Soederberghia groenlandica, : Lehman, 1959
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2007.00320.x |
|
persistent identifier |
https://treatment.plazi.org/id/AB75B326-FFE2-0178-9373-A4F55BB5F8FE |
|
treatment provided by |
Felipe |
|
scientific name |
Soederberghia groenlandica |
| status |
|
Neurocranial remains have not been reported previously for Soederberghia . Lehman (1959) indicated that braincases were not preserved in his specimens, and suggested that they were probably unossified. Re-examination of the material studied by Lehman confirms that there are no remains of endocrania preserved in situ. However, an irregularly shaped endochondral ossification located just posterior to the skull roof of a specimen available to Lehman (MGUH VP 3120) might be neurocranial in origin. It is pierced by a foramen, perhaps for the exit of a cranial nerve. A new specimen of Soederberghia (MGUH VP 28400) preserves the braincase in articulation with the dermal skull roof, and this specimen forms the basis of most of the following description.
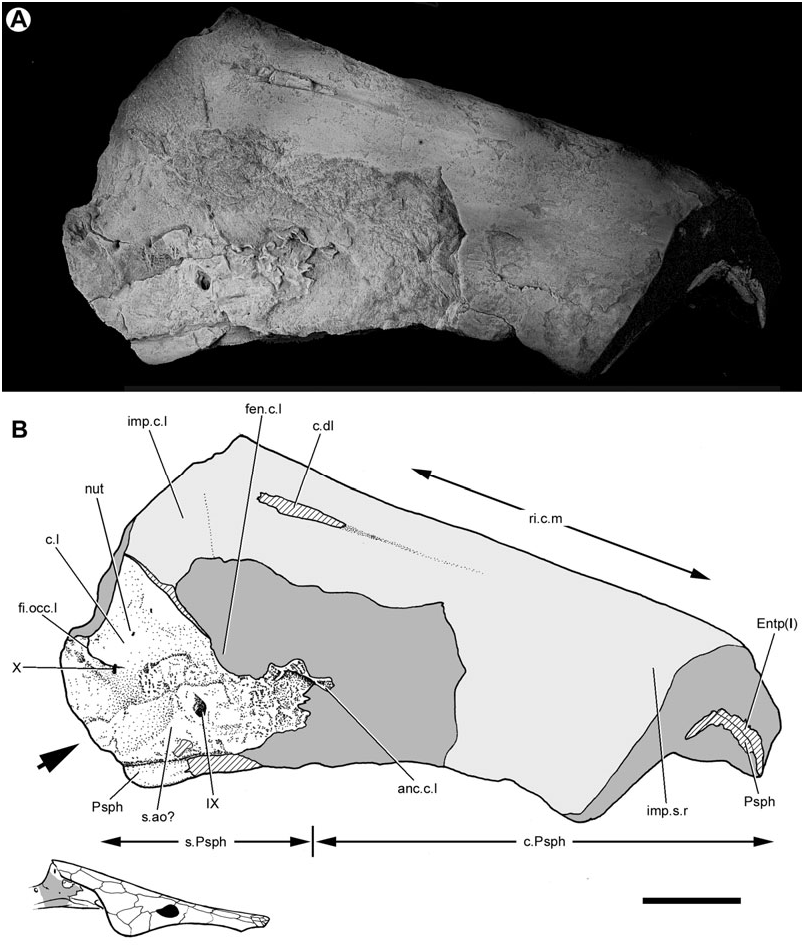
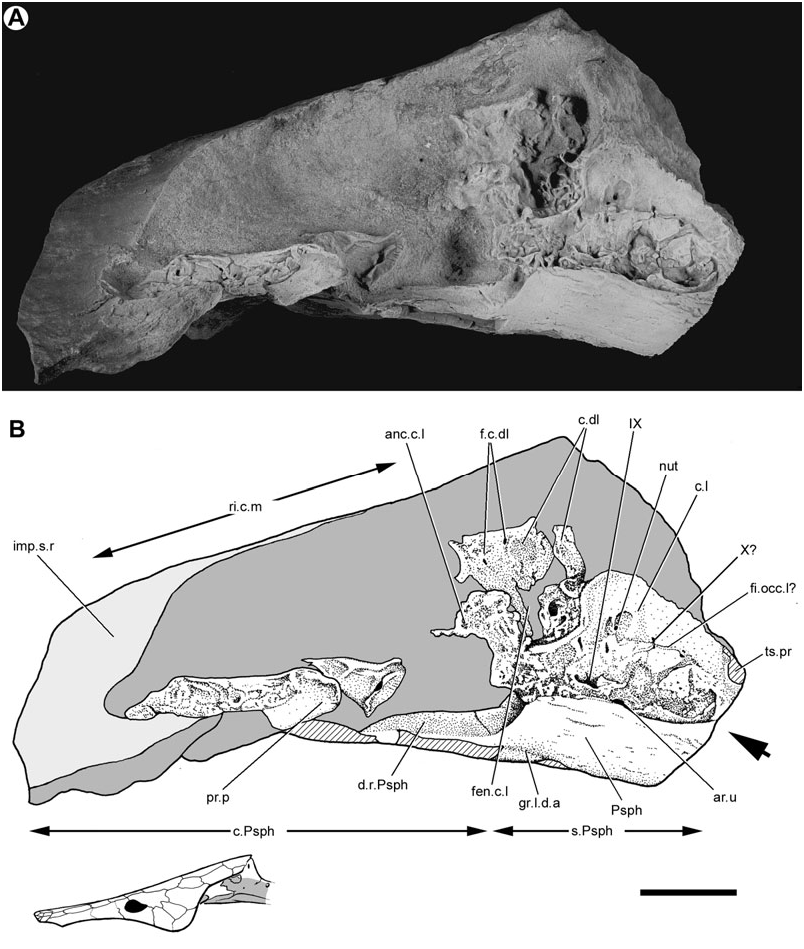
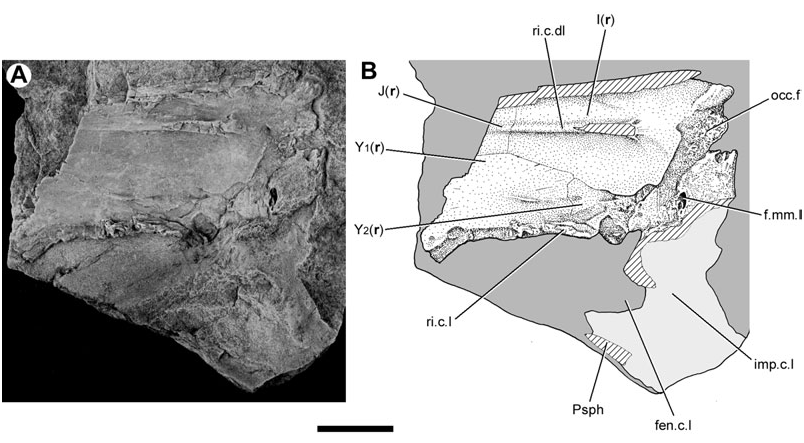
Both lateral surfaces of this neurocranium have been prepared. The right face is marred by several longitudinal cracks ( Fig. 1 View Figure 1 ), whereas the left face is distorted and partly obscured by the parasphenoid ( Fig. 2 View Figure 2 ). Nevertheless, it is possible to synthesize observations from both sides of the braincase to reconstruct the intact structure ( Fig. 3 View Figure 3 ).
Occipital region
The division between the occipital and otic regions of the neurocranium is marked by the lateral occipital fissure (fi.occ.l, Figs 1 View Figure 1 , 3 View Figure 3 ), which is best preserved on the right side of the specimen. A disrupted suture on the opposite surface may represent its antimere (fi.occ.l?, Fig. 2 View Figure 2 ). The fissure extends anteroventrally just beyond the vagus foramen, terminating well before the dorsal margin of the parasphenoid.
On the right side of the neurocranium, the foramen jugulare is present as an expansion of the lateral cranial fissure near its point of ventralmost extent. The vagus nerve (X, Figs 1 View Figure 1 , 3 View Figure 3 ) and the posterior cerebral vein would have exited through this foramen ( Bertmar, 1965; Miles, 1977), which on the left side of this specimen does not show a complete separation between venous and nervous portions. The foramen tentatively interpreted as this feature on the opposite side of the neurocranium (X?, Fig. 2 View Figure 2 ) also shows incomplete subdivision. There is no groove for the ramus supratemporalis N. X posterior to the foramen.
There are no clear foramina for the occipital artery. Grooves that might have accommodated this vessel cannot be identified, and there are no notches in the margin of the parasphenoid that reveal its trajectory. It is probable that the condition in Soederberghia was similar to that of ‘ G. ’ whitei and H. gogoensis , in which the course of this vessel was largely extramural ( Miles, 1977).
A boss, located at the posterodorsal corner of the occipital region, is visible on the left side of the specimen (ts.pr, Figs 2 View Figure 2 , 3 View Figure 3 ). The endochondrally ossified core of this feature was exposed prior to preparation, suggesting that its most distal portions are missing. This feature may represent the transverse process of a neural arch which has fused to the neurocranium, as is the case in some specimens of ‘ G. ’ whitei (e.g. BMNH P52583; Miles, 1977). Alternatively, this process could be an outgrowth of the occipital region itself, as in ‘ Chirodipterus ’ australis and Gogodipterus ( Miles, 1977; Long, 1992a).
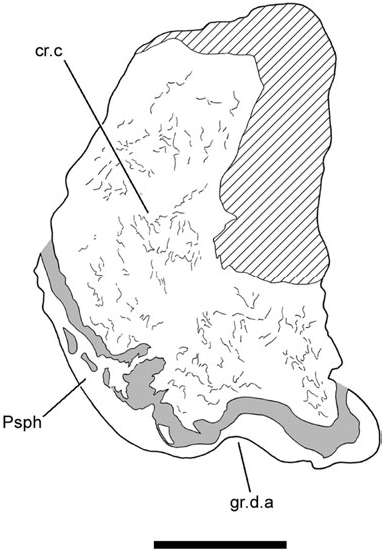
The specimen is broken just before the presumed posterior termination of the occipital region, revealing a cross-section of the neurocranium ( Fig. 4 View Figure 4 ). Dense endochondral bone is exposed across the entirety of the break (cr.c, Fig. 4 View Figure 4 ). This area of bony infilling occludes the notochordal canal, and is consistent both morphologically and topologically with the cranial centrum described for ‘ G. ’ whitei ( Miles, 1977: fig. 10). Apart from these two genera, such a feature is not known to be present in any other Devonian dipnoan.
This cross-section also reveals a median groove on the ventral surface of the neurocranium ( Fig. 4 View Figure 4 ). A similar feature is found in ‘ G. ’ whitei ( Miles, 1977: fig. 13) and G. minutidens ( Schultze, 1969: fig. 15). It has been suggested that the feature in Griphognahtus received a ligament from the dorsal aorta ( Miles, 1977), but Gardiner (1984: 206) has expressed reservations about this interpretation (also see Discussion accompanying character 8 below). However, the depression in Soederberghia may only reflect the median thickening on the dorsal surface of the parasphenoid, which is closely applied to this region of the braincase.
Otic region
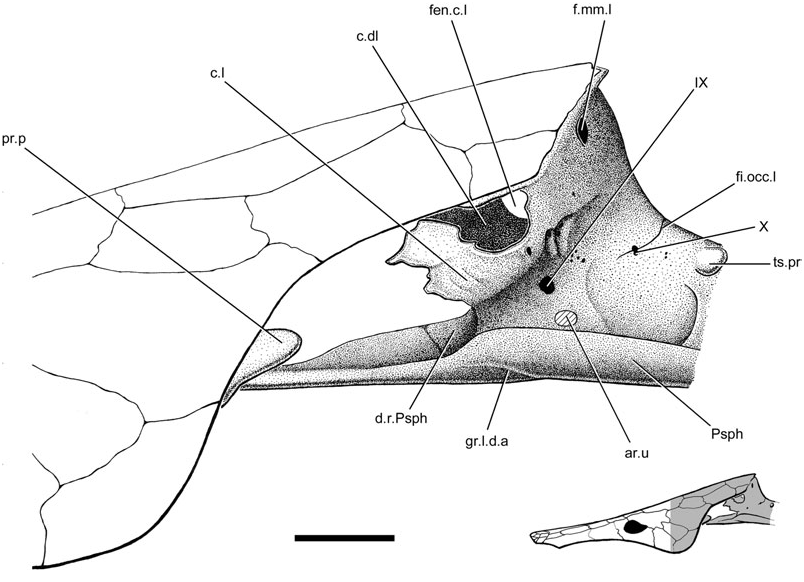
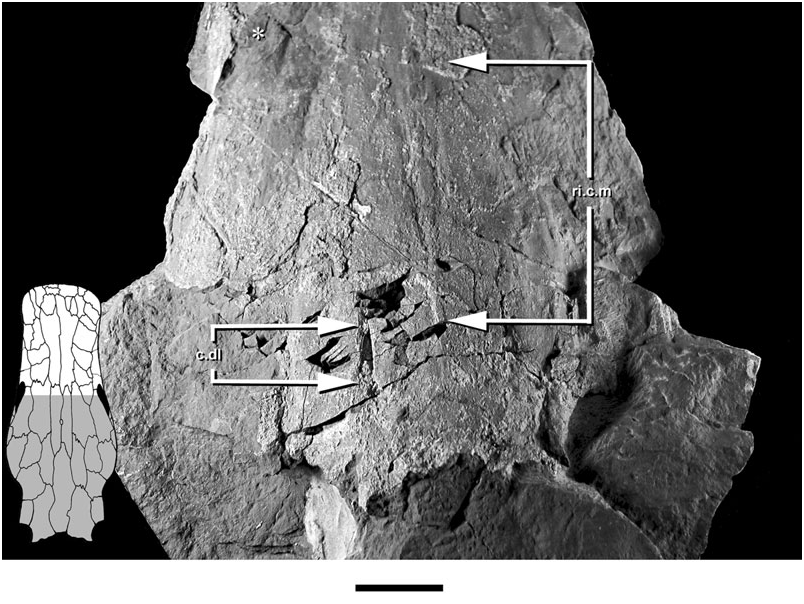
The neurocranium is suspended from the visceral surface of the dermal skull roof by a series of endoskeletal cristae. The paired lateral (c.l., Figs 1–3 View Figure 1 View Figure 2 View Figure 3 ; imp.c.l, Figs 1 View Figure 1 , 5 View Figure 5 ) and dorsolateral cristae (c.dl, Figs 1 View Figure 1 , 2 View Figure 2 , 5 View Figure 5 , 6 View Figure 6 ) are confined to the otic region. The unpaired median crista is unossified in Soederberghia , although features of the visceral skull roof (ri.c.m, Figs 1 View Figure 1 , 2 View Figure 2 , 6 View Figure 6 ) reveal its position, indicating that its intersection with the dermatocranium was limited to the area above the orbitotemporal region.
The dorsolateral and lateral cristae are both ossified, although the latter are incompletely so. Only the left dorsolateral crista has been prepared, and is complete despite fracturing. The attachment ridges preserved on the visceral surface of the skull roof confirm that the posterior margin visible in the specimen is the genuine termination of the crista. Like that of ‘ Griphognathus ’ whitei, the dorsolateral crista of Soederberghia is low, indicating that the neurocranium was suspended a short distance below the skull roof. This is different from the arrangement in ‘chirodipterids’ and ‘dipnorhynchids’, in which high dorsolateral cristae result in deep chambers between the skull roof and the neurocranium, presumably to accommodate the powerful jaw adductor muscles associated with durophagy ( Campbell & Barwick, 1982, 1987). Neither ‘ G. ’ whitei nor Soederberghia possess a dentition consistent with a crushing habit, and it is not surprising that their adductor vaults are less expansive than those of durophagous lungfishes ( Schultze, 1992a: fig. 13).
The dorsolateral crista shows no sign of a large dorsal embayment, like that of the ‘chirodipterids’ ‘ Chirodipterus ’ australis ( Miles, 1977) , Gogodipterus ( Long, 1992a) and Pillararhynchus ( Barwick & Campbell, 1996) . Two small foramina (f.c.dl, Fig. 2 View Figure 2 ), one comparable with a similar perforation described in some specimens of ‘ G. ’ whitei ( Miles, 1977: fig. 14), are found near the dorsal margin of the crista.
The leading edge of the dorsolateral crista is inclined anteriorly, an exceptional orientation among early lungfishes. This arrangement is only found elsewhere in ‘ G. ’ whitei ( Miles, 1977: fig. 14) and Orlovichthys ( Krupina et al., 2001: fig. 3B), although the dorsolateral cristae of the latter are very different from those of the two denticulated forms. More typically, the anterior margin of the dorsolateral cristae will be swept posteriorly at a conspicuous angle ( Long, 1992a: fig. 4). The unusual orientation of the cristae in these three genera is probably a function of their reduced height, as the leading edge of the crista typically curves anteriorly in the region just ventral to its intersection with the dermal skull roof.
The dorsolateral crista would have been confluent anteroventrally with the posterior portion of the orbitotemporal region, which was cartilaginous in Soederberghia . It is not possible to determine if a pronounced ridge over the anterior semicircular canal was present anterior to and continuous with the dorsolateral crista, as in ‘ G. ’ whitei ( Miles, 1977: fig. 14a), Holodipterus gogoensis ( Miles, 1977: fig. 22), C. wildungensis ( Säve-Söderbergh, 1952: pl. 4) and Orlovichthys ( Krupina et al., 2001: fig. 3A). A series of delicate perichondral ossifications located ventral to the dorsolateral crista bear perforations that superficially resemble foramina for the trigeminal (V) and facial (VII) nerves. However, this interpretation seems unlikely, as these nerves typically exit the neurocranium closer to the anterior margin of the dorsolateral cristae in other Devonian lungfishes ( Miles, 1977: fig. 35). It is more probable that this area represents the incompletely ossified dorsolateral wall of the otic region.
The lateral crista of Soederberghia is continuous with the lateral wall of the otic region and sweeps dorsolaterally to intersect the dermal skull roof above the opercular embayment. It is most robustly ossified posteriorly, where it extends anterodorsally from the neurocranium, joining the margin of bones Y 2 (tabular) and I (postparietal) at the posterolateral corner of the dermal skull roof. A perforation (f.mm.l, Figs 3 View Figure 3 , 5 View Figure 5 ), consistent in location with the feature identified by Miles (1977) as the lateral foramen of the masseter fossa, pierces the lateral crista near its intersection with the posterior margin of the skull roof.
Apart from their strongly developed posterior regions, the lateral cristae of Soederberghia are weakly ossified. A thin flange (ri.c.l, Fig. 5 View Figure 5 ) projects ventrally from the lateral margin of bones Y 2 and Y 1 of the dermal skull roof, and is discontinuous with the better-defined posterior contact of the lateral crista with the skull roof. This ridge is positioned directly above a projection (anc.c.l, Figs 1 View Figure 1 , 2 View Figure 2 ) of perichondral bone that extends anterodorsally from the otic region. This extension of the lateral crista fails to reach the skull roof dorsally, terminating with an irregularly shaped border, and is separated from the more robustly ossified posterior region of the crista by a large embayment (fen.c.l, Figs 1–3 View Figure 1 View Figure 2 View Figure 3 , 5 View Figure 5 ).
Weak development of the dorsolateral cristae is not unique to Soederberghia among Devonian dipnoans. Both H. gogoensis and ‘ G. ’ whitei have poorly mineralized cristae, but only ‘ G. ’ whitei has well-developed fenestrae ( Miles, 1977; Pridmore et al., 1994). The extent of ossification in Soederberghia is considerably less than that described for either of these species. Indeed, mineralization of the lateral cristae in Soederberghia is so sparse that the expansive gaps in its lateral cristae are not completely enclosed by endoskeletal bone. It is probable that these embayments in the lateral cristae of Soederberghia are more extensive equivalents of the fenestrae of ‘ G. ’ whitei.
The lateral wall of the otic region is distorted due to compression ( Figs 1 View Figure 1 , 2 View Figure 2 ). The left side of the specimen bears a subcircular unossified area (ar.u, Figs 2 View Figure 2 , 3 View Figure 3 ), located just anterior to the border with the occipital region and immediately dorsal to the margin of the parasphenoid. Positionally, this feature corresponds to the articular facet for the first branchial arch, an identification made more inviting by the fact that its placement posterior to the level of the foramen for N.IX might plausibly be viewed as a derived arrangement shared with ‘ G.’ whitei ( Miles, 1977: figs 14A, 8). Although this initial resemblance is impressive, there are problems with this interpretation. Most notably, there is no clear evidence of an equivalent structure on the opposite side of the specimen. While there is a slight swelling with a broken surface in the appropriate position on the right side that might correspond to the adotic swelling of other lungfishes (s.ao?, Fig. 1 View Figure 1 ), there is nothing on its surface that makes a convincing candidate for an epibranchial facet. The condition of the facet for the first epibranchial in Soederberghia is best considered uncertain pending the discovery of further neurocranial material for this genus. There is a possibility that the facet was located more anteriorly, on unossified portions of the neurocranium. Some support is lent to this interpretation by the conditions in G. minutidens , which is discussed later in this paper (see Reinterpretation of the braincase of Griphognathus minutidens ).
Numerous foramina are present in the otic region, although most of them cannot be identified with certainty. Multiple foramina are clustered near the intersection of the lateral crista with the lateral face of the neurocranium. These are small and have no regular equivalents in other lungfishes, nor are they consistent in location or form between the two sides of the braincase. It is probable that they are nutritive foramina (nut, Figs 1 View Figure 1 , 2 View Figure 2 ). A large foramen (IX, Figs 1–3 View Figure 1 View Figure 2 View Figure 3 ) is that for glossopharyngeal nerve (IX).
The trajectory of the jugular vein in Soederberghia is difficult to trace, as there is no deep groove or fold in the lateral wall of the otic region marking its course. This differs considerably from the arrangement in ‘ G. ’ whitei, in which the jugular vein is embraced by the walls of the otic region, forming a well-defined trough.
Orbitotemporal and ethmoid regions
No neurocranial ossification is present in the orbitotemporal region of Soederberghia ( Figs 1–3 View Figure 1 View Figure 2 View Figure 3 ), suggesting that it was cartilaginous in life. The lateral wall of the orbitotemporal region typically consists of a thin perichondral coat in lungfishes with mineralized braincases, and has little if any endochondral ossification dorsally ( Säve-Söderbergh, 1952; Miles, 1977). This area is weakly mineralized in ‘ Griphognathus ’ whitei ( Miles, 1977). The lack of ossification in the orbitotemporal region of Soederberghia highlights an apparent trend in the reduction or complete loss of mineralization in those areas that are poorly ossified in ‘ G. ’ whitei, a phenomenon that is also clear in the lateral cristae.
The head of MGUH VP 28400, including the neurocranium and palate, is broken approximately at the level of the orbits. Another specimen, MGUH VP 28398 ( Fig. 7 View Figure 7 ), overlaps with MGUH VP 28400 in the orbital region, and provides information on the more anterior portions of the skull. As in MGUH VP 28400, the orbitotemporal region of the smaller MGUH VP 28398 is unmineralized. However, this specimen does preserve some endoskeletal ossifications in the ethmoid region.
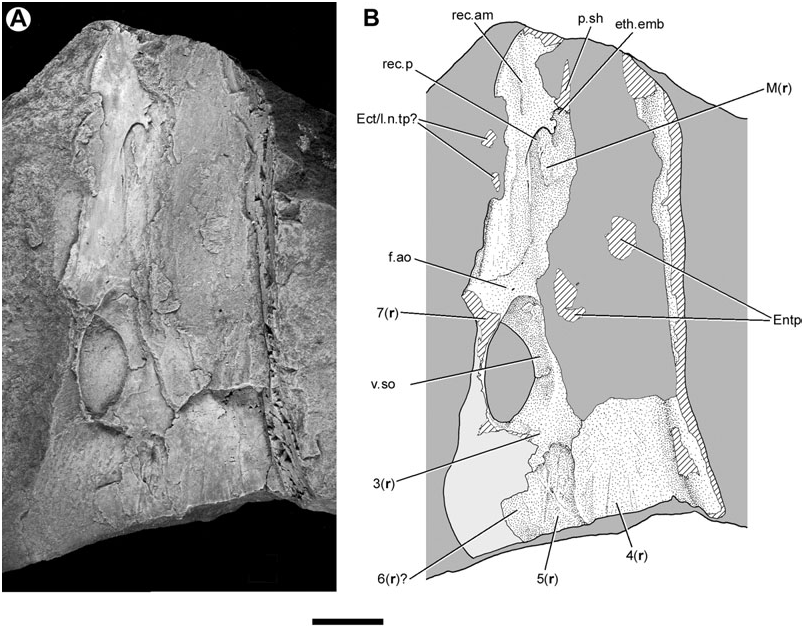
In the snout, a layer of perichondral bone merges laterally with the dermal bones bearing the infraorbital canal. This ossification forms a shelf-like projection that extends medially as a thin sheet. Its free posterior margin is marked by a rounded embayment that defines an incomplete ellipse with its long axis orientated anteroposteriorly. This border is interpreted as the anterior margin of the posterior recess of the nasal cavity (rec.p, Fig. 7 View Figure 7 ), which is of similar shape and position in ‘ G. ’ whitei ( Miles, 1977: figs 57, 58, 63). As in other lungfishes, the nasal capsules are open ventrally ( Jarvik, 1942; Miles, 1977).
The nasal cavity in Soederberghia is incompletely defined posteriorly and medially, indicating that the ventral surface of the ethmoid region lacked a complete perichondral coat. There is no sign of endochondral ossification between the thin perichondral sheet and the skull roof, nor does it appear that there is endoskeletal bone that roofs this cavity and separates it from the dermal bones of the skull roof.
Several features mark the surface of the ethmoid ossification anterior to the cavity for the nasal capsule. Anteromedial to this embayment is a smaller and more irregular excavation (eth.emb, Fig. 7 View Figure 7 ). This feature does not have a clear equivalent in other lungfishes ( Thomson & Campbell, 1971; Miles, 1977; Novitskaya & Krupina, 1985), and might simply represent the uneven termination of the ethmoid ossification. A well-ossified but damaged ridge, running subparallel to the long axis of the skull, is located just anterior to this embayment (p.sh, Fig. 7 View Figure 7 ). It is continuous with the ethmoidal ossification, and is similar to the shelf for the first dermopalatine found in ‘ G. ’ whitei ( Miles, 1977). A broad, shallow groove (rec.am, Fig. 7 View Figure 7 ) extends from the level of the anterior margin of the cavity for the nasal capsule and terminates on the anterolateral margin of the snout. This depression is well defined posteriorly, while its more anterior portions are faint. This feature is interpreted as the anteromesial recess of the nasal cavity. It is topologically consistent with this structure in ‘ G. ’ whitei ( Miles, 1977: fig. 57), although it occupies a more lateral position in Soederberghia . If this identification is correct, this depression would have been positioned dorsal to the anterior nasal opening. Although the location of this broad groove might suggest that it accommodated a dermal bone bearing a tooth-ridge of the sort seen in ‘ G. ’ whitei ( Miles, 1977: fig. 57), this alternative interpretation is doubtful, as such plates are borne on a thickening of endoskeletal bone – not depressions – in that taxon ( Miles, 1977: fig. 91).
There is no clearly defined depression that can be identified as the anterolateral recess. This feature marks the position of the excurrent nostril, and is well developed in Ganorhynchus splendens (Gross, 1965) and Dipnorhynchus sussmilchi ( Thomson & Campbell, 1971) . Such a depression has been described for ‘ G. ’ whitei, but it is not always separated from the anteromesial recess in this species ( Miles, 1977: 128).
Palatoquadrate
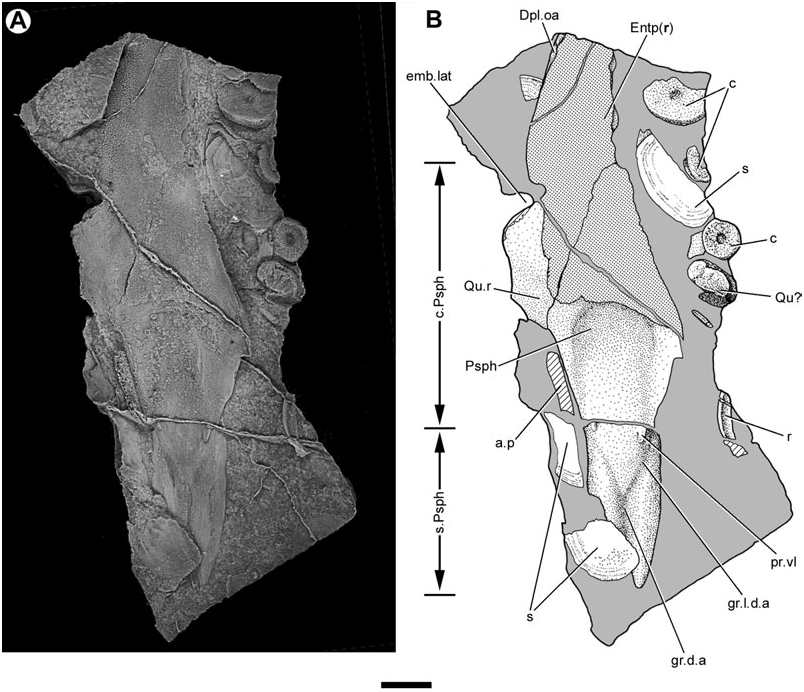
The palatoquadrate of Soederberghia is largely unossified. A bone found in association with an entopterygoid and parasphenoid might represent the mineralized head of the quadrate (Qu?, Fig. 8 View Figure 8 ). This endochondrally ossified bone bears a long, strapshaped facet that lacks a perichondral coat and resembles the articular face of the quadrate of Griphognathus ( Miles, 1977: figs 30, 33). The perichondral surface of this bone is furrowed.
Entopterygoids
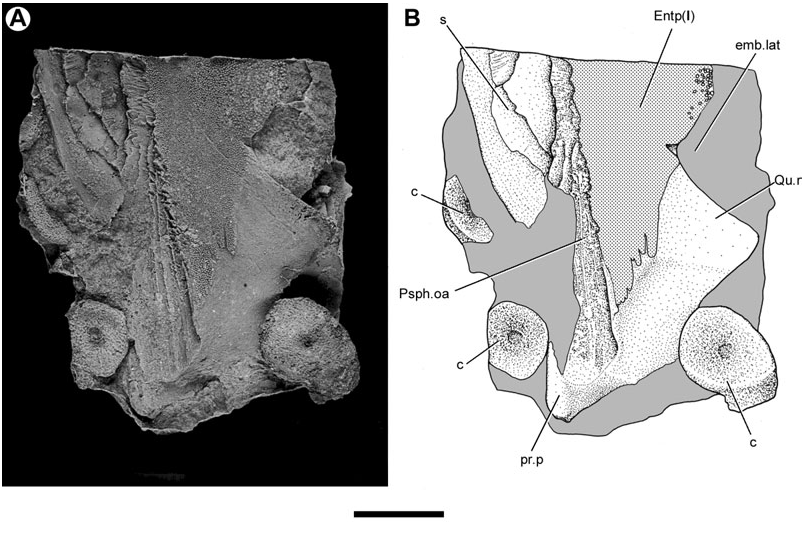
Lehman (1959) provided a brief description of the entopterygoids (‘ptérygoïdes’) of Soederberghia based on two poorly preserved specimens (MGUH VP 3042, MGUH VP 3067). New acid-etched material (MGUH VP 28397; Entp, Figs 8–10 View Figure 8 View Figure 9 View Figure 10 , 12 View Figure 12 ) complements that available to Lehman, permitting these bones to be described more completely.
The entopterygoids of Soederberghia are elongated, corresponding to the lengthened snout of this genus. The lateral margin of the entopterygoid has a conspicuous embayment (emb.lat, Figs 8 View Figure 8 , 9 View Figure 9 ), similar to that noted for ‘ Griphognathus ’ whitei ( Miles, 1977: fig. 79) and Fleurantia ( Fig. 11A View Figure 11 ). This excavation would have accommodated the falcate preglenoid process of the lower jaw. The quadrate ramus (Qu.r; Figs 8 View Figure 8 , 9 View Figure 9 ) forms the posterior margin of this embayment and marks the widest point of the palate. The lateral margin of the quadrate ramus is gently concave, and meets the posterolateral margin at a distinct angle. The entopterygoid terminates posteromesially with a blunt prong (pr.p, Fig. 9 View Figure 9 ). This posteriorly orientated projection closely resembles a similar feature in Fleurantia ( Fig. 11A View Figure 11 ). This differs from ‘ G. ’ whitei, where the posteriormost portion of the entopterygoids is located laterally and there is no distinct posterior prong ( Miles, 1977: fig. 79; the posterior regions of the entopterygoid are incompletely known in ‘ G. ’ sculpta and G. minutidens ). The entopterygoid joins its fellow along a median suture, forming a ventrally concave vault as in many other denticulated lungfishes, including ‘ G. ’ whitei ( Miles, 1977: fig. 79; Campbell & Barwick, 1987: fig. 5) and ‘holodontids’ ( Pridmore et al., 1994).
Almost the entirety of the buccal surface of the entopterygoids is covered with denticles. Only two areas are non-denticulated: the quadrate ramus, and an elongate, depressed band of smooth bone located on the lateral margin of the entopterygoid anteriorly (Dpl.oa, Fig. 8 View Figure 8 ). This latter feature matches the anterolateral embayment in an entopterygoid figured by Lehman (1959: fig. 15), and corresponds to the overlap area of the first dermopalatine in ‘ G. ’ whitei ( Miles, 1977: fig. 79). A similar feature is found in G. minutidens ( Schultze, 1969: fig. 14). The dermopalatines themselves have not been recovered for Soederberghia .
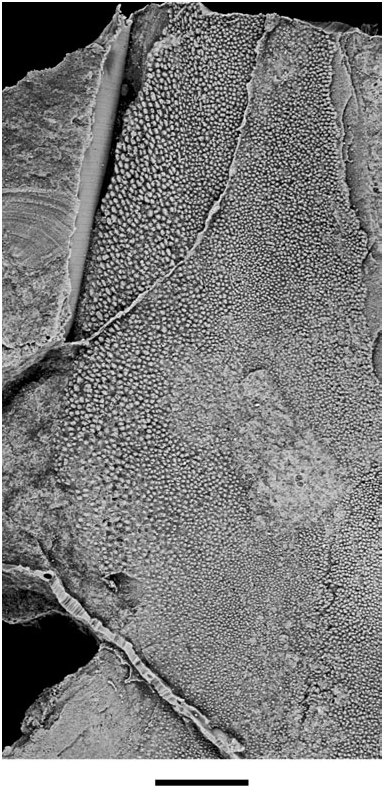
Across the remainder of the entopterygoid, the denticles form an extensive field that is continuous with that of the parasphenoid. Most denticles are small and appear unorganized. However, those located in a broad strip along the lateral margin of the entopterygoid immediately anterior to the excavation for the preglenoid process are more robust ( Figs 9 View Figure 9 , 10 View Figure 10 ). Some of these denticles are arranged in radial rows ( Fig. 10 View Figure 10 ). Individual denticles in these rows have their long axes aligned with the axis of radiation, but show no obvious increase in size with decreased distance from the entopterygoid margin. This ordered arrangement of denticles in Soederberghia is reminiscent of the pattern seen in Fleurantia . However, Soederberghia lacks greatly enlarged tooth rows and a high degree of denticle organization across the majority of the palate, both of which characterize Fleurantia ( Cloutier, 1996a: fig. 14).
The restriction of organized denticles to the margins of the entopterygoids provides insights into dental patterning in Soederberghia . The radiating arrangement of the marginal denticles suggests that they were added at specific loci along the edge of the entopterygoid, and that they are in fact true teeth ( Reif, 1982; Ahlberg et al., 2006). These teeth would come to occupy more interior positions with progressive marginal growth of the palate. When shed, their place on the palate would be filled with randomly arranged denticles, thus obliterating the radiating pattern with increased distance from the palatal margin. This inferred mode of growth matches that of Fleurantia , which has true teeth placed laterally in radial rows as well as more medially located fields of unorganized denticles ( Smith, 1988).
The overlap area for the parasphenoid (Psph.oa, Fig. 9 View Figure 9 ) is visible on the ventral surface of an isolated entopterygoid. This depression bears a series of anteroposteriorly orientated striations that correspond to a similarly textured surface on the dorsolateral surface of the parasphenoid corpus. It is difficult to determine the mesial extent of the overlap area, but those portions that are visible indicate that the entopterygoid failed to contact its partner of the opposite side over much of the length of the parasphenoid corpus. This is confirmed by specimens that preserve the impression of the dorsal surface of articulated palates (MGUH VP 3042; Lehman, 1959: pl. 6). The palate reconstructed by Lehman (1959: fig. 15) more accurately portrays the arrangement of bones as they would appear in dorsal view, not ventral view (‘vue buccale’) as his figure caption indicates.
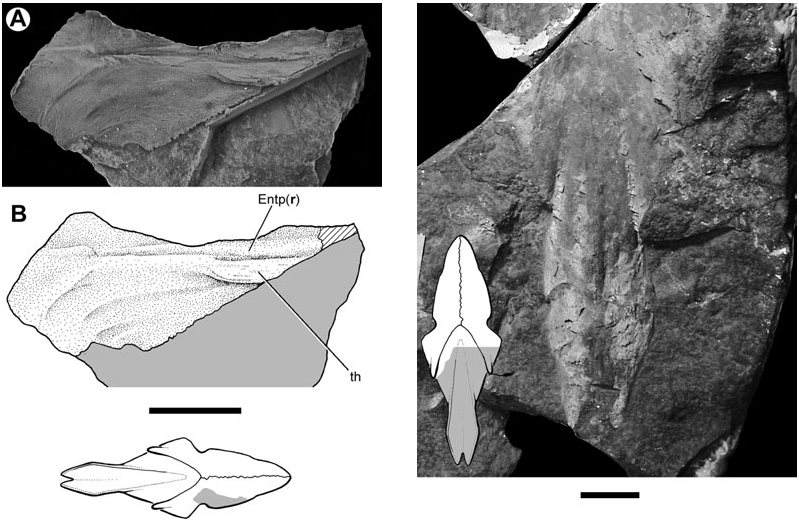
The dorsal surface of the entopterygoid is less completely known than its ventral face. A thickened ridge, which is continuous with the posterior prong, runs along the mesial margin of this bone. This thickening is presumably homologous to the ridge of the quadrate ramus as identified by Miles (1977) for Holodipterus gogoensis and ‘ Chirodipterus ’ australis . The dorsal surface of the entopterygoid bears a series of crescentic fossae in the region immediately anterior to the lateral embayment for the preglenoid process of the lower jaw ( Fig. 12 View Figure 12 ). A thickened area (th, Fig. 12 View Figure 12 ) might correspond to the well-developed ridges found in other lungfishes that mark the lateral termination of the endoskeletal subocular shelf ( Miles, 1977: figs 76, 77; Pridmore et al., 1994: fig. 30; Barwick & Campbell, 1996: fig. 4; Campbell & Barwick, 1998: fig. 4C).
Parasphenoid
The parasphenoid (Psph, Figs 1–4 View Figure 1 View Figure 2 View Figure 3 View Figure 4 , 8 View Figure 8 , 13) of Soederberghia consists of an elongated posterior stalk (s.Psph, Figs 1 View Figure 1 , 2 View Figure 2 , 8 View Figure 8 , 13) and a rhomboidal anterior corpus (c.Psph, Figs 1 View Figure 1 , 2 View Figure 2 , 8 View Figure 8 , 13). The anterior angle of the corpus is more acute than that of ‘ Griphognathus ’ whitei ( Miles, 1977: fig. 75) or G. minutidens ( Schultze, 1969: fig. 15). The semi-articulated palate of MGUH VP 28397 indicates that the parasphenoid extends beyond the level of the lateral embayment of the entopterygoid in ventral view, contrary to Lehman’s (1959: fig. 15) reconstruction, which shows the palate as it would appear in dorsal view (see above). This is different from the condition in the species of Griphognathus , in which the parasphenoid terminates before surpassing the lateral excavation of the entopterygoids ( Schultze, 1969: fig. 15; Miles, 1977: fig. 6). However, the fleurantiids Jarvikia ( Lehman, 1959: fig. 24; Clement & Boisvert, 2006: fig. 4) and Fleurantia ( Fig. 11A View Figure 11 ) have parasphenoids that are at least as anteriorly extensive as that of Soederberghia . There is no indication of a buccohypophysial canal in any of the Soederberghia parasphenoids examined.
Sections through the corpus and the stem indicate that both regions are constructed of thin, compact bone. Posteriorly, the parasphenoid cradles the otic and occipital regions of the neurocranium, resulting in a trough-like profile in transverse section ( Fig. 4 View Figure 4 ). The corpus is depressed on its ventral face, contributing to the strong arching of the palate. This pattern of welldeveloped dorsal and ventral concavities is concordant with the condition exhibited by ‘ G. ’ whitei ( Miles, 1977: fig. 75).
Denticles are present on the buccal surface of the parasphenoid, but are limited to the area of the corpus anterior to its lateral angles. The posterior margin of the denticle field is gently concave. In both G. minutidens ( Schultze, 1969: fig. 15) and ‘ G. ’ whitei ( Miles, 1977: fig. 6), the border of the denticulated region of the parasphenoid is convex posteriorly, although the precise shape of the posterior margin is variable in the Gogo species. The parasphenoid corpus posterior to the denticle field is covered with smooth bone and is depressed relative to the denticle field, particularly medially.
Two short ridges (pr.vl, Fig. 8 View Figure 8 ) are present on the ventral surface of the parasphenoid in the region where the stalk intersects the corpus, with one located at each lateral margin. They appear to have no equivalent in other lungfishes. The parasphenoid stalk is bifurcate posteriorly, forming two caudally directed processes. These extensions differ from the peculiarly shaped prongs of ‘ G. ’ whitei ( Miles, 1977: fig. 6), but are roughly equivalent in profile to those of several Late Devonian forms (e.g. Howidipterus and Barwickia ; Long, 1992b: figs 8, 17). It is unclear if these prongs would have contacted the first vertebral centrum, as in ‘ G. ’ whitei ( Miles, 1977). A median groove runs between these two processes and divides approximately at the midlength of the stalk, defining a ‘Y’- shaped depression with each of the lateral branches approximately aligned with the posterolateral margins of the parasphenoid corpus. The median notch and groove is interpreted as a depression for the dorsal aorta (gr.d.a, Figs 4 View Figure 4 , 8 View Figure 8 ). The anterior divergence of the median groove records the bifurcation of the dorsal aorta into the lateral dorsal aortae (gr.l.d.a, Figs 2 View Figure 2 , 3 View Figure 3 , 8 View Figure 8 ). The parasphenoid of Jarvikia has a similar arrangement of well-defined grooves ( Fig. 11B View Figure 11 ). Midline gutters are found on the parasphenoid stalks of many other Late Devonian lungfishes, including Andreyevichthys (Krupina, 1987: fig. 2), Howidipterus and Barwickia ( Long, 1992b: figs 8, 17). However, unlike those of Jarvikia and Soederberghia , the median grooves of these ‘phaneropleurids’ extend on to the parasphenoid corpus. While there are clear grooves for the lateral dorsal aortae that branch from the midline gutter near the intersection of the parasphenoid corpus and stalk in Andreyevichthys (pers. observ. of PIN 2921/1003), there is no obvious indication of any such branching in published figures of either Barwickia or Howidipterus ( Long, 1992b) .
The inferred course of the dorsal aorta in Andreyevichthys , ‘ G. ’ whitei, Jarvikia and Soederberghia is unusual among Devonian dipnoans where the condition can be assessed reliably. Typically, the dorsal aorta splits posterior to the termination of the parasphenoid, with the lateral dorsal aortae continuing lateral to the parasphenoid in grooves on the ventral face of the otic and occipital regions of the neurocranium ( Säve-Söderbergh, 1952; White, 1965; Miles, 1977; Pridmore et al., 1994; Barwick & Campbell, 1996; Schultze, 2001). The consistent presence of this circulatory pattern in what are typically considered plesiomorphic dipnoans suggests that the course of these vessels over the parasphenoid stalk is derived.
The visceral surface of the parasphenoid in Soederberghia is known primarily from impressions ( Fig. 13; Lehman, 1959: pl. 6; MGUH VP 3042). Two well-defined ridges, aligned with the lateral margins of the stem, extend across the dorsal surface of the posterior half of the corpus (d.r.Psph, Figs 2 View Figure 2 , 3 View Figure 3 ). Similar ridges are visible in sectioned material of ‘ G. ’ whitei ( Miles, 1977: figs 27, 28). Between these ridges, and across the entirety of the stalk, the visceral surface of the parasphenoid is concave and receives the ventral surfaces of the otic and occipital regions of the neurocranium ( Fig. 4 View Figure 4 ). There is a narrow depressed strip that runs along the midline of this trough anteriorly. The most notable features of the visceral surface of the parasphenoid corpus are the dorsolaterally orientated overlap areas for the entopterygoids. These bands extend along the margins of the corpus from its lateral angle to its apex and have a roughened surface like their counterparts on the entopterygoids.
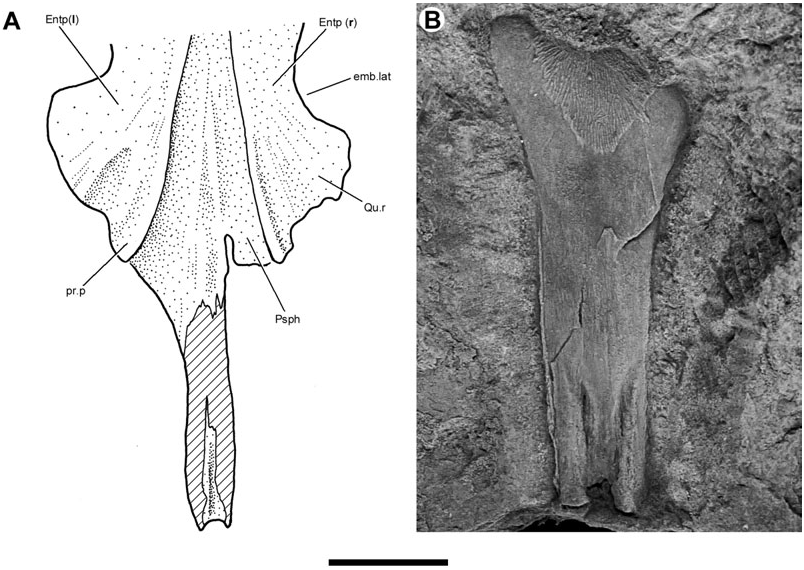
Reinterpretation of the braincase of Griphognathus minutidens : Description of the neurocranial anatomy of Soederberghia and comparison with that of ‘ Griphognathus ’ whitei has led to a reconsideration of the braincase of the type species of Griphognathus , G. minutidens ( Fig. 14 View Figure 14 ), the only other ‘rhynchodipterid’ for which endocranial remains have been described. Available specimens of this species are small, and neurocrania are invariably crushed, making interpretations of this material difficult. The only detailed account of the braincase in G. minutidens ( Schultze, 1969; this material was occasionally referenced by Miles, 1977) was made when intact braincases were known for only two Devonian lungfishes: Chirodipterus wildungensis and Dipterus . The wealth of new data that have appeared subsequently ( Thomson & Campbell, 1971; Bernacsek, 1977; Miles, 1977; Song & Chang, 1991; Wang et al., 1993; Pridmore et al., 1994; Barwick & Campbell, 1996; Krupina et al., 2001) provides a new comparative context for revisiting some aspects of neurocranial anatomy in G. minutidens .
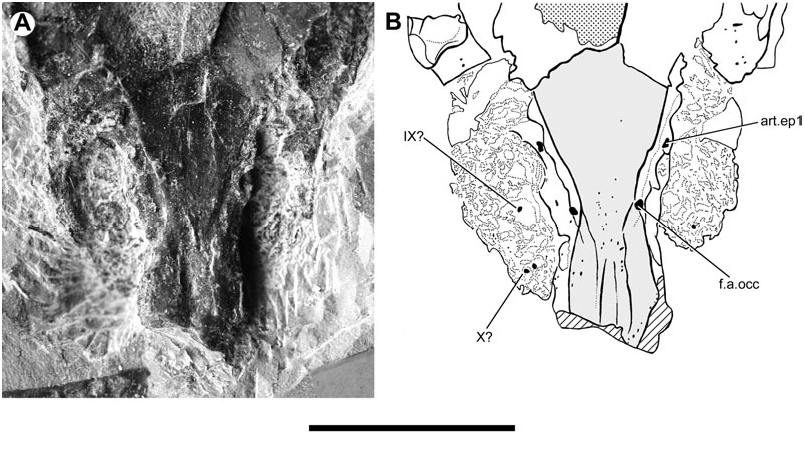
The most immediate difference between the neurocrania of Soederberghia and ‘ G. ’ whitei and that of G. minutidens is the presence of an enclosed canal for the occipital artery in the latter species. The ventral foramen for the canal in G. minutidens occupies an anterior position, near the intersection of the parasphenoid corpus and stalk. This placement differs from the more posterior location seen in ‘holodontids’ ( Pridmore et al., 1994) and most ‘chirodipterids’ ( Säve-Söderbergh, 1952; Miles, 1977) but is similar to that found in ‘ Chirodipterus ’ liangchengi ( Song & Chang, 1991). However, placement of this foramen within the well-defined groove for the lateral dorsal aorta of G. minutidens leaves little doubt that Schultze’s (1969) interpretation is correct.
An additional unossified feature is present on the ventral surface of the braincase of G. minutidens , anterior to the foramen for the occipital artery but lateral to the groove for the lateral dorsal aorta (art.ep1, Fig. 14 View Figure 14 ). The structure was identified as a foramen for a ventral branch of N.VIII ( Schultze, 1969), which seems to have been directly informed by White’s (1965: figs 43, 46) interpretation of a similar structure in Dipterus . However, better preserved material of other dipnoans clearly shows that the structure equivalent to the putative foramen for N.VIII in G. minutidens and Dipterus is an unfinished area for articulation with the first epibranchial (cf. Miles, 1977: 78). Furthermore, it is clear that the unfinished area in G. minutidens is borne on a small prominence; this is particularly clear on the left side of the specimen ( Fig. 14 View Figure 14 ).
The recognition of an anteriorly located epibranchial facet in G. minutidens provides clues about the possible condition of the equivalent structure in Soederberghia . There is an unossified area on the ventrolateral surface of one side of the neurocranium of Soederberghia that could be identified as such a facet, but arguments against this interpretation have been given above. The braincase of Soederberghia is proportionally similar to that of G. minutidens (compare Fig. 3 View Figure 3 with Schultze, 1969: fig. 10), which suggests that the epibranchial facets in both genera may have occupied similar positions. In Soederberghia , this corresponds to the unossified region of the neurocranium located anterior to the intersection between the parasphenoid stalk and corpus, which might account for the absence of a convincing epibranchial facet in the available material of this genus ( Figs 1 View Figure 1 , 2 View Figure 2 ).
More detailed information on the braincases of early lungfishes also permits a further interpretation of the parasphenoid of G. minutidens , including details of the course of the lateral dorsal aortae. The neurocranium figured by Schultze (1969: fig. 15) bears a depressed area that delimits the margins of the posterior half of the parasphenoid corpus and much of the stalk. The stalk was clearly broad and cradled the ventral portions of the otic and occipital regions of the braincase, as in Soederberghia and ‘ G. ’ whitei. Grooves on the ventrolateral surface of the otic region have been correctly identified as accommodating the lateral dorsal aortae by Schultze (1969). However, these depressions do not appear to have extended as far posteriorly as his figures indicate. This earlier interpretation requires that these vessels passed between the dorsal surface of the parasphenoid and the ventral surface of the braincase only to re-emerge at the intersection of the stalk and corpus, an exceptional arrangement unknown in any lungfish. An alternative interpretation, partly informed by the conditions in Soederberghia and G. minutidens , is that the lateral dorsal aortae travelled over the ventral surface of the parasphenoid, passing on to the neurocranium at approximately the level of the intersection between the parasphenoid stalk and corpus. The trajectory of the grooves for the lateral dorsal aortae in G. minutidens suggest that these vessels diverged from one another anterior to the occiput as in Soederberghia , but unlike ‘ G. ’ whitei, where the split occurred behind the braincase.
Schultze (1969) indicated a series of foramina in the wall of the otic region of G. minutidens that he interpreted as giving passage to cranial nerves. These features roughly correspond in the positions expected for the exits of these nerves ( Fig. 14 View Figure 14 ). However, the walls of the braincase are crushed and poorly preserved, and these identifications must be considered tentative.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |