Sinacroneuria obscura Li & Murányi, 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4299.1.4 |
|
publication LSID |
lsid:zoobank.org:pub:F54BD34D-0B2A-4D33-AC18-446E57B2F079 |
|
DOI |
https://doi.org/10.5281/zenodo.6039403 |
|
persistent identifier |
https://treatment.plazi.org/id/03B88799-FFBC-FB1A-FF7C-FB92FD2BFEA0 |
|
treatment provided by |
Plazi |
|
scientific name |
Sinacroneuria obscura Li & Murányi |
| status |
sp. nov. |
Sinacroneuria obscura Li & Murányi View in CoL , sp.n.
( Figs. 1–37 View FIGURES 1 – 6 View FIGURES 7 – 8 View FIGURES 9 – 10 View FIGURES 11 – 16 View FIGURES 17 – 21 View FIGURES 22 – 32 View FIGURE 33 View FIGURES 34 – 37 )
Type materials: Holotype male ( HIST), China: Guangxi Zhuang Autonomous Region, Shangsi County, Shiwandashan National Forest Park, Pearl River above tourist route bridge, 375 m, 21°59.913'N, 107°54.283'E, 2015 GoogleMaps . III.27, leg. J. Kontschán, J.Y. Li, S. Li, W.H. Li, D. Murányi, G.Q. Wang. Paratypes: same locality and date: 2 male and 1 female penultimate larvae, 2 male exuviae ( HNHM: No. PLO86) GoogleMaps ; same locality but collected on 2015. III.28: 2 male and 1 female exuviae ( HIST), 1 adult male, 1 female larva, 5 male and 1 female exuviae ( HNHM: No. PLO100); Shiwandashan National Forest Park, small forest brook, 365 m, 21°50.574'N, 107°51.802'E, 2015 GoogleMaps . III.28, leg. J. Kontschán, W.H. Li, D. Murányi, G.Q. Wang: 1 male exuviae ( HNHM: No PLO115).
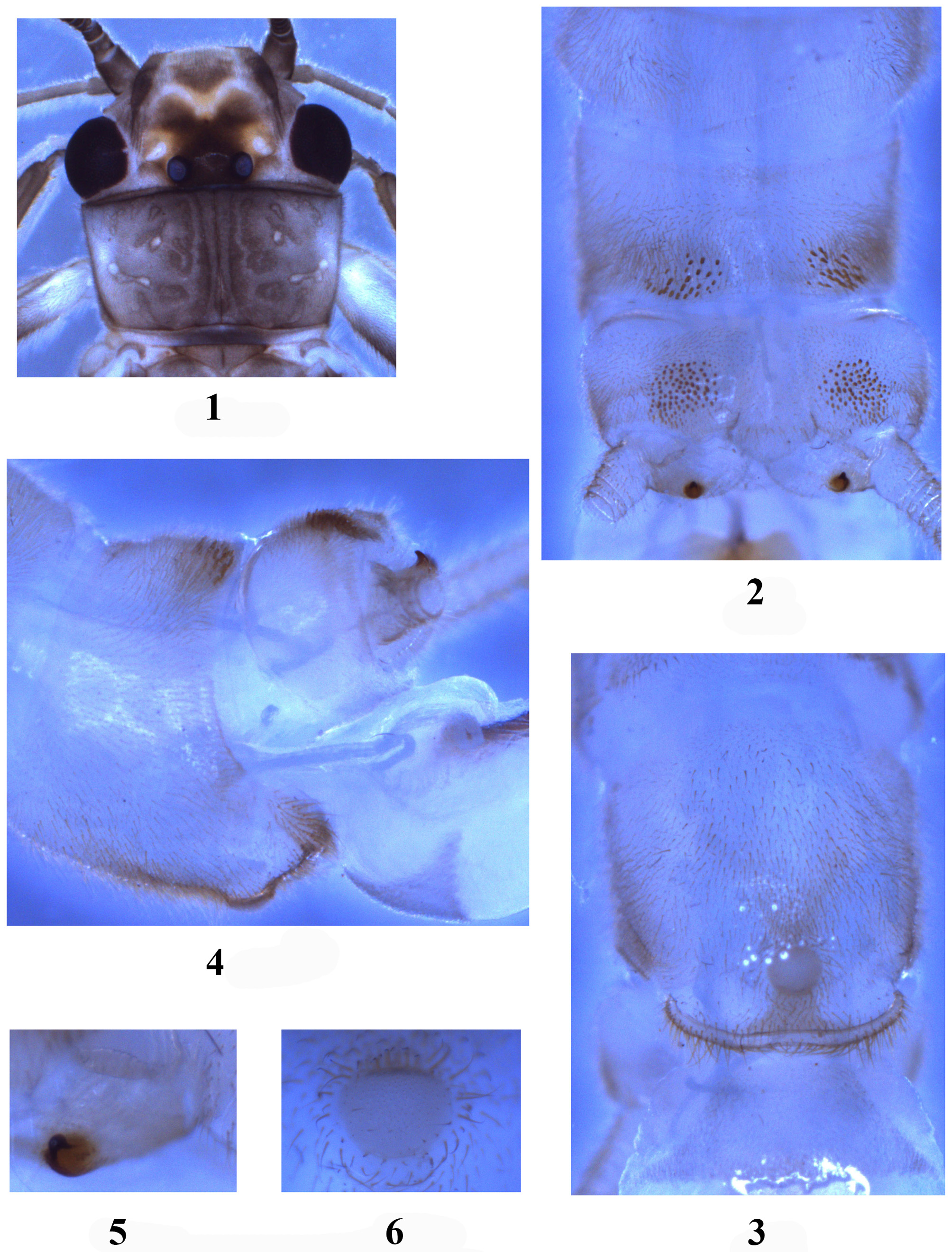
Adult. General body color yellow brown. Biocellate, vestigial anterior ocellus a small dark spot. Distance between ocelli almost two times the diameter of the ocellus. Head with dark brown area covering ocellar triangle, slightly extending laterally under pale M line; lappets dark and triangular mark forward of M-line brown to brownish ( Fig. 1 View FIGURES 1 – 6 ); compound eyes black; antennae brown. Pronotum brownish with dark rugosities ( Fig. 1 View FIGURES 1 – 6 ); wing membrane transparent, veins brown; legs brownish. Abdominal segments pale brown to brownish.
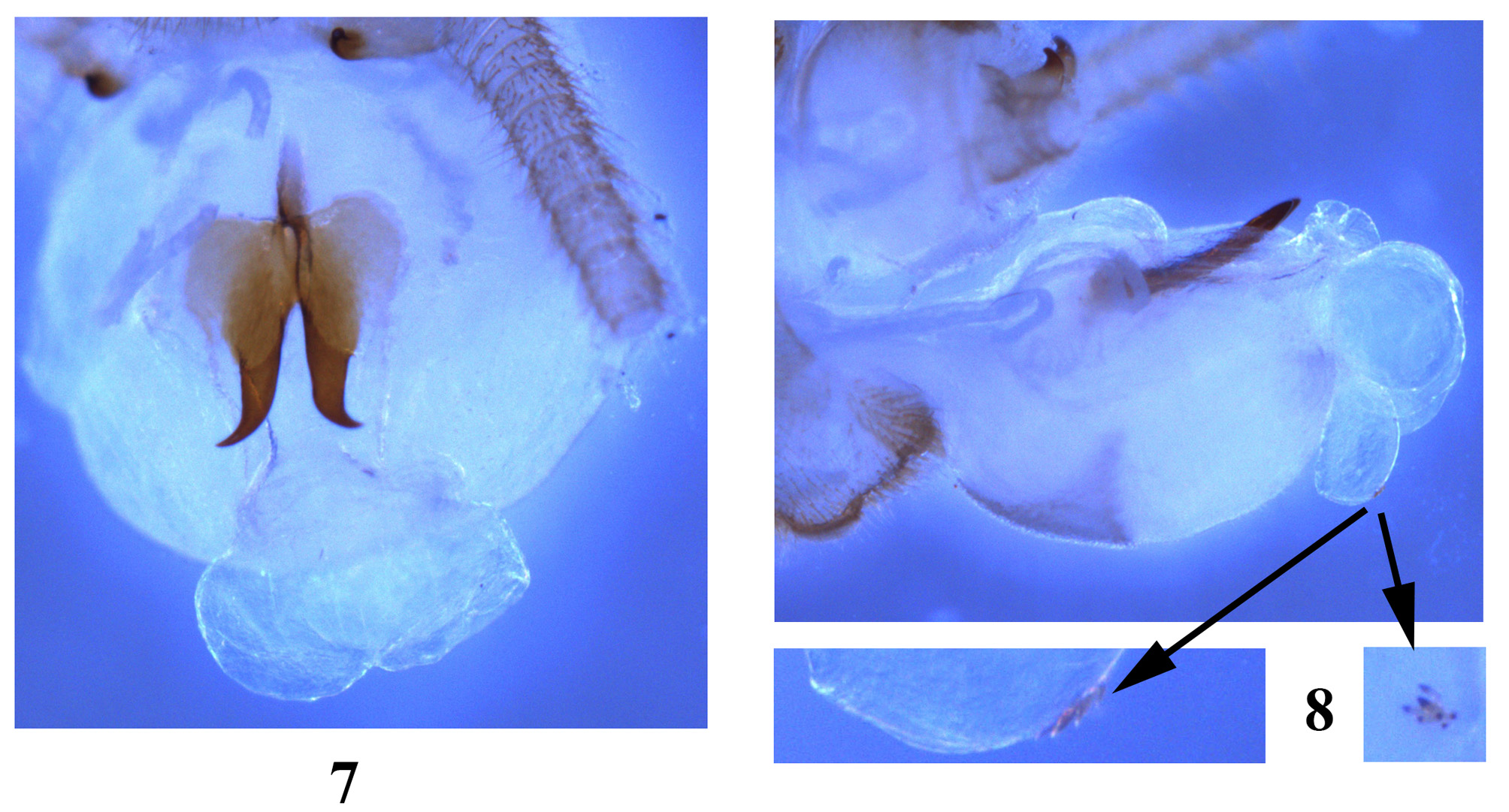
Male. Forewing length holotype ca. 14.9 mm, paratype 15.0 mm. Tergum 9 posterior margin slightly produced, with two far sensilla patches, each consisting of many larger triangular sensilla basiconica ( Fig. 2 View FIGURES 1 – 6 ). Sensilla basiconica patch of tergum 10 divided medially distance between patches ca. 1.5X diameter of the patch ( Fig. 2 View FIGURES 1 – 6 ). Paraprocts heavily sclerotized, with small, nipple like tips ( Figs. 2, 5 View FIGURES 1 – 6 ). Sternum 9 with sclerotized posterior margin fringed with short hairs, hammer oval-shaped, slightly elevated in lateral view ( Fig. 3 View FIGURES 1 – 6 ); hammer bare but surrounded by strong hairs, anterior hairs heavier; below hammer is a patch of brown hairs giving appearance of brown pigmentation ( Fig. 4 View FIGURES 1 – 6 ). Aedeagus ( Figs. 4 View FIGURES 1 – 6 , 7–8 View FIGURES 7 – 8 ): basoventral area with large patch of setal hairs, apical membrane forming a large dorsal and a small ventral globes; median sclerite absent, replacing with a patch of tiny spines on the ventral globe; Y-arms about 3 times as long as stem, basal trunk of Y-arms nearly parallel-sided, the apex of Y-arms sharp and horn-like, curved outward and upward. ( Fig. 8 View FIGURES 7 – 8 ).
Female. Unknown.
Larva. Body length of the female larva 17 mm, male exuviae 12-13 mm, female exuviae 17–18.5 mm; no last instar male larva available. General colour pale but with distinct dark markings on dorsal surface ( Fig. 9 View FIGURES 9 – 10 ); ventral surface generally pale ( Fig. 10 View FIGURES 9 – 10 ). Head yellow with transverse dark stripe posterior to M-line, anterolateral edges are also dark; occipital area not elongated, eyes normal sized. Biocellate, distance between ocelli about twice as wide as diameter of one ocellus. Lacks occipital ridge and row of occipital setae, occipital suture indistinct; only few, relatively long, pale setae occur posterior to eyes, while most of the head covered by fine, black clothing hairs ( Fig. 21 View FIGURES 17 – 21 ). Antenna yellowish, palpi pale. Postmentum undivided ( Fig. 27 View FIGURES 22 – 32 ), glossa and paraglossa usual for the family ( Figs. 28–29 View FIGURES 22 – 32 ). Lacinia bidentate, apical tooth much longer than subapical tooth, marginal fringe consist of only 8–9 strong setae; galea as long as apical tooth of lacinia, bear only apical setae ( Figs. 22–23 View FIGURES 22 – 32 ). Mandible wide, having two molar and two incisor dens, molar brush short but dense ( Figs. 24–26 View FIGURES 22 – 32 ). Labrum mostly dark brown, with downcurved posteromedial lobe bearing dense setae ( Figs. 30–31 View FIGURES 22 – 32 ); hypoharynx simple, with short apical setae ( Fig. 32 View FIGURES 22 – 32 ). Pronotum with rounded corners, less than twice wider than long, marginal row of setae laterally incomplete; all thoracic segments covered only by fine, black clothing hairs, and dark coloration is limited to marginal areas ( Fig. 9 View FIGURES 9 – 10 ). Mesosternum with long furcasternal pit, furcasternal arms short and laterally positioned ( Figs. 10 View FIGURES 9 – 10 , 12 View FIGURES 11 – 16 ). Thoracic gills short and with dense chloride cells ( Fig. 11 View FIGURES 11 – 16 ); prothorax with one anterior and one posterior supracoxal gill; mesothorax with two anterior sternal gills one of which is deeply divided, one anterior and one posterior supracoxal gill; metathorax with two anterior sternal gills one of which is deeply divided, one anterior and two posterior supracoxal gill ( Fig. 12 View FIGURES 11 – 16 ). Proventricular teeth uniform and regularly placed ( Figs. 17– 18 View FIGURES 17 – 21 ). Legs long, dorsal row of swimming hairs scarce and indistinct on femora, more developed on tibiae; surface setation consist of black clothing hairs and scarce, pale and moderately long setae ( Figs. 15–16 View FIGURES 11 – 16 ). Abdomen with terga 1–3 and 7–8 mostly pale, while terga 4–6 and 9–10 contrastingly dark ( Fig. 9 View FIGURES 9 – 10 ). All terga bear dense apical row of shorter and very long setae mixed, that are pale and easily broken; similar setae are placed in an irregular, transverse median row on the terga, while most of the surface is covered with fine, black clothing hairs, easily detached but represented as fine punctuations ( Fig. 19 View FIGURES 17 – 21 ). Clothing hairs similar on sterna, but the apical row medially widely interrupted on sterna 2–7, complete on sterna 8–10 in case of males while on female sternum 8 it is narrowly interrupted by the shallow genital notch ( Fig. 20 View FIGURES 17 – 21 ); median row of setae complete only on sternum 10.
Paraproct simple, bears small anal gill ( Figs. 9–10 View FIGURES 9 – 10 , 14 View FIGURES 11 – 16 ). Cercus long, lacks swimming hairs but elongated hairs of the medial row are forming distinct tufts on the inner surface of cercomeres; further portions of the row consist of long but not erect, pale setae mixed with long, fine hairs ( Figs. 13–14 View FIGURES 11 – 16 ).
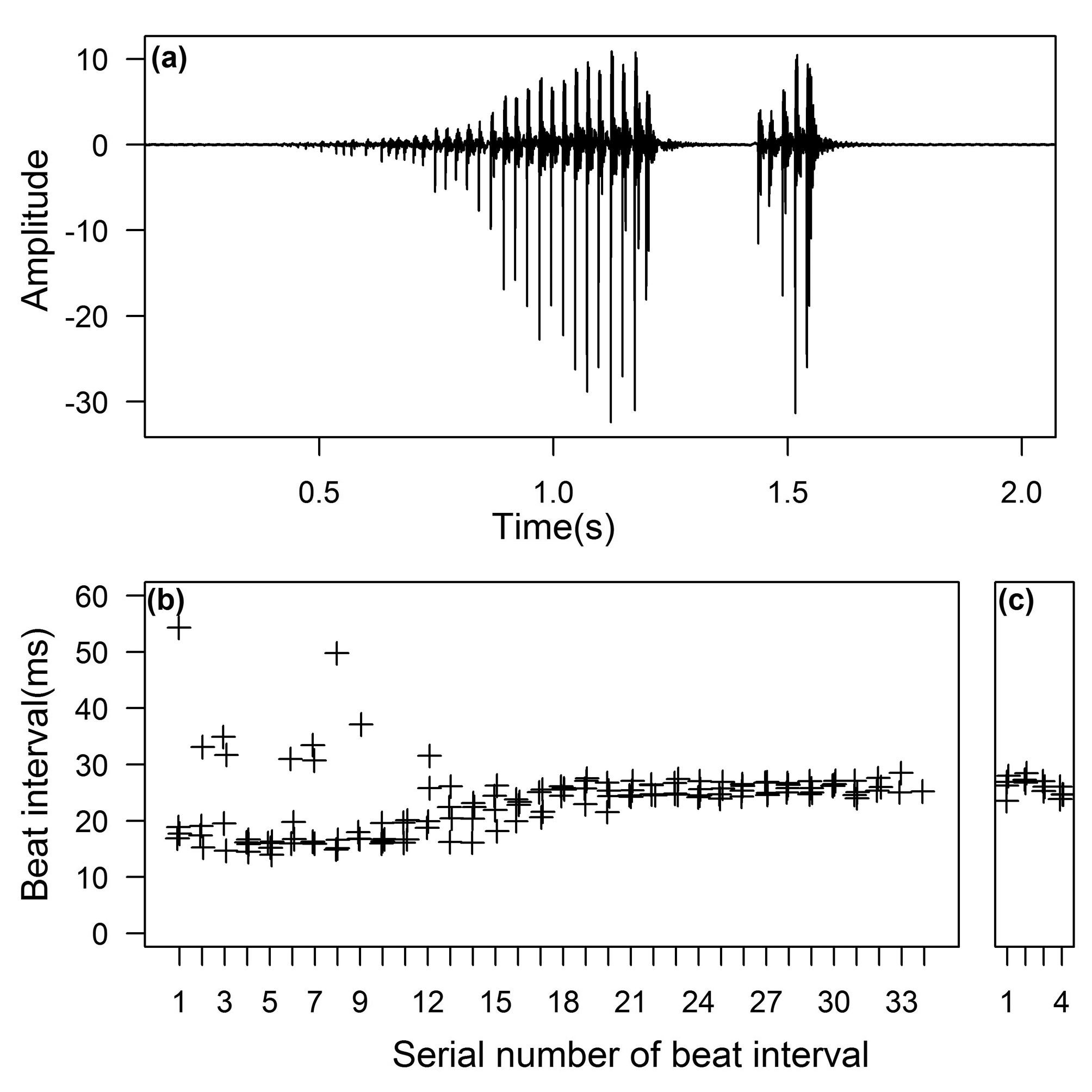
Drumming. Four drumming calls of only one male were recorded; therefore the present description of the male vibrational signal of the species should be regarded as preliminary revealing only the major rhythmic features of the vibratory male calls of this species. The duration and count values given in parentheses report the range measured in the signals of the studied specimen recorded at an ambient air temperature of 19.4o C. The drumming call of S. obscura consists of two beat groups ( Fig. 33 View FIGURE 33 a). The first longer beat group (32-35 beats, duration between 732-838 ms) is followed by a second shorter beat group (5 beats invariably, duration 103-107 ms) after an interval shorter than the first beat group (inter beat group interval varied between 239-273 ms). In both beat groups, beat amplitude increases during the course of the group. In the first beat group beat intervals vary between 15-20 ms during the first low amplitude part (values above 30 ms occurs probably as a result of missing beats) and between 20-25 ms during the final high amplitude part ( Fig. 33 View FIGURE 33 b). A short transitional part in the middle of the beat group can be observed where beat intervals show a tendentious increase. The second beat group beat intervals vary from 20-25 with an uncertain decreasing tendency ( Fig. 33 View FIGURE 33 c).
Distribution and ecology. The new species was collected from Pearl River, a submontane river and a small forest brook in the Shiwandashan National Forest Park, Guangxi Zhuang Autonomous Region of southcentral China. The Shiwandashan Mountains are part of a moderately high coastal range on the border of China and northeastern Vietnam, being relatively isolated from the main mountain chains of both Indochina and the southern Chinese mountain ranges. At the type locality, the Pearl River flows in dense, evergreen submontane forest at moderately low elevation, having fast flow with several cascades, and a rocky bed with very few organic deposits and coarse sand patches among the large rocks and stones ( Figs. 34–36 View FIGURES 34 – 37 ). The stonefly fauna of the river is rather rich, besides the new species, twelve species (two Leuctridae , four Nemouridae , six Perlidae ) were found, among them further three are new and will be described in a future paper, including a new Rhopalopsole Klapálek, 1912 species having been published recently ( Li et al. 2017). The forest brook, a tributary of the Pinglong River, where we found a single exuviae, has reduced flow and is shallow but similar rocky bed as the Pearl River ( Fig. 37 View FIGURES 34 – 37 ). Here the new species was found together with a another Perlidae , one Leuctridae and two Nemouridae species. According to high number of exuviae observed on emergent rocks, the new species appears relatively common in the Pearl River. Peak emergence apparently was completed by the time of our late March collecting, as we found only a single last instar larva despite several hours of searching.
Etymology. The name obscura (from the Latin word obscurus, meaning obscure) refers to the reduced anterior ocellus. Used as an adjective, gender feminine.
Remarks. The new species is similar to S. biocellata Stark & Sivec, 2008 from Vietnam, by sharing similar head pattern and two ocelli, and to S. bicornuata Stark & Sivec, 2008 from Sichuan Province of southwestern China by absence of median sclerite in ventral surface of aedeagus. It may be easily separated by the latter by nearly divided sensilla basiconica patch of tergum 9 and parallel-sided basal trunk of Y-arms ( Figs. 2 View FIGURES 1 – 6 , 7 View FIGURES 7 – 8 ). The sensilla basiconica patch of tergum 9 in both S. biocellata and S. bicornuata are apparently fused ( Figs. 2, 5 View FIGURES 1 – 6 in Stark & Sivec 2008, Fig. 3 View FIGURES 1 – 6 a in Li et al. 2014). Only the larva of S. obscura and S. acuticornis sp. n. are known. The two species are being rather similar, but can be separated on the basis of different abdominal dorsal pattern ( Fig. 9 View FIGURES 9 – 10 , compared to Fig. 14.4 in Shimizu et al. 2005).
| HNHM |
Hungarian Natural History Museum (Termeszettudomanyi Muzeum) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |