances, which can be large or smaller, located either evenly ( Fig. 249 View FIGURES 248–249 ), or concentrated around KTCs ( Figs 274–275 View FIGURES 274–275 ), or, vice verse, located at maximum distance from KTCs forming rows ( Figs 315–316 View FIGURES 315–316 ); in some species rows of protuberances are fused into integral ridges, which form a net-like relief with KTC in each cell ( Figs 377–378 View FIGURES 377–378 ).
Among the species examined, one species Thraulodes viviparus sp. n. is probably ovoviviparous; in a single examined female imago of this species, abdomen is filled by completely grown eggs lacking chorion.
Systematic position and diagnosis of Thraulodes
Thraulodes belongs to subsequently subordinated taxa: Leptophlebiidae > Atalophleboadentata Kluge 2009 > Atalophlebopectinata Kluge 2009 > Atalophleboculata Kluge 2009 > Atalophlebomaxillata Kluge 2009 > Atalophlebolinguata Kluge 2009. General system of the taxon Atalophlebolinguata remains poorly elaborated. One of the taxa established within Atalophlebolinguata is Hagenulus /fg1, or the tribe Hagenulini Kluge 1994b ( Kluge 2008) .
Hagenulus /fg1
Tribe Hagenulini Kluge 1994b: 250 ;
Hegenulus/fg1: Kluge 2008: 387.
Autapomorphy. Patella-tibial suture is lost on all legs of all stages—larva ( Figs 21–24 View FIGURES 21–24 ), subimago ( Fig. 31 View FIGURES 25–34 ) and imago ( Figs 28–30 View FIGURES 25–34 ).
Other characters. Hind wing has more or less prominent costal projection; vein Sc terminates on costal margin just distad of costal projection, far from wing apex ( Fig. 41 View FIGURES 35–41 ) (apomorphy, independently occurred in various taxa). Imaginal and subimaginal claws of all legs are ephemeropteroid (plesiomorphy).
Distribution. Neotropical Region.
Composition. The taxon Hagenulus /fg1 comprises Hagenulus /fg2 (incl. Borinquena , Turquinophlebia , Poecilophlebia , Careospina , Traverina , Hagenulopsis , Neohagenulus ), Ulmeritus /g1 (incl. Ulmeritoides ), Miroculus/ g1, Hermanellandria taxon nov. and some other taxa. The new taxon Hermanellandria comprises Thraulodes and Hermanellonota:
Hermanellandria taxon nov.
Thraulodes group: O’Donnell & Jockusch 2008: 663;
Circumscriptional name: Hermanellandria taxon nov.;
Hierarchical name: Hermanella /f1= Thraulodes /g1;
Possible rank-based name: subtribe Hermanellina .
Autapomorphy of Hermanellandria. Each of two penis apices is produced into a slender sclerotized tubular process— telopenis, inside which the genital duct passes; apex of telopenis is obliquely truncated and pointed. In imago telopenes are sharply bent at their bases and/or shifted from apices of penis lobes, so that are directed either medially (in most Thraulodes ), or laterally (in Farrodes ), or ventrally (in Hermanellognatha), or even ventrallyanteriorly, i.e. toward penis base. Unlike imago, in mature larva telopenes represent apical continuations of penis lobes, being directed caudally, so that gonopores occupy apical position; larval protopenis has developed gonopores with ducts lined by larval cuticle ( Figs 107, 109 View FIGURES 106–111 , 347 View FIGURES 346–347 , 500–501 View FIGURES 498–507 , 607 View FIGURES 603–612 ; Kluge 2008: Figs 9–11 View FIGURES 5–13 , 15 View FIGURES 14–20 , 27–28, 32, 34 View FIGURES 25–34 ). In subimago telopenes either have the same position as in imago, or position intermediate between larval and imaginal ones ( Kluge 2008: Fig. 12 View FIGURES 5–13 ). Subimaginal telopenes are sclerotized, while the rest subimaginal cuticle of penis is membranous.
In Hermanellognatha and Simothraulopsis each telopenis represents a tube with apical opening. In all Thraulodes each telopenis is a roll, i.e. a tube cleft up to the base. In Farrodes telopenes lack gonoducts, and genital openings are located at their bases.
Telopenes can be confused with superficially similar processes, which occur in some other taxa and arise from penis apex toward its base; but unlike telopenes, penial processes of other Leptophlebiidae do not bear genital ducts and in larval stage develop in the same position as they are in imago.
Distribution. Neotropical Region.
Composition. The taxon Hermanellandria (or Hermanella /f1= Thraulodes /g1) comprises Thraulodes and Hermanellonota Kluge 2008 (or Hermanella /f2=g1). In its turn, Hermanellonota is divided into Simothraulopsis Demoulin 1966 , Farrodes Peters 1971 and Hermanellognatha Kluge 2008 (or Hermanella /f3=g2).
Comments. O’Donnell and Jockusch (2008) established the « Thraulodes group» based on similarity of histone H3 and 28S ribosomal DNA of three examined species, Thraulodes sp., Farrodes sp. and Traverella albertana Edmunds (which belongs to the « Hermanella complex», i.e. to Hermanellognatha Kluge 2008). These authors stated that their analysis did not support hypothesis about relationship of these species with Meridialaris lineage, which was proposed by Flowers and Dominguez (1991). O’Donnell and Jockusch (2008) also wrote that «this grouping is additionally supported by the similarity of maxillary palps in Thraulodes spp. and Farrodes spp.», but did not report concrete synapomorphies in the maxillary palp structure. The taxon with the new circumscriptional name Hermanellandria corresponds to the « Thraulodes group» established by O’Donnell and Jockusch (2008).
The idea about relationship of Thraulodes with the Notogean genera Meridialaris and Massartellopsis suggested by Flowers and Dominguez (1991) was based on parsimony analysis of a limited number of characters of several New World genera. Generally speaking, the method of parsimony analysis is not based on any scientific theory; a tree built by this method depends on occasional set of characters, their arbitrary formulation and an arbitrarily chosen method of calculation, but not on revealed apomorphies ( Pesenko 1989). I the tree suggested by Flowers and Dominguez (1991: Fig. 3 View FIGURES 1–4 ), the branch comprising Thraulodes , Meridialaris and Massartellopsis and the subordinated branch comprising Thraulodes and Meridialaris were argued by characters «1» (wide labrum), «3» (hood of labrum), «30» (dorsal setae on fore tibia), «50» (spines on penis) and «25» (absence of setae on submentum). Each of these characters was also reported for some other taxa, so no one autapomorphy of these branches was reported; the character «3» was also reported as lost in Thraulodes ( Flowers & Dominguez 1991: Table 1 View TABLE 1 ). The character «spines on penis» was reported, besides Thraulodes , Meridialaris and Massartellopsis , for Massartella Lestage 1930 , for all taxa belonging to Hermanellognata, for Traverina Peters 1971 and for Hagenulus Eaton 1882 ; among Hagenulus , it was erroneously reported as absent in H. morrisonae Peters & Alayo (in Peters 1971) , while actually this species has diminished spines ( Kluge 1994b: Fig. 37 View FIGURES 35–41 ). These «spines» of Thraulodes and «spines» of Hermanellognatha are homologous, being telopenes with sperm canal inside (see above). In contrast to them, the «spines» of Traverina and Hagenulus are a pair of simple denticles on ventral side of penis far from the genital opening. The «spines» of Meridialaris , Massartellopsis and Massartella are various small processes on margins of genital opening; in various species these processes are soft or sclerotized, pointed or blunt, stout or hair-like; no one of these processes contains sperm canal, neither in imaginal penis, not in larval protopenis. So the idea about a relationship of Thraulodes with the Meridialaris lineage is groundless.
Earlier, Flowers (1987) proposed a relationship between Thraulodes and Atopophlebia Flowers 1976 . Unfortunately, I am unable to examine all stages of development of Atopophlebia , being limited by a single undetermined female larva from Peru. Its examination reveals the following characters common for Atopophlebia and Thraulodes : horseshoe row of hairs on dorsal side of glossa (see above); dense setation on distal part of outer side of mandible (the same in some other taxa—see above); absence of patella-tibial suture on at least middle leg of larva (the same in all other Hagenulus /fg1 and some other taxa). The penis of Atopophlebia has a pair of apical processes directed ventrally; their structure and development are not described, so it is unclear, if they are telopenes, or not. At present, the position of Atopophlebia in Hermanellandria is an open question.
Hierarchical name: Thraulodes /g2;
Rank-based name: Thraulodes Ulmer 1920: 33 (imago); Needham & Murphy 1924 (imago, larva); Traver & Edmunds 1967 (imago, larva, eggs; species revision)
Type species: Thraulus laetus Eaton 1884 .
Diagnosis.
Autapomorphies:
(1) Bases of gonostyli are brought together and inserted in a common unpaired gonostylar cavity of styliger; dorsal margin of styliger is projected caudally, and the gonostylar cavity is exposed ventrally-caudally ( Figs 102 View FIGURES 101–105 , 110 View FIGURES 106–111 , 112 View FIGURES 112–120 ).
(2) Telopenis (see autapomorphy of Hermanellandria) is tubular with a longitudinal cleft up to its base, i.e. represents a roll ( Figs 103–105 View FIGURES 101–105 ) (in contrast to integral tube of Hermanellognatha and Simothraulopsis ).
(3) On hind tibia, stout setae of outer margin (initial for Leptophlebiidae ) form two separated rows, outer-anterior and outer-posterior ones; long hairs (also initial for Leptophlebiidae ) are situated between these two rows of stout setae and form one more row posteriad of them; row of recurved hairs is located on anterior side of tibia, more distally from inner margin than the inner-anterior row of stout setae, which is present on middle and hind legs ( Fig. 89 View FIGURES 86–93 ).
Characters of unclear phylogenetic status:
(4) Outer side of each mandible with distal setal bunch, but without middle setal bunch: long hairs forming a row or a field on outer margin of mandible, are denser and longer nearer the base of incisor ( Figs 17–18 View FIGURES 14–20 ). (the same in some other taxa—see above).
(5) Long setae of dorsal side of glossa form irregular row or stripe of a characteristic horseshoe shape ( Fig. 20 View FIGURES 14–20 ) (the same in Atopophlebia —see above).
(6) Swimming larva makes lateral movements with its abdomen (in contrast to most other Ephemeroptera , which make dorso-ventral swimming movements). Among the taxa whose larval swimming was observed, Kimminsula makes the same movements as Thraulodes .
Plesiomorphy:
(7) Hind wing always retains furcations of RS and MP (while furcation of MA is lost in all Ephemeroptera ) ( Fig. 41 View FIGURES 35–41 ).
TABLE 1. Inequality of larval legs in Thraulodes.
| Fore leg | Middle leg | Hind leg | ||
|---|---|---|---|---|
| Size | longest | |||
| Form | widest proximally | widest at middle | widest at middle | |
| Proximal blank | + | – | – | |
| Outer-anterior stripe of stout setae | + | + | + | |
| Femur | Outer-posterior stripe of stout setae Outer stripe of thin hairs | + long, dense | + long, dense | + shorter, sparser |
| Row of recurved hairs | – (+) | + (–) | + (–) | |
| Stout setae on inner margin | + | + | + | |
| Stout setae on anterior surface | + | + | + | |
| Setae on posterior surface | – (+) | – (+) | + | |
| Outer-anterior row of stout setae | – | – | + | |
| Outer-posterior row of stout setae | – | – | + | |
| Tibia | Inner-anterior row of stout setae Inner field of stout pointed setae | vestigial or absent dense, especially apically | sparse sparse | + sparse |
| Posterior pointed pectinate setae | – | – | + | |
| Outer hairs | + | + | + | |
| Inner-anterior row of recurved hairs | – (+) | + | + |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |