Arumatia dubia ( Caudell, 1904 ) Ghirotto & Crispino & Engelking & Neves & Góis & Chiquetto-Machado, 2022
|
publication ID |
https://doi.org/ 10.5852/ejt.2022.827.1849 |
|
publication LSID |
lsid:zoobank.org:pub:8B6F1573-B627-4C62-94CA-DB0F1146ED2C |
|
DOI |
https://doi.org/10.5281/zenodo.6798865 |
|
persistent identifier |
https://treatment.plazi.org/id/03CF87FA-8530-FFCF-D447-CEAAFDAED38A |
|
treatment provided by |
Felipe |
|
scientific name |
Arumatia dubia ( Caudell, 1904 ) |
| status |
gen. et comb. nov. |
Arumatia dubia ( Caudell, 1904) gen. et comb. nov.
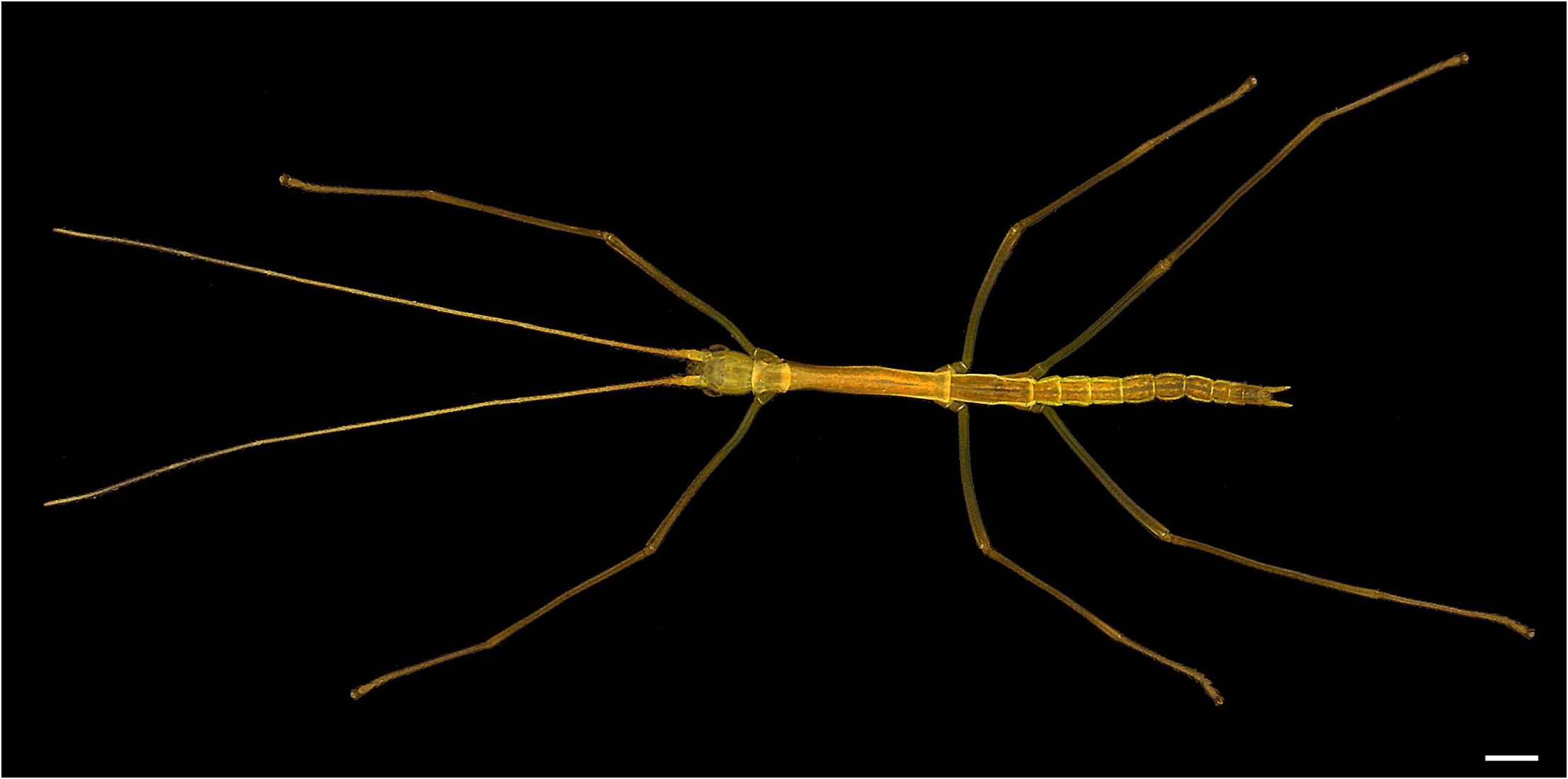
Figs 1–13 View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig , 46 View Fig , 52 View Fig , 53A–D View Fig
Bacunculus dubius Caudell, 1904: 186 View in CoL .
Bacunculus fragilis Brunner von Wattenwyl, 1907: 336 View in CoL . Syn. nov.
Echetlus evoneobertii Zompro & Adis, 2001: 294 . Syn. nov.
Heteronemia dubia View in CoL – Zompro 2001: 223. — Otte & Brock 2005: 158. Heteronemia fragilis View in CoL – Zompro 2001: 223. — Otte & Brock 2005: 158. Echetlus evoneobertii – Zompro 2004b: 138. — Otte & Brock 2005: 132. — Zompro & Domenico 2005: 257. — Zompro et al. 2006: 131 (specimen record for Australia in error, misidentification).
Candovia evoneobertii View in CoL – Brock & Hasenpusch 2007: 7, 70; 2009: 151. — Araujo & Garraffoni 2012: 235.
Diagnosis
Females
From Arumatia fulgens gen. et comb. nov., A. dubia gen. et comb. nov. differs by the shorter subgenital plate not exceeding half the length of the tergum X, the slightly shorter cerci (not reaching the length of terga VIII–X combined) and the thinner body. From A. anyami gen. et sp. nov. it differs by the lack of a black spot on the ventral region of the prothorax, the longer median segment, equal in size to the metanotum (vs ¾ the length of metanotum), the shorter subgenital plate, larger epiproct, posterior margin of anal segment less emarginate, tergum VII having a widened and incurved posterior margin and shorter gonapophyses VIII, and narrower gonoplac. Some mouthparts differ between the two species, because A. dubia has a different microtrichia pattern as a band on the galea and not a circle, has less and stouter setae on the lacinia, a narrower galea and narrower labial palp segments than those of A. anyami . From A. crassicercata gen. et sp. nov. it differs by the lack of a praeopercular organ, the longer median segment, much longer cerci and thinner legs with less prominent keels. Arumatia dubia also has fairly less and stouter setae on the lacinia and shorter microtrichia of the galea than A. crassicercata , and the microtrichia are arranged in a band, not a circle. From A. aramatia gen. et sp. nov. it differs by the shorter, less elongate head, the more elongate galea, the longer median segment, as long as the metanotum, the shorter terga VIII–X, the emarginate posterior margin of tergum X, the slightly shorter subgenital plate, the lack of a praeopercular organ and the shorter gonapophyses VIII. From A. motenata gen. et sp. nov.
and A. diamante gen. et sp. nov. it differs by the shorter, less elongate head, the more elongate galea, the shorter microtrichia of the galea, the longer median segment, as long as the metanotum, the presence of stronger spiniform setae on the carinae of the tarsi and on the apex of mid and hind tibiae, the absence of an apical sessile spine on the apex of the ventral, antero- and posteroventral carinae of the tibiae, the slightly longer cerci, the shorter epiproct, the longer subgenital plate, the absence of a praeopercular organ, the narrower gonoplac and the shorter gonapophyses VIII.
Eggs
The egg of Arumatia dubia gen. et comb. nov. has a smoother capsule than that of A. anyami gen. et sp. nov., and a lumpier and grosser texture (rather than finely punctuated) than that of A. motenata gen. et sp. nov. or A. crassicercata gen. et sp. nov. The egg also has a reticular, netted aspect of the keels of the capitulum rather than radial on the capitulum of the eggs of A. aramatia gen. et sp. nov., A. motenata and A. diamante gen. et sp. nov. as well as a widened posteriormost region of the micropylar plate and dorsal region less convex in lateral view than that of A. crassicercata .
Type material
PARAGUAY • 1 ♀, holotype of Bacunculus dubius ; Sapucay ; W.T. Foster leg.; “10-3[?]1/[?]”, “ Bacunculus dubia ♀ type. Caudell; Type No. 8027. U.S.N.M.”; USNM 8027 View Materials (examined from photo) .
SOUTH AMERICA • 1 ♀, holotype of Bacunculus fragilis ; “America merid.” [ South America ]; Bol. leg.; MNMS (examined from photo) .
BRAZIL • 1 ♀, holotype of Echetlus evoneobertii ; São Paulo, Boa Esperança do Sul ; 18 Feb. 1998; J.A. Cerignoni leg.; MZUSP 0035 (examined) • 1 ♀, eggs, paratypes of Echetlus evoneobertii ; São Paulo, Piracicaba, ESALQ / USP; Sep. 2000; “criado em laboratório em folhas de Eucalyptus ” [raised in lab with Eucalyptus leaves]; MZUSP 0036 (examined) • 1 ♀, paratype of Echetlus evoneobertii ; same collection data as for preceding; MZUSP 0037 (examined) • 1 egg, paratype of Echetlus evoneobertii ; same collection data as for preceding; ANIC (examined from photo) • 1 ♀, paratype of Echetlus evoneobertii ; same collection data as for preceding; ANIC (not examined) • 5 ♀♀, paratypes of Echetlus evoneobertii ; same collection data as for preceding; MZUSP (not traced) • 2 ♀♀, paratypes of Echetlus evoneobertii ; São Paulo, Boa Esperança do Sul; 18 Feb. 1998; J.A. Cerignoni leg.; ZMUK 457-1 , 457-3 (not traced) • 5 eggs, paratypes of Echetlus evoneobertii ; same collection data as for preceding; ZMUK 457-7 (not traced).
Other material examined
BRAZIL – Paraná • 1 ♀; Londrina ; 6 Apr. 1998; E.P. Frazão leg.; ESALQENT 000438 . – Distrito Federal • 1 ♀; Brasília, urban area near Parque Olhos D’água; 15°44′56.6″ S, 47°53′25.1″ W; 2020; J. Góis and P. Souza leg.; MZUSP GoogleMaps . – Mato Grosso do Sul • 2 ♀♀; Chapadão do Sul, margin of Sucuriú River; 19°28′31.3″ S, 52°32′17.7″ W; 30 Nov. 2020; E.B. Crispino and D. Castro-Pereira leg.; in cerradão [deciduous forest]; MZUSP V0534 , V0535 GoogleMaps • 2 ♀♀, nymphs, eggs; same collection data as for preceding; MZUSP GoogleMaps . – Minas Gerais • 3 ♀♀; Paraopeba ; 18 Jun. 1988; J.A. Cerignoni leg.; in Eucalyptus sp. ; ESALQENT 000439 , 000444 , 000451 • 1 ♀; Delfinópolis, Pousada Cachoeira Paraíso, near Serra da Canastra; 20°20′35.5″ S, 46°47′14.0″ W; E.W. Engelking leg.; in cerrado [savannah formation]; UNESP GoogleMaps • 1 ♀; Araguari, near Emborcação Dam; 18°27′23.0″ S, 48°00′40.7″ W; 20 Jan. 2021; V.M. Ghirotto leg.; in cerradão [deciduous forest]; MZUSP V0547 GoogleMaps • eggs; same collection data as for preceding; from female V0547 ; MZUSP GoogleMaps • 1 ♀, first instar nymph; Minas Gerais, Araguari ; reared by V.M. Ghirotto 2021 from female V0547; MZUSP V0556 • 1 ♀, second instar nymph; same collection data as for preceding; MZUSP V0557 • 1 ♀; same collection data as for preceding; MZUSP V0676 . – São Paulo • 1 ♀; Piracicaba, ESALQ; Jun. 1992; J.A. Cerignoni leg.; host plant Psidium guajava ; Echetlus evoneobertii ♀ det. O. Zompro Nov. 2003; ESALQENT 000443 • 1 ♀; Piracicaba; Jun. 2001; S.S. Prado leg.; ESALQENT 000442 • 1 ♀; Piracicaba ; 16 May 2000; R.A. Polanczyk leg.; ESALQENT 000436 • 1 ♀; Piracicaba , ESALQ; 18 May 1998; S.R. Magro leg.; ESALQENT 000450 • 1 ♀; Piracicaba, ESALQ, Dept. Entomology; 17 May 1998; V.D.A. dos Reis leg.; ESALQENT 000440 • 3 ♀♀; Limeira; 23 Jan. 2004; P. Milano leg.; ESALQENT 000446 , 000447 , 000448 • 1 ♀; Limeira; 10 Jun. 1999; “criação particular” [culture stock] P. Milano; ESALQENT 000445 • 1 ♀; Descalvado ; 26 Apr. 1968; Renato leg.; ESALQENT 000441 • 1 ♀; “R. Preto” [Ribeirão Preto]; Lordello leg.; “4-51”; ESALQENT 000437 • 1 ♀; “Boa Esperança” [ Boa Esperança do Sul ]; 21 Apr. 1998; R.B.Q. Silva leg.; ESALQENT 000449 • 8 ♀♀; Ibaté ; Jan.–Jul. 2019; reared by V.M. Ghirotto, culture obtained at ESALQ entomology lab; MZUSP 1222 View Materials , 1224 View Materials , 1227 View Materials , V0314 , V0315 , V0333 , V0415 , V0503 • 1 ♀, first instar nymph; same collection data as for preceding; MZUSP V0272 • eggs; same collection data as for preceding; MZUSP • 2 ♀♀; same collection data as for preceding; 2021; MZUSP V0542 , V0558 • eggs; same collection data as for preceding; MZUSP • 2 ♀♀; Assis , Campus UNESP ; 22°39′00.2″ S, 50°26′18.8″ W; Aug. 2018; P.W. Engelking leg.; UNESP WE059 GoogleMaps • 2 ♀♀; Echaporã , Trilha da cachoeira Stn Rosa; 22°25′23.8″ S, 50°11′60.0″ W; Sept. 2020; P.W. Engelking and G.A. Nunes leg.; UNESP WE060 GoogleMaps .
Remarks
The Australian specimen of Arumatia dubia gen. et comb. nov. reportedly housed at the Zoological Museum of the Christian-Albrechts University of Kiel in Germany (as Candovia evoneobertii ) could not be traced (Thies Büscher, pers. comm., 2021) and no description or illustration of this specimen was provided by Zompro et al. (2006). It is very likely that this specimen was misidentified and represents some distinct species native to Australia (Paul Brock, pers. comm., 2021).
Redescription
Female
MEASUREMENTS (in mm, N = 3). Body (without cerci) 69.1–72.4, head 4–4.1, antennae 43.5–48.5, pronotum 2.4–2.6, mesonotum 15.7–16.6, metanotum 4.5, median segment 4.5, abdomen (excluding median segment, with cerci) 37.6–39.4, cercus 4.7–5.0, profemur 15.9–16.4, protibia 16.5–17.2, mesofemur 11.2–11.8, mesotibia 10.8–11.6, metafemur 14.2–14.9, metatibia 15.5–16.9.
COLOUR ( Figs 1–2 View Fig View Fig , 13 View Fig ). Body generally light to dark green, yellow, beige, salmon, orange, brown, grey or black with or without light granulations on body and legs (mostly femora) and with or without irregular stains of different tones ( Fig. 13 View Fig ). Some individuals with longitudinal band running along entire body, slightly darker than body to black ( Fig. 13A, F View Fig ). Eyes same colour as rest of body but darker, or reddish brown. Antennae darker ventrally. Apical palp segments and other mouthparts in same colour as body or brownish to beige.
HEAD ( Figs 3–4 View Fig View Fig ). Elongate, smooth, with sparse setae mostly on dorsal region, vertex flat but gently convex at posterior margin to fit underneath the pronotum, frontal convexity developed and frontal suture round ( Fig. 3A–D View Fig ). Eyes small and slightly elongate, approximately 0.2× as long as head. Cervix covering more than half of head, cervical sclerites elongate and weakly sclerotized. Gula somewhat spatulate, bearing setae and covering ca more than half of cervix ( Fig. 3D View Fig ). Subgena narrow with posterior projection ca as high as eye. Submentum narrow and only slightly curved backwards, mentum simple, prementum somewhat wide. Glossa elongate and rounded, paraglossa larger, roundly falcate and almost reaching labrum. Lacinia with three distal teeth, one large medially and two smaller laterally, with mesal edge bearing bundles of ca 12 large setae from base to sclerotized portion ( Fig. 4D View Fig ). Lobe over base of lacinia almost indistinct, showing as small and gentle round bump. Galea elliptical and bearing long setae ( Fig. 4F View Fig ), apically with dense tuft of hairy, large microtrichia forming band along apical edge. Dorsally and posteriad to this patch ca 29 distinct circular granules of same size as base of setae ( Fig. 4E View Fig ). Galealobulus present, small and widely round, basally fused to galea ( Fig. 4D, F View Fig ). Palpal segments cylindrical. Clypeus wide, approximately elliptical, anterior surface with two parasagittal
furrows and anterior margin medially with round, wide notch ( Fig. 3C View Fig ). Labrum strongly notched anteromedially, asymmetric with right lobe significantly larger than left one ( Fig. 3C View Fig ). Left mandible with two smooth and straight edges, dorsal one cutting and sharp, and ventral one blunt, mesal surface with convex globose protuberance near ventral cutting edge, and sinuous furrow between both edges ( Fig. 4A, C View Fig ). Right mandible with sharp dorsal cutting edge, straight but interrupted at border with ventral edge by round edge, ventral edge irregular, level and molariform with two larger projections, mesal surface without protuberances ( Fig. 4A–B View Fig ). Both mandibles with dense row of long setae at base of dorsal margin. Antennae filiform, extending approximately until posterior region of third abdominal segment and exceeding forelegs, scapus ca 1.9× as long as wide, basally constricted in dorsal view and slightly compressed dorsoventrally, pedicellus subglobose, large, more than half the length of scapus; composed of 68–72 segments. Antennomeres bear three types of setae ( Fig. 3E–F View Fig ), a very short, small and densely distributed one, a transparent and porrect one, and a black, slightly more elongate, straight one. From 47 th segment, smaller setae becoming sparser, on segments 62–67 smaller type very sparse and almost absent and on segments 67–72 absent. Other two types of setae gradually becoming thinner
and more elongate towards last segments. Antennal bump very discrete on dorsal surface of posterior region of 12 th antennomere ( Fig. 3F View Fig ).
THORAX ( Figs 1–2 View Fig View Fig , 5 View Fig ). Smooth with scattered setae inserted in paler small bumps. Pronotum slightly longer than wide and slightly constricted pre-medially, anteriorly and posteriorly convex in lateral view, transverse sulcus conspicuous and straight, gently curved in lateral edges, longitudinal median sulcus distinct. Paranota curved and ca 3× as long as wide, procoxopleurite apically round ( Fig. 5A– B View Fig ). Probasisternum tapering towards anterior, profurcasternum round ( Fig. 5C View Fig ). Mesothorax 6× as long as prothorax, as wide as prothorax anteriorly and slightly widening towards posterior region. Mesonotum with pair of distinct lateral carinae, mesepisternum lanceolate and regularly widening posteriorly, mesepimeron slightly elongate, pointing towards posterior and slightly exceeding end of mesothorax. Mesocoxopleurite indistinct, mesofurca Y-shaped. Metathorax continuing pair of lateral carinae of mesonotum, metepisternum long and similar to mesepisternum. Metepimeron extremely elongate, extending across entire length of median segment, posteriorly pointing and slightly exceeding posterior region of median segment. Metacoxopleurite very discrete but elongate, metafurca Y-shaped. Metanotum and median segment of about same length ( Fig. 5E View Fig ). Median segment anteriorly marked by two parasagittal ovoid stains ( Fig. 5E View Fig ), continuing pair of thoracic lateral carinae. Posterior margin of metanotum and anterior margin of median segment slightly widened ( Figs 1 View Fig , 13 View Fig ).
LEGS ( Figs 1–2 View Fig View Fig , 6 View Fig ). Slender. Hindlegs slightly extending beyond epiproct but not cerci, anterior legs ca as long as hindlegs, midlegs distinctly shorter. Midlegs slightly longer in some individuals. Coxae smooth ( Figs 5B–C View Fig , 6A View Fig ). Profemora of around same length as mesothorax, mesofemora about 0.7 × as long as
profemora and metafemora slightly shorter than mesothorax. Protibia varying from slightly shorter to longer than profemur, mesotibia ca as long as mesofemur, metatibia slightly longer than metafemur. Profemur with distinct basal curvature ( Fig. 6A View Fig ). Femora and tibiae with five carinae without setae between them ( Fig. 6B View Fig ). Carinae of profemora and protibiae distinctly keeled, mid and hind femora and tibiae weakly keeled. Antero- and posteroventral carinae of femora with apical toothed prominence. Carinae of all femora, tibiae and tarsi bear row of short setae, last two setae of all five carinae of meso- and metatibiae stouter and spiniform ( Fig. 6B–F View Fig ). In tarsi, setae on ventral carinae of probasitarsi longer (but not stouter), while setae on ventral, antero- and posteroventral carinae of meso- and metabasitarsi stouter and spiniform, distributed across entire length except for apex of mesobasitarsi and on basal half to three quarters of metabasitarsi, stronger on ventral carinae ( Fig. 6D–F View Fig ). Some individuals with weaker,
not spiniform setae on antero-ventral carinae of metabasitarsi. All basitarsi very elongate, significantly longer than respective following tarsomeres combined ( Fig. 6B–E View Fig ), with hairy setae restricted to apical portion on ventro-lateral patches ( Fig. 6C–D View Fig ). Remaining tarsomeres with setae on ventro-lateral patches in portions not covered by euplantulae. Tarsomeres I–III with discrete dorsal round apical projection. Arolium round and broad, bearing setae dorsally. Pretarsal claws symmetrical with setae dorsally and laterally. Euplantulae well developed in all tarsomeres, composed of two symmetrical pads separated by median groove in tarsomeres I–III, of single pointing domed pad in tarsomeres IV and of single flattened lobed pad in tarsomeres V, present only apically at tarsomeres I–II, covering ca half length of tarsomeres III, covering two thirds length of tarsomeres IV and covering almost entire ventral surface of tarsomeres V ( Fig. 6D View Fig ).
ABDOMEN ( Figs 1–2 View Fig View Fig , 7–8 View Fig View Fig ). External surface as in thorax, bearing few setae across its entire length. Median segment ca as long as metanotum ( Fig. 5E View Fig ). Combined length of segments II–X slightly longer than combined length of head, thorax and median segment. Terga II–VII and sterna II–VIII bearing discrete lateral carinae near lateral margins. All segments longer than wide ( Fig. 1 View Fig ). Segment II as long as VII and slightly shorter than III, segments III–VI slightly increasing in size in relation to anterior segment, tergum VIII significantly shorter than preceding segment and slightly longer than IX, tergum X slightly longer than IX. Terga III–VI wider than II, VII and VIII. Terga IX and X slightly narrower
than preceding segments. Lateral borders of tergum VII gradually widening and curving downwards and inwards near posterior margin ( Fig. 7B–E View Fig ). Tergum X conical in lateral view ( Fig. 7B View Fig ), in dorsal view just slightly narrower towards apex, posterior margin emarginate with broad round indent showing epiproct ( Fig. 7A View Fig ). Epiproct domed in dorsal view, dorsoventrally flattened, distinctly prominent, exceeding posteriormost margins of tergum X and visible dorsally and laterally ( Fig. 7A–B View Fig ). Paraprocts elongate, posteriorly acute, straight, setose on posterior margin, laterally bearing cerci and not concealing them from ventral or lateral view ( Fig. 7C–E View Fig ). Cerci extremely elongate, straight and basally narrower, fitting paraprocts, pointing to posterior, gradually tapering, slightly longer than terga IX, X and epiproct combined ( Fig. 7 View Fig ). Cerci bearing four types of setae (as in nymphal stages, see Fig. 11F–G View Fig ), three very similar to those of antennae, densely covered in very short, small setae similar to those of antennae, sparser transparent setae inclined towards posterior and black and slightly more elongate straight setae and with inner and ventral surfaces also bearing several sensory hairs significantly thinner than other setae. Praeopercular organ absent (see praeopercular region in Fig. 7C–E View Fig ). Subgenital plate roundly lanceolate to slightly more acute, reaching medial region of tergum X and bearing two parasagittal carinae beginning at anterior margin and running half length of segment before becoming flatter and more
setose, gradually tapering towards posterior and totally covering gonapophyses and gonoplac ( Fig. 7C– E View Fig ). Gonapophyses and gonoplac flattened, dorsoventrally for gonapophyses VIII, lateroventrally for IX and laterally for gonoplac ( Figs 7E View Fig , 8 View Fig ). Gonapophyses VIII and IX short and of similar length, reaching ca ¾ of length of tergum IX; VIII linear to lorate, blunt to tapering towards posterior, IX lorate to conical, tapering towards posterior ( Fig. 8 View Fig ). Gonapophyses IX ventrally folded to fit within gonapophyses VIII. Gonangulum distinctly reduced, flat and not lobed ( Fig. 8C View Fig ). Gonoplac significantly elongate, widened basally, subsequently linear to lorate, bearing setae, exceeding both gonapophyses and reaching ca anterior margin of tergum X ( Fig. 8 View Fig ). Distinct triangular sclerotization pointing towards posterior and slightly towards ventral present at dorsal face of gonapophyses, separating them from ventral wall of segment IX. Inside oviduct around median region of segment VIII with triangular and slightly sclerotized structure dorsoventrally flattened and pointing towards posterior.
Egg ( Fig. 12 View Fig )
Measurements in mm (N = 10): length 2.5, height 1.7–1.8, width 1.4–1.5. Relatively small, capsule ovoid, constricted at opercular collar, laterally compressed and ca 1.8× as long as wide and 1.4× as long as tall ( Fig. 12A–D View Fig ). Capsule surface slightly roughly textured with lumpy or net appearance. Colour varying in shades of orangish or reddish brown, rarely greyish, with lighter band on dorsal, anterior and posterior regions, surrounding operculum and polar region ( Fig. 12A–D View Fig ). Micropylar plate very elongate, occupying large area of dorsal region and varying around 0,6–0,8 × as long as capsule and 2,8–5,7× as long as wide, elliptical with round margins and almost parallel-sided with posterior portion gently but noticeably widened ( Fig. 12C–D, G View Fig ). Micropylar plate with lighter elevated outer circle delimiting darker inner flat region confluent with micropylar cup ( Fig. 12C–D View Fig ). Internal micropylar plate closed, accompanying external micropylar plate, interior surface smooth. Micropylar cup small, rounded, only slightly elevated, dark and anteriorly merged to widened polar edge of micropylar plate. Median line short and of same colour and elevation as elevated margin of micropylar plate, almost reaching polar area ( Fig. 12C–D View Fig ). Opercular collar narrower (constricted) than rest of capsule, smooth and lighter, with minute and delicate bristles surrounding edge ( Fig. 12F View Fig ). Operculum elliptical with irregular reticular and sinuous elevation, with amber texture composing non-stalked capitulum ( Fig. 12A–F View Fig ). Very few eggs present flat, smooth and amber operculum ( Fig. 12G–H View Fig ).
Nymphs ( Figs 2B View Fig , 9–11 View Fig View Fig View Fig )
FIRST- INSTAR NYMPH ( Figs 1 View Fig , 9–10 View Fig View Fig , 11A–B View Fig ). Entirely green with black blurred longitudinal lateral line running along head behind eyes, further crossing prothorax and extending to anterior portion of mesothorax. Dorsally green but lighter than lateral and ventral areas delimited by lateral black line. Body with sparse setae and surface with smooth and flat scaly texture. Head and eyes large. Head globose, just slightly elongate ( Fig. 9 View Fig ). Gula not sclerotized, visually absent, cervix more than half length of head, slightly wider than that of adults. Subgena similar in shape to that of adults. Labrum notched and asymmetric, similar to that of adults but right lobe only slightly larger than left one. Lacinia similar to that of adults but with more blunt teeth and few, ca 3 setae on mesal edge ( Fig. 10A–B View Fig ). Galea similar to that of adults but narrower and with ca 8 circular granules, microtrichia present but diminutive and discrete ( Fig. 10B View Fig ). Galealobulus present, similar but slightly smaller than in adults ( Fig. 10A–B View Fig ). Left mandible with sharp dorsal cutting edge with two dentations ventrally, ventralmost one continuous with less sharp and slightly blunt ventral edge, mesal surface without protuberances but with sclerotization in centre away from edges ( Fig. 10C View Fig ). Right mandible with sharp dorsal cutting edge presenting two broad dentations, ventralmost one larger and continuous, with less sharp and slightly blunt ventral cutting edge, basally presenting level blunter surface corresponding to projections of molariform right ventral edge in adults, mesal surface without protuberance but with broad central sclerotization almost reaching edges ( Fig. 10C View Fig ). Antennae longer than body, filiform, with seven flagellomeres, each one very long and of similar length. Legs very elongate, proportionally much longer than those of adults. Profemoral
basal curvature very discrete ( Fig. 9 View Fig ). Carinae of legs similar to those of adults, distinct and bearing row of somewhat straight and long setae, also on basitarsi. Stouter setae absent. All basitarsi extremely elongate, more than half length of corresponding tibia ( Fig. 9 View Fig ). Tarsomeres with indistinct to discretely budded euplantulae on posterior margin of tarsomeres III and IV, with stiff setae instead ( Fig. 11A View Fig ). Unguitractor plate broad. Median segment distinct but not separated from thorax and about as long as metanotum ( Fig. 9 View Fig ). Upon hatching, abdominal segments somewhat ovoid, none significantly longer than wide but most becoming longer with development of first instar. Anal segment emarginate, with broader posterior margin than in adults. Epiproct large, round, bearing setae and visible in dorsal view. Cerci longer than tergum X but not longer than IX and X combined ( Fig. 9 View Fig ), on basal third presenting only long sensory hairs at all sides, and on remaining length presenting only black, thicker and fairly straight setae. Paraprocts fused, broad and elongate, with posterior margin emarginate and blunter than in adults, bearing setae ( Fig. 11B View Fig ). Sternum VIII budded, short, visible as central, forwadly curved lobe near anterior margin of segment VIII ( Fig. 11A View Fig ). Both gonapophyses absent but round light outline of budded gonapophyses noticeable, becoming more evident as first instar nymph develops ( Fig. 11A View Fig ).
SECOND- INSTAR NYMPH. Similar to first instar, except for: labrum distinctly asymmetric. Mesal surface of mandibles more sclerotized, reaching edges. Around 18 antennomeres, barely distinct, antennae reaching abdominal segment VII. Profemoral basal curvature slightly more prominent. Carinae of meso-
and metatibiae with one or two larger apicalmost setae. Basitarsi significantly longer than following tarsomeres but shorter than half length of corresponding tibia. Subgenital plate small and lobed. Gonapophyses budded, distinctly visible as bean-shaped protuberances with paler colour.
THIRD- INSTAR NYMPH. Similar to second instar, except for: head and body slightly more elongate and more similar to those of adults. Meso- and metatibiae with two apicalmost larger setae on each carina. Thorax relatively wider than in adults (similar to later instar nymphs, see Fig. 13A View Fig in comparison with adults in Fig. 13B–E View Fig ). Meso- and metabasitarsi bear stouter spiniform setae on ventral carinae. Euplantulae developed but small. Subgenital plate and gonapophyses budded, subgenital plate not covering gonapophyses VIII (smaller than those of Fig. 11C View Fig ). Cerci shorter than terga IX and X combined.
FOURTH- INSTAR NYMPH. Similar to third instar, except for: head very similar to that of adults. Colouration pattern more similar to that of adults, as dorsal and lateral areas of body acquiring same colour tone. Basitarsi significantly longer than following tarsomeres. Gonapophyses very short, only VIII covered by subgenital plate. Subgenital plate short, only reaching slightly anterior to or just to base of gonapophyses IX, gonoplac larger and exceeding gonapophyses IX ( Fig. 11C View Fig ).
FIFTH- INSTAR NYMPH. Subadult. Generally more similar to adults. Similar to fourth instar, except for: thorax slightly wider than in adults ( Fig. 13G–H View Fig , compare with adults in Fig. 13B–E View Fig ). Gonapophyses and gonoplac slightly longer. Subgenital plate exceeding base of gonapophyses IX, from not totally covering them nor gonoplacs to covering all valves except apex of gonoplac ( Fig. 11D–E View Fig ).
Distribution ( Fig. 52 View Fig )
Arumatia dubia gen. et comb. nov. occurs in different biomes of South America: savannah formations ( Fig. 53C–D View Fig ) and seasonal deciduous forests ( Fig. 53A–B View Fig ) of the Cerrado domain ( Batalha 2011) in the Southeast and Central-West regions of Brazil and the Humid Chaco of Paraguay. All these biomes share significant characteristics and are part of the South American Dry Diagonal ( Vanzolini 1963; Prado & Gibbs 1993; Collevatti et al. 2020) with markedly dry seasons and deciduous vegetation. It also occurs in Cerrado seasonal forests in contact areas with the Atlantic Forest. In Brazil, A. dubia is known from Paraná, São Paulo, Minas Gerais, Mato Grosso do Sul and Goiás States, and in Paraguay from Paraguarí Department ( Fig. 52 View Fig ). One examined specimen is recorded from Londrina (Paraná), where the Atlantic Forest dominates, but there are savannic and deciduous seasonal forest (Cerrado) enclaves in the region ( Estevan et al. 2016), meaning that this record does not exclude the hypothesis that A. dubia is endemic to savannah, shrubland and deciduous seasonal forest ecoregions in the Brazilian Cerrado and Paraguayan Chaco. The currently known distribution is very likely to increase with further sampling effort. We disregard previous records of the species from Australia agreeing with Brock & Hasenpusch (2009) (see Remarks).
Sexuality of the species
All specimens of Arumatia dubia gen. et comb. nov. reared by us from different localities are parthenogenetic and no males are known so far. Additionally, we did not find any specimen that could represent a male of A. dubia in the visited collections matching the known or an expected distribution of the species. This is also true for iNaturalist photographic records. It is quite likely that Arumatia gen. nov. presents exclusively natural parthenogenetic populations or even entire species, which is a possible and previously recorded condition for phasmids such as is known for most or some species in the genera Acanthoxyla in New Zealand ( Uvarov 1944; Myers et al. 2013) or Bacillus Berthold, 1827 in Europe ( Berthold 1827; Scali et al. 2003). All sightings of A. dubia in nature consisted of females even when found in abundance.A factor that could be related to the evolution of parthenogenesis in this lineage is the short egg incubation period observed for A. dubia (2–3 months). The eggs of facultative parthenogenetic individuals or populations of sexual phasmid species take considerably longer to hatch ( Bedford 1978; VMG, PWE & PABAN pers. obs.). Another possible factor is the absence of a praeopercular organ, contrasting with its presence in A. aramatia gen. et sp. nov. and A. motenata gen. et sp. nov., both species with known males. This organ serves as an attachment for the male vomer so a historical absence of males could have led to the loss of such a structure. Arumatia anyami gen. et sp. nov., A. diamante gen. et sp. nov. and A. crassicercata gen. et sp. nov. are also known by females only, the former also lacking a praeopercular organ. This might suggest parthenogenesis to be present in other species of Arumatia gen. nov. as well.
Biology
In Chapadão do Sul (MT), Assis (SP) and Araguari (MG), specimens were found in natural areas feeding on plants of the subfamily Mimosoideae (Fabaceae) . In captivity, they also fed on the Fabaceae plants Anadenanthera Speg. spp. and Parapiptadenia rigida (Benth.) Brenan (angico trees), Chamaecrista flexuosa (L.) Greene (sensitive pea), the Myrtaceae plants Psidium guajava L. (guava) and Eugenia uniflora L. (Brazilian cherry), and the Sapindaceae plant Serjania Mill. sp. , all native to Brazil and occurring in the Cerrado and adjacent biomes.
Several reared specimens were observed to drastically change colour between green, orange or brown. This change was observed both gradually during moults and in adult specimens that changed colour in
a few days. The female V0561 from Ibaté (SP) was orangish when pre-subadult ( Fig. 13A View Fig ) and turned green when adult ( Figs 2D–E View Fig , 13B View Fig ). The female V0547 from Araguari (MG) was light green when found already as an adult ( Fig. 2C View Fig ) and later turned greenish brown ( Fig. 1A View Fig , adult individual) and to orangish brown before dying ( Figs 6A View Fig , 7A–C View Fig , 13I View Fig ).
The female V0561 was kept in an open area from the fifth instar until adult from 16 April 2021 to 17 August 2021 with access to ca 10 plant vases, including the host plant species C. flexuosa and P. guajava . The insect was observed every day and night. It hid in typical camouflage posture during the day ( Fig. 2D–E View Fig ) and foraged at night, never going further than one meter from the host plants. At night, even if not walking away from the spot where it stayed by day, it kept its body away from the resting surface and held the antennae up and apart (similar to specimens in Fig. 2A, C View Fig ). During daytime, it usually hid in different spots, among leaves and branches either atop, on the side or under branches, near the surface of cactuses ( Fig. 2E View Fig ) or large leaves, on the side of vases and less frequently on a thin branch with all legs close to the body ( Fig. 2D View Fig ). The guava plant was strongly preferred, as the phasmid totally ignored the sensitive pea most of the nights. The insect fed on parts of random leaves of the guava plant, and a few times on some leaves and flowers of the sensitive pea.
When handled, both nymphs and adults of Arumatia dubia gen. et comb. nov. display agitated behaviour, quickly walking frenetically away without stopping, which also happens frequently when the rearing cage is opened.
Eggs of this species have a somewhat short incubation period with most eggs hatching within 50–70 days and having a high hatching rate (> 70%). Eggs require infrequent watering to hatch with higher rates resulting from spraying 2–3× to 0.5–1× a week, which configures a lesser humidity requirement in comparison with many other phasmids from Southeast Brazil (pers. obs.). Some eggs were tracked for development: seven of a few dozen eggs laid in 18–30 January 2021 by female V0547 from Araguari hatched 6–10 April 2021; only four nymphs survived and moulted from first to second instar from 2 to 5 May 2021; from second to third in the period 23–25 May 2021; from third to fourth on 18 June 2021; from fourth to fifth in the period 10–14 July 2021; and from fifth to sixth in the period 9–12 August 2021, when they became adults. Three nymphs that hatched around 10 December 2021 from the stock from Ibaté were also tracked, presenting a quicker development probably due to the warmer season in which they developed: they moulted to second instar from 28 December to 4 October; to third instar in the period 9–16 October; to fourth from 16 to 30 October; to fifth from 30 October to 12 November; and to sixth from 11 to 25 November.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Arumatia dubia ( Caudell, 1904 )
| Ghirotto, Victor Morais, Crispino, Edgar Blois, Engelking, Phillip Watzke, Neves, Pedro Alvaro Barbosa Aguiar, Góis, Júlia de & Chiquetto-Machado, Pedro Ivo 2022 |
Candovia evoneobertii
| Araujo & Garraffoni 2012: 235 |
| Brock & Hasenpusch 2009: 151 |
| Brock & Hasenpusch 2007: 7, 70 |
Heteronemia dubia
| Zompro et al. 2006: 131 |
| Otte D. & Brock P. 2005: 158 |
| Otte D. & Brock P. 2005: 158 |
| Otte D. & Brock P. 2005: 132 |
| Zompro & Domenico 2005: 257 |
Echetlus evoneobertii
| Zompro O. & Adis J. 2001: 294 |
Bacunculus fragilis
| Brunner von Wattenwyl K. 1907: 336 |
Bacunculus dubius
| Caudell A. N. 1904: 186 |