Paropsisterna selmani Reid
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3681.4.4 |
|
publication LSID |
lsid:zoobank.org:pub:7D873D35-CBBD-4AA7-8D7C-9ECE13780025 |
|
DOI |
https://doi.org/10.5281/zenodo.6159860 |
|
persistent identifier |
https://treatment.plazi.org/id/6F42DB12-3333-2A26-FF02-F9A6FEDFFF51 |
|
treatment provided by |
Plazi |
|
scientific name |
Paropsisterna selmani Reid |
| status |
|
Paropsisterna selmani Reid & de Little, sp. nov.
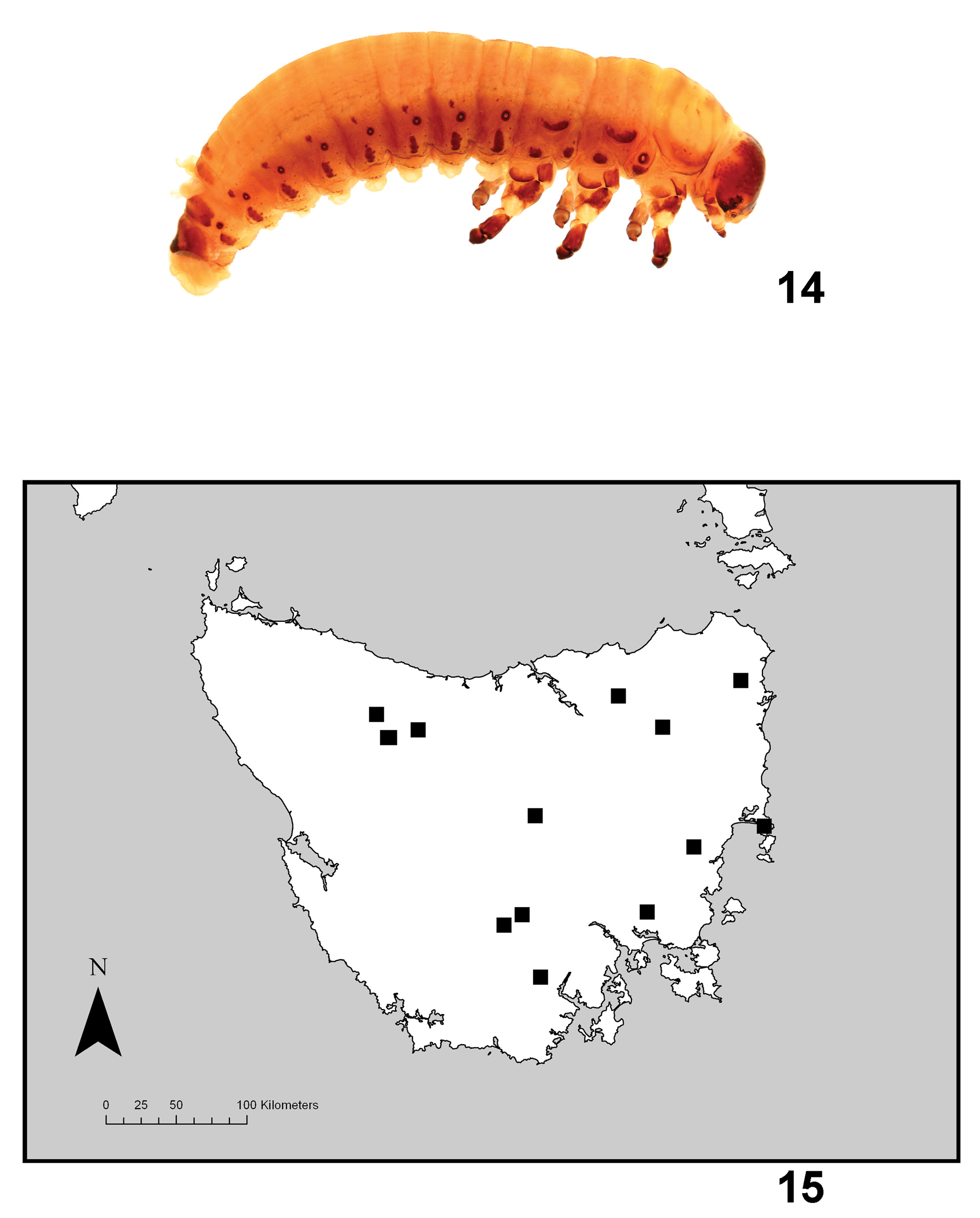
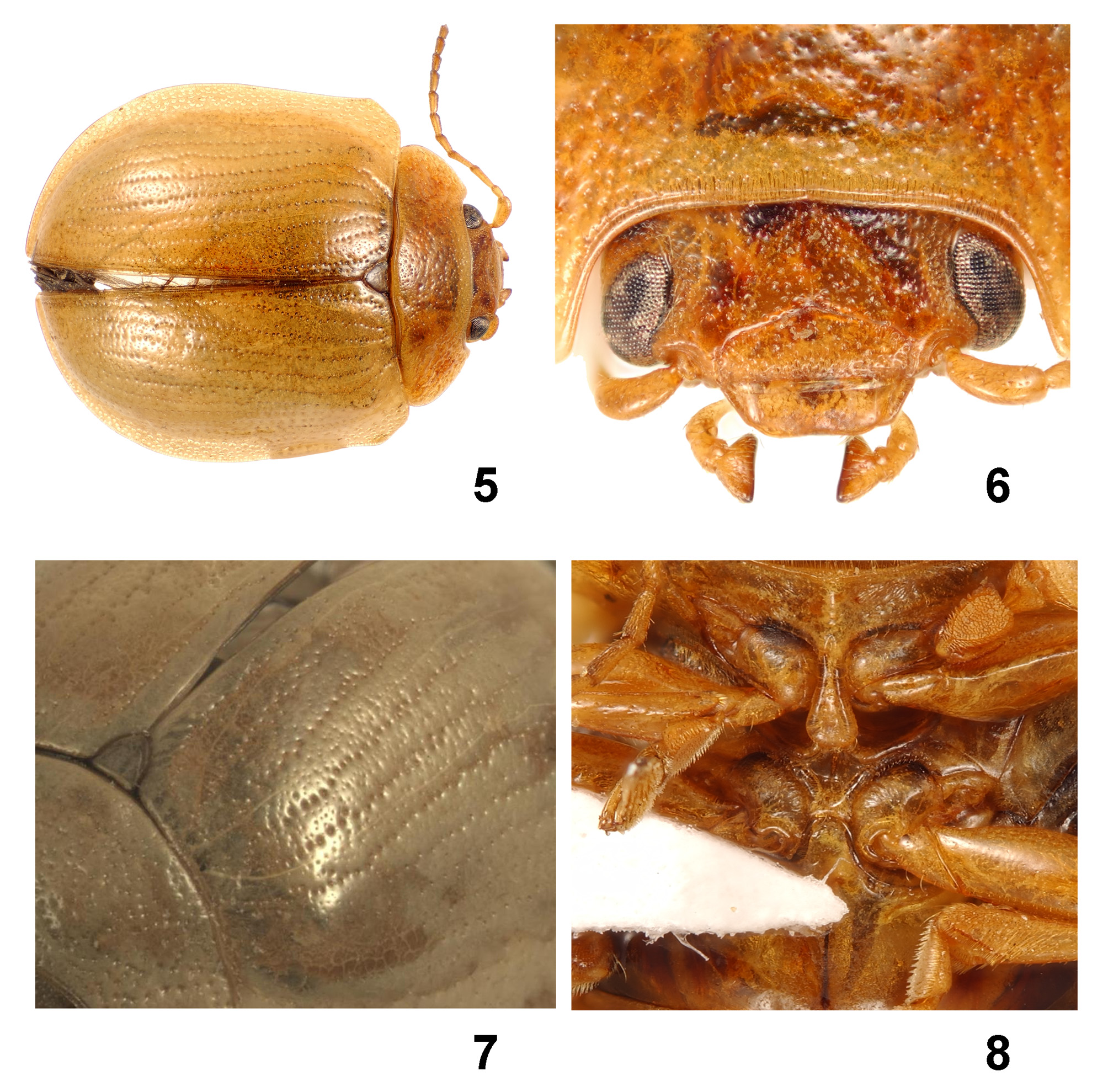
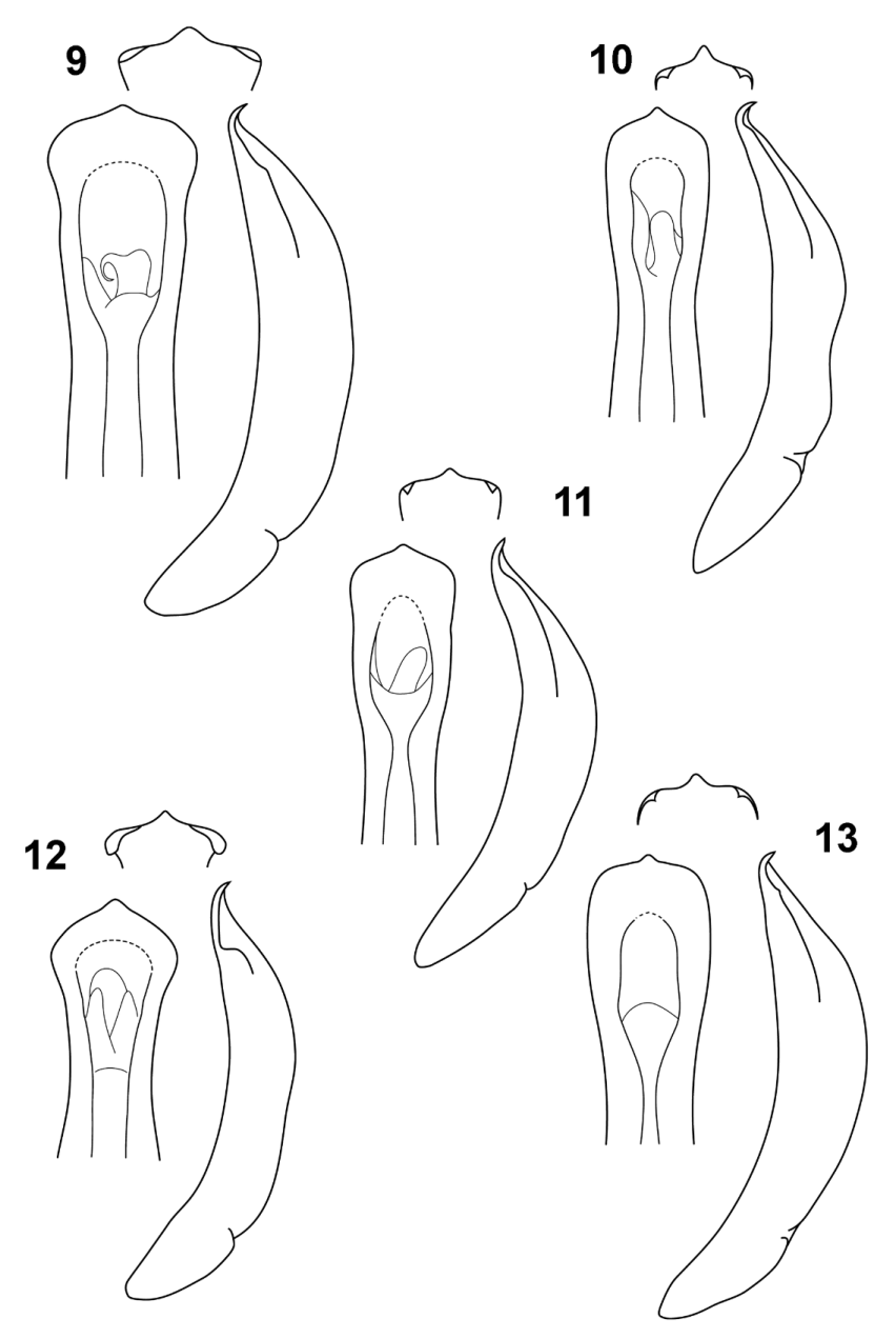
( Figs. 1–3 View FIGURES 1 – 4 , 5–9 View FIGURES 5 – 8 View FIGURES 9 – 13 , 14–15 View FIGURE 14 )
Material examined. Types. Holotype: 3/Vale of Belvoir, Tas., 41°33.005´S 145°53.549´E, 14.iii.2010, A. Throssell, Bush Blitz Survey/ ( TMAG); Paratypes (30): Australia: 3, Ƥ: /Glen Huon [43°05’S 146°51’E], Euc. nitens , 9.xi.2004, D de Little/ ( TMAG); Ƥ/ Myrtlebank [41°17’S 147°21’E], 28.xii.1992, D de Little/ ( TMAG); Ƥ, ditto except 1.xii.1992 ( AMS); Ƥ:/ Ragged Jack [41°29’S 147°38’E], 24.xii.1992, D de Little / ( TMAG); Ƥ: /Vale of Belvoir, 41°33.357´S 145°52.940´E, Col. K Bonham 15.iii.2010, Bush Blitz Survey/ ( TMAG); Ireland: 3, 2 Ƥ: /Co. Kerry, 22.x.2007, A Whelton, CSL 20720078, BMNH 2010- 157 Ex. Enq. FERA / ( NHML); 9 3, 11 Ƥ/Kerry, on Euc. nitens , vi.2007, F. Horgan ( AMS, NHML, NMD, NMNH, SAM). Non-types. 9 first, 4 second, 4 third and 5 fourth instar larvae, reared by DWdL from adults collected at Myrtlebank, Tasmania ( AMS).
Diagnosis. Paropsisterna selmani has the following unique combination of attributes. Elytra of live adult with diagnostic distribution of gold or yellow spots and stripes, including lateral stripe on 7th–8th intervals and ring of elongate spots on apical half of combined elytra ( Figs 1–3 View FIGURES 1 – 4 ); dead adult yellowish-brown with four anteriorly projecting patches of black at base of head, and lateral margins of metaventrite and metanepisterna brown; frons and disc of pronotum with mixture of two sizes of punctures, coalescent towards sides; anterior angles pronotum strongly anteriorly produced; elytra with laterally prominent obtuse anterior angles (c. 120°); elytra with strongly punctured scutellary striole and 9 distinct striae, strial punctures as large as on pronotal disc, those of at least striole and stria 1 (but often 2–5 as well) slightly irregularly placed (not neatly linear); middle third of penis evenly broad in lateral view, contracting apically to sharply reflexed thin tip, short and bluntly triangular in apical view; preapical part of penis abruptly expanded, with slight nick in lateral view at base of expansion and edges narrowly ventrally reflexed in apical view; spermatheca absent.
Description. Adult. Length: 3, 7– 8.5 mm; Ƥ, 8–9 mm; body broadly ovate (length to width ratio 1.3) and convex (length to height ratio 2.6), with highest point at about half body length; head slightly wider than half pronotal width; elytra at anterior angles 1.3x pronotal width. Mature live adult beetles with distinctive but variable colour pattern, 3 generally darker and with more red pigmentation than Ƥ, elytral colour darker after hibernation (pers. obs. DWdL): anterior of head, pronotum, outer margins of elytra, most of venter, antennae, palpi and legs yellowish-brown; base of head (behind eyes) black, with 4 anteriorly projecting poorly defined extensions of the black base, one behind each eye, two divergent at middle (black areas often hidden by overlapping pronotum); head and pronotal disc sometimes partly or entirely reddish-brown; scutellum and basal third of elytral suture often dark brown; each elytron usually with short reddish to gold streak at base of suture (mostly in second interval from suture), constantly with gold to greenish-yellow streak from humerus to apex in 7th–8th intervals, series of 7–8 short elongate gold spots on apical half in a semi-circle, starting at 2nd interval, reaching 4th and curving back to 2nd, the spots often fused, forming a circle when elytra united; outer margin of lateral gold stripe sometimes dark brown; remainder of elytral disc varying from entirely red to purplish-brown with scattered pale brown spots; lateral margins of metaventrite and most of metanepisterna usually stained dark brown. Immature live adults less intensely coloured, with pink elytral disc, black border to disc replaced by pale blue and gold spots replaced by pale yellow. Dead mature adult entirely yellowish-brown except: base of head black and lateral margins of metaventrite and metanepisterna brown as described above. Pubescence: head with depressed areas adjacent to eyes with erect setae, two long setae projecting anteriorly from sides of apical margin of clypeus; rest of head, all of pronotum (including hypomera) and elytra (including epipleura) glabrous; prosternum glabrous except setose anterior margin and apex of prosternal process; mesepimera and mesoventrite glabrous except process; metanepisterna with sparse minute setae; metaventrite glabrous; abdominal ventrites glabrous except lateral margins and apex ventrite V densely setose; antennomeres 4–11 with smooth impunctate midline and raised setae either side of this; femora almost glabrous; tibiae setose, densely towards apices; male with ovate pads of spatulate setae on pro- and meso- first tarsomeres.
Head: eyes small, separated by c. 4x greatest widths; frontoclypeus irregularly punctured, with mixed large (slightly larger than eye facets) and small (slightly smaller than eye facets) punctures, often coalescent in small pits, densely and finely punctured in depressed areas adjacent to eyes, more evenly sized and not coalescing at base of head (in black area); interspaces finely microreticulate; antennae c. half body length, all antennomeres elongate, 2 shortest, 11 longest; 1 and 3 almost as long as 11, 4–11 distinctly flattened, 5–10 slightly asymmetric; labrum microreticulate, finely and sparsely punctured, apex shallowly concave; securiform apical maxillary palpomere broader in male, with sharper lateral angles.
Thorax: pronotum strongly transverse, width c. 2.3x length, posterior angles broadly rounded, anterior angles strongly anteriorly produced; pronotal margins entirely finely beaded; disc of pronotum (area behind vertex) punctured as head but punctures larger and interspaces larger, punctures increasing in size and conflation towards sides of pronotum, which are rugose with irregular slightly ridged intervals; pronotal surface shining but finely microreticulate; prosternum elevated as smooth ridge along midline, posteriorly bifurcating to follow lateral margins of process (middle of process depressed); prosternal process elongate spatulate, reaching well beyond posterior margins of procoxae; scutellum elongate semi-ovate, strongly microreticulate, minutely punctured; elytra broadest at about middle, but with laterally prominent obtuse anterior angles (c. 120°); humeri prominent, at about 3/5 width from suture to lateral margin; elytra with strongly punctured scutellary striole and 9 distinct striae, strial punctures as large as on pronotal disc, those of at least striole and stria 1 (but often 2–5 as well) slightly irregularly placed (not neatly linear); striae 2 & 3 and 4 & 5 usually anastomosed before apex, striae 6–9 ending in area of scattered large punctures at elytral apex; intervals 1–9 and strip outside stria 9 with scattered mixed punctures, similar to pronotal disc; slightly reflexed outer margins of elytra with broad strongly punctate area, punctures more evenly spread and larger than on pronotal disc; mesoventrite deeply hollowed anteriorly to accommodate prosternal process, small tubercle either side of hollow; mesoventrite process strongly elevated, anterior face deeply concave and posterior face (apex) deeply concave; metaventrite smooth except finely wrinkled and punctate in lateral angles, process flat or slightly elevated; inner margins of protibiae slightly concave before apex; apical third of meso- and metatibiae with dorsal seta-fringed excavation to accommodate tarsi; male pro- and meso- first tarsomeres ovate, female elongate-triangular; claws with acute tooth on middle of ventral surface.
Abdomen: ventrites I–IV shining but finely microreticulate, close and finely punctured at base, wrinkled at sides, with sparse fine punctures elsewhere; ventrite V as I–IV, but without lateral wrinkles and more closely punctured, with subapical transverse setose groove in both sexes; apex of male ventrite V truncate with slight median convexity, apex of female broadly convex; middle third of penis evenly broad in lateral view, contracting apically to sharply reflexed thin tip, short and bluntly triangular in apical view; pre-apical part of penis abruptly expanded, with slight nick in lateral view at base of expansion and edges narrowly ventrally reflexed in apical view; spermatheca absent; vaginal palpi one-segmented, flat, apically setose.
Larva ( Fig. 14 View FIGURE 14 ). Four larval instars. First instar: length 3–3.5mm, head capsule 0.8–0.85mm wide; integument pale yellowish-green with scattered dark microspicules and brown dorsal and lateral sclerites present, as follows: most of head capsule (clypeal area and genae pale), antennae, maxillary palpi; prothorax: lateral edges D-DL-EP, trochantin, P; meso- and metathorax: DLpi, DLe, Sp (mesothorax only), EPa, EPp, trochantin, P; abdominal segments 1–6: DPe (minute), DLpi, Sp, EP, P; abdominal segment 7: DA, DP, DLpi, Sp, EP, P; abdominal segment 8: D-DL, Sp, EP, P; abdominal segment 9: D-DL-EP; lateral surfaces of legs. Ventral surface with minute pale sclerites. Vertex and frons entirely microtuberculate, sides slightly wrinkled; stemmata arranged 5+1; antennae short, 3rd segment not longer than 2nd; antenna inserted on elevated flange, inner edge of flange as long as antenna; 3 pairs of eggbursters short, narrowly acute, on DLpi of meso- and metathorax and abdominal segment 1; annular spiracles present on mesothorax and abdominal segments 1–8; pair of elongate eversible dorsal glands, bulbous at base, between abdominal segments 7 and 8; paired eversible thin-walled ampullae in middle of venter of abdominal segments 1–7.
Second to fourth instars: lengths: second 5mm, third 9mm, fourth 12.5mm (straightened); head capsule widths: second: 1.15–1.2mm; third 1.5–1.6mm; fourth 1.9–2.1mm; coloured and sclerotised as first instar, except brown areas generally darker and with lateral grey-green stripe each side, from head to abdominal apex; DLpi missing from meso- and metathorax, DPe missing from abdominal segments 1–6, P reduced to small spots, eggbusters absent, microtubercles of head less distinct.
Notes. Etymology: named for the late Brian Selman of University of Newcastle-on-Tyne, UK (deceased 2009), who worked for many years on the Australian Chrysomelinae . He was Honours degree supervisor for CAMR and taxonomic advisor to DWdL.
The diagnostic bright colours of Paropsisterna selmani fade rapidly after death, although they may be partially restored by soaking in water.
When it was discovered causing damage to plantations in Tasmania, P. selmani was identified as P. gloriosa (de Little 2011) and this name has also been applied to the Irish population (Anonymous 2008; Fanning et al. 2009; Withers 2011), although later corrected to ‘nr gloriosa ’ ( Horgan 2011). Our studies show that P. gloriosa belongs to a morphological species complex, involving P. nobilitata ( Erichson, 1842) , P. annularis ( Blackburn, 1899) and P. debilis ( Chapuis, 1877) , distinguished from P. selmani by linearly punctured elytral striae, black spots behind the eyes, either a discrete black median mark or no mark at the base of the head, and large golden elytral blotches in the living animals. The male genitalia of this species complex and P. selmani are similar, but the penis of P. s e l m a n i is larger, with short reflexed apex and narrowly folded apical margins, and is not strongly constricted towards the base in either lateral or dorsal view (compare Figs. 9–13 View FIGURES 9 – 13 ). A further distinction is that most dead specimens of the P. nobilitata species complex show some darker translucent patches on the elytral disc, whereas the elytral disc of dead P. s e l m a ni is uniformly coloured. The distributions and hosts are also slightly different. Paropsisterna gloriosa is a widespread species on mainland Australia, from central Victoria to the Blue Mountains, NSW, collected from Eucalyptus radiata and E. polyanthemos (label data in AMS), neither of which is recorded as a host of P. selmani (see below). Most of the photographs of P. ‘ nobilitata’ posted on websites appear to belong to this species (pers. obs., CAMR). Paropsisterna annularis is only known from the Canberra region, with an unidentified host species of Eucalyptus . The identification of P. nobilitata feeding on plantation eucalypts at Crookwell, NSW, is almost certainly an error and may refer to either P. annularis or P. gloriosa ( Edwards & Wanjura 1991) . Paropsisterna nobilitata is probably endemic to Tasmania where it has been collected on a wide variety of eucalypts in subgenera Symphyomyrtus and Eucalyptus s. str. (label data in AMS, ANIC & TMAG). Paropsisterna debilis is endemic to Western Australia where it has been collected feeding on Eucalyptus globulus (label data in AMS), a plantation species introduced from eastern Australia. Gut analyses suggest P. debilis feeds on Corymbia species ( Jurado-Rivera et al. 2009).
Paropsisterna selmani appears to be a Tasmanian endemic which has become established in Ireland. In Tasmania, P. s e l m a n i is widespread in the eastern and central parts of Tasmania ( Fig. 13 View FIGURES 9 – 13 : this map is based on the type material plus field records by DWdL), dominated by dry eucalypt forest and woodland ( Harris & Kitchener 2005). It has been collected from the native Tasmanian species Eucalyptus brookeriana , E. dalrympleana View in CoL , E. rubida View in CoL and E. gunnii View in CoL , all of which are in subgenus Symphyomyrtus , as well as the exotic plantation species E. nitens View in CoL (all records pers. obs. DWdL). Plantations of E. nitens View in CoL were developed throughout Tasmania in the mid 1980s and P. selmani became common and well-established in them from about 1992 (pers. obs. DWdL; J. Elek pers. com. to DWdL, 2013), having previously been collected rarely. In Ireland, P. s e l m a n i has been recorded on E. glaucesens , E. globulus View in CoL , E. gunnii View in CoL , E. johnstonii View in CoL , E. moorei View in CoL , E. nicholii View in CoL , E. nitens View in CoL , E. parvula View in CoL , E. pauciflora View in CoL ssp. niphophila, E. perriniana View in CoL , E. pulverulenta View in CoL , E. vernicosa View in CoL and E. viminalis View in CoL . These host associations cut across proposed phylogenetic groups within Eucalyptus View in CoL ( Brooker 2000; Steane et al. 2011).
In Tasmania, adult P. selmani beetles emerge from hibernation in spring, feed on foliage and mate, and oviposition continues through summer to early autumn (pers. obs., DWdL). Eggs are pale yellow and laid side to side in rafts of 5–15 on a leaf lamina. Larvae feed on foliage and when fully developed drop to the ground where pupation occurs in the soil under host trees. The larvae may be diagnostically coloured, as indicated by a study of Paropsisterna species ( Reid 1983, in which P. nobilitata is either P. gloriosa or P. a n n u l a r i s). Pupae are unknown but unlikely to be distinguishable from related species of the P. nobilitata species-group ( Reid 1992, as Chrysophtharta ). Teneral adults emerging in autumn feed prior to overwintering (J. Elek, pers. comm. to DWdL, 2013; Horgan 2011).
Paropsisterna selmani is a eucalypt plantation pest in both Tasmania and Ireland where it causes significant leaf loss (de Little 2011; Horgan 2011). Paropsisterna selmani is slowly spreading in Ireland but at present remains confined to counties Kerry and Cork ( Horgan 2011). However, it has recently been photographed in London, UK (Anonymous, 2012), where eucalypts are common garden trees. Based on behaviour of related species in exotic locations ( White 1973; Tribe & Cillie 1997; Millar et al. 2009), P. s el m a ni can be expected to spread wherever its host eucalypts are growing within flight range. All stages are also easily transported and short stretches of sea may not be an effective barrier ( White 1973). The species poses a significant pest risk to the Eucalyptus View in CoL -based industries of Europe.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.