Macrobiotus engbergi, Stec & Tumanov & Kristensen, 2020
|
publication ID |
https://doi.org/ 10.5852/ejt.2020.614 |
|
publication LSID |
lsid:zoobank.org:pub:8F2CC4F8-D4F8-4870-A0C7-3D8D78F2D5EC |
|
DOI |
https://doi.org/10.5281/zenodo.3718299 |
|
persistent identifier |
https://treatment.plazi.org/id/C592B357-37F6-4C92-B16A-C28EDB17A231 |
|
taxon LSID |
lsid:zoobank.org:act:C592B357-37F6-4C92-B16A-C28EDB17A231 |
|
treatment provided by |
Plazi |
|
scientific name |
Macrobiotus engbergi |
| status |
sp. nov. |
Macrobiotus engbergi View in CoL sp. nov.
urn:lsid:zoobank.org:act:C592B357-37F6-4C92-B16A-C28EDB17A231
Figs 1–9 View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig
Etymology
We take great pleasure in dedicating this new species to the friend of the third author, Lars Engberg Hansen, who is a teacher emeritus from Qeqertarsuaq and Alluitsup Paa in Greenland and is always happy to help with collecting samples of mosses and lichens for us.
Material examined
112 animals (including 9 simplex) and 108 eggs. Specimens mounted on microscope slides in Hoyer’s medium (98 animals + 103 eggs), fixed on SEM stubs (10+ 5) and processed for DNA sequencing (4+ 0).
Holotype
GREENLAND • ♀; Alluitsup Paa ; 60°28′1.5″N, 45°34′27.8″W; 25 m a.s.l.; mixed sample of moss and lichen collected from rock in arctic tundra; IZiBB, slide GL.052.22 . GoogleMaps
Paratypes
GREENLAND • 107 paratypes; same collection data as for holotype; IZiBB, slides GL.052.17 to 052.24, SEM stub 17.08 GoogleMaps • 108 eggs; same collection data as for holotype; IZiBB, slides GL.052.09 to 052.16, SEM stub 17.08 GoogleMaps .
Description
Animals (measurements and statistics in Table 2)
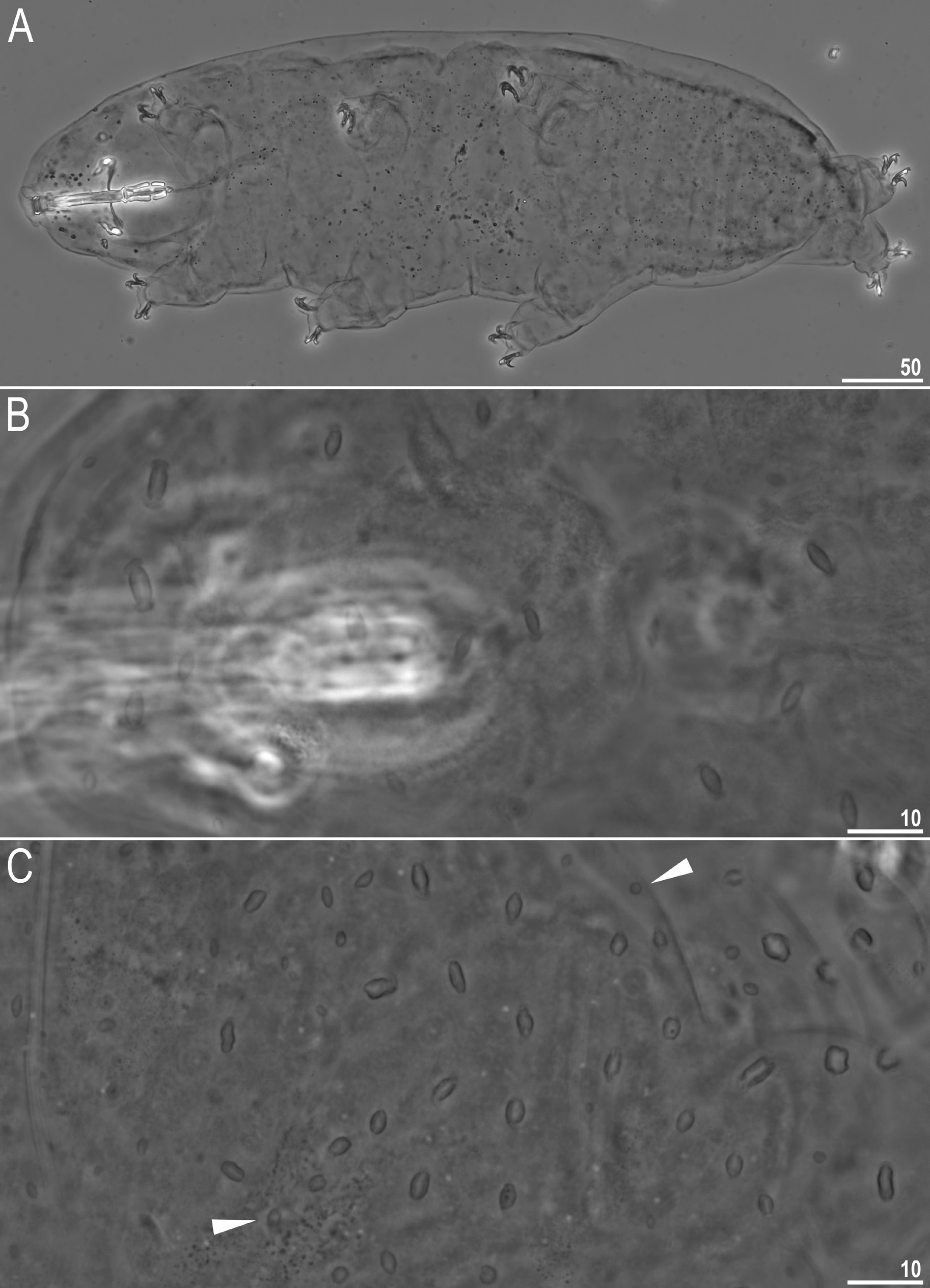
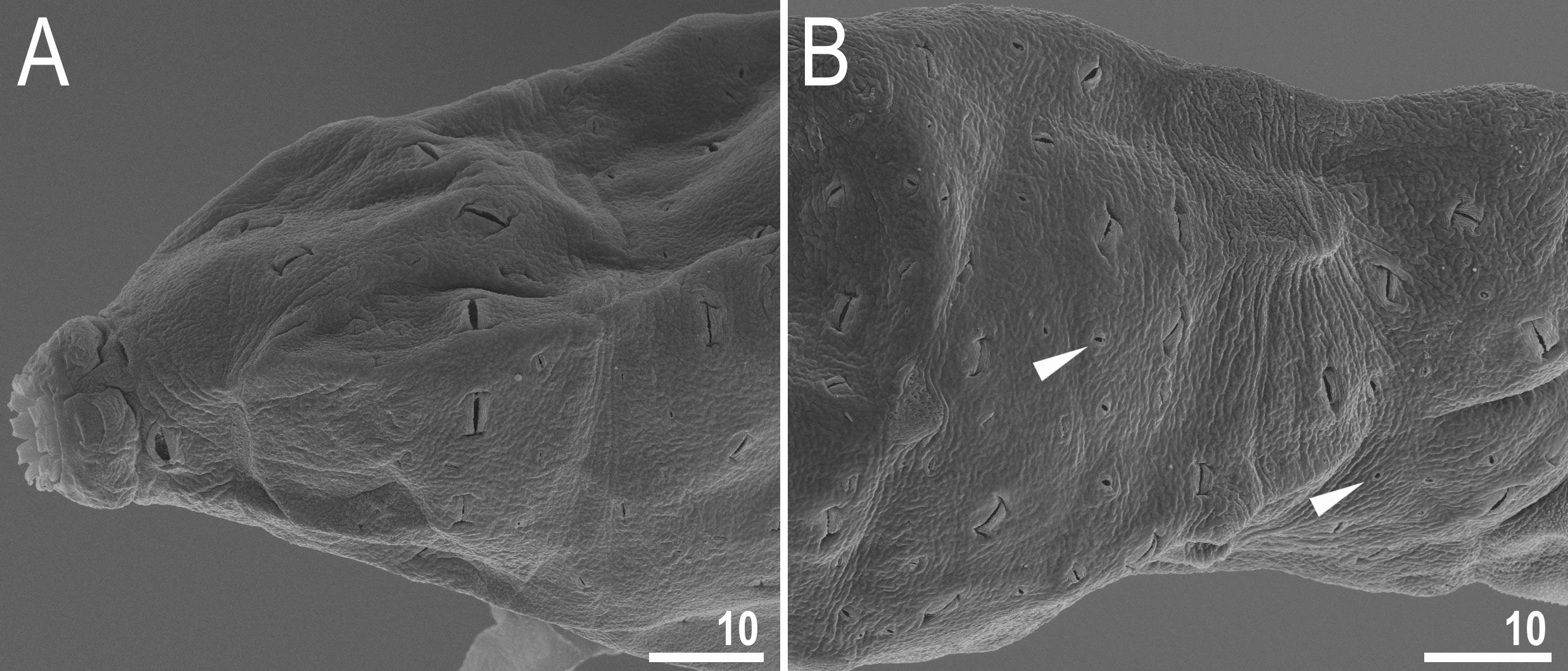
Body transparent in juveniles and whitish in adults, after fixation in Hoyer’s medium transparent ( Fig. 1A View Fig ). Eyes present, visible also in specimens mounted in Hoyer’s medium. Cuticle porous with two types of pores: large (up to 5.0 μm in diameter) lenticular pores of shape resembling paper wrapped candy, with transversal wrinkles in extremities distributed randomly on entire body cuticle and being the biggest on anterior and posterior dorsal region ( Figs 1 View Fig B–C, 2); and small round cuticular pores (0.3–0.7 μm in diameter) scattered in between lenticular pores ( Figs 1C View Fig , 2B View Fig ). Patches of granulation on all legs present ( Fig. 3 View Fig ). A patch of clearly visible granulation is present on the external surface of legs I–III ( Fig. 3 View Fig A– B). A pulvinus present on internal surface of legs I–III, together with a faint cuticular fold covered with faint granulation and paired muscles attachments which are present just below claws ( Fig. 3 View Fig C–D). Both structures are visible only if the legs are fully extended and well oriented on slide. Granulation on legs IV always visible and consists of a single large granulation patch on each leg ( Fig. 3 View Fig E–F).
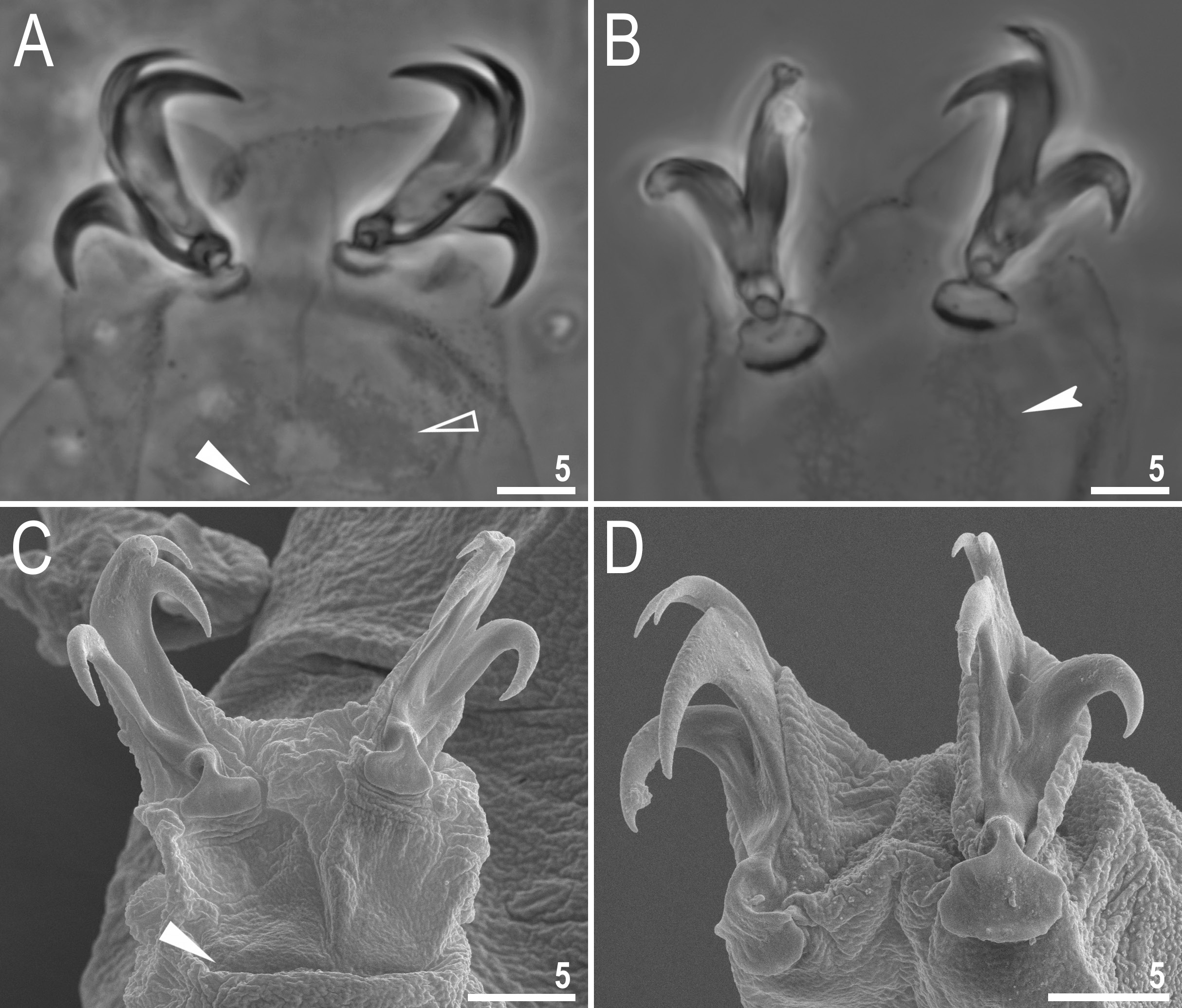
Claws stout, of the hufelandi type ( Fig. 4 View Fig ). Primary branches with distinct accessory points, a common tract and with an evident stalk connecting the claw to the lunula ( Fig. 4 View Fig ). Lunulae on all legs smooth ( Fig. 4 View Fig ). Cuticular bars under claws are absent. Double muscle attachments are faintly marked under LCM but clearly visible under SEM ( Fig. 4A, C View Fig ). The horseshoe structure connecting the anterior and the posterior claw is present and is visible only in PCM ( Fig. 4B View Fig ) and sometimes also on legs I–III, but in this case inverted and not connecting the external and the internal claw ( Fig. 4A View Fig ).
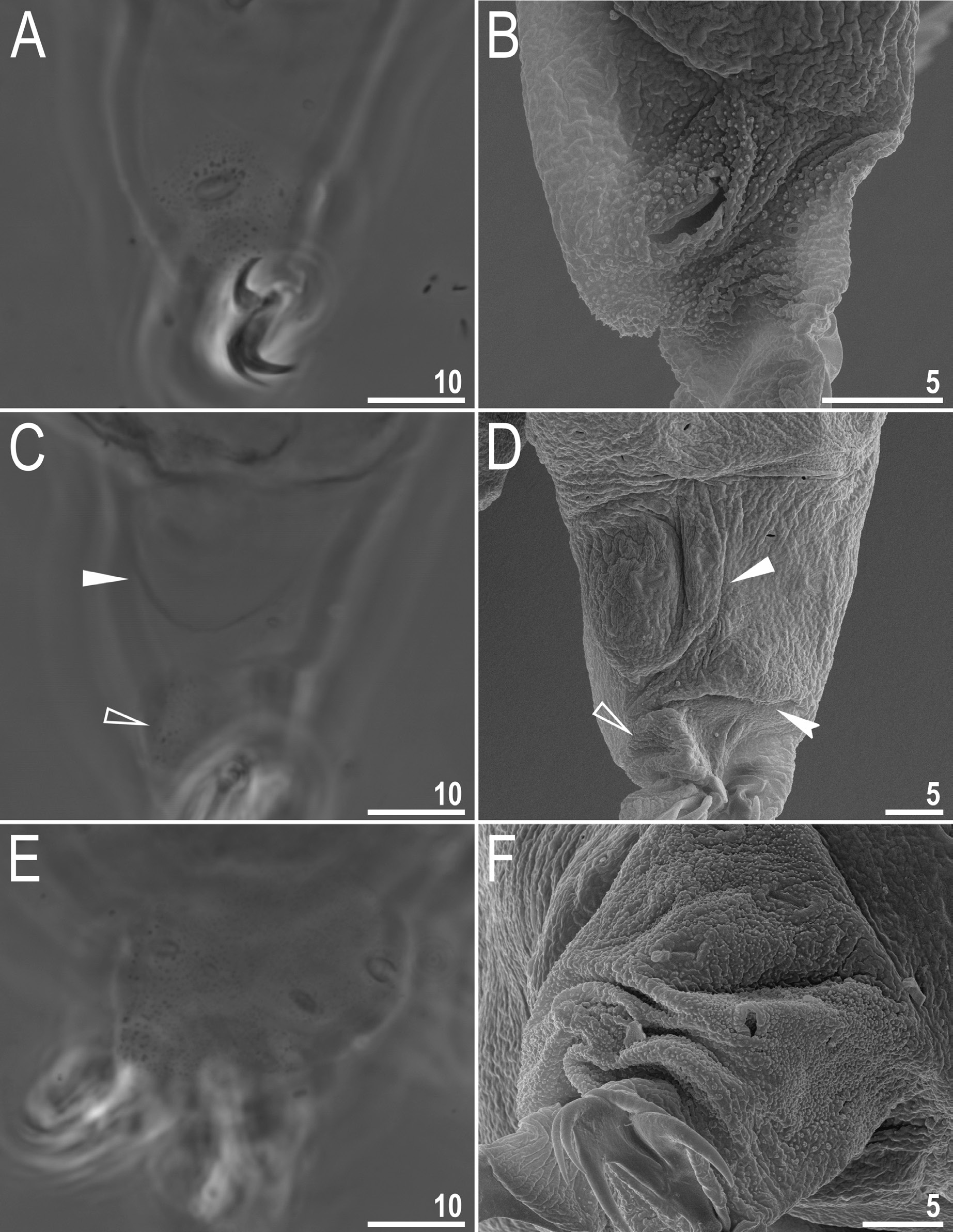
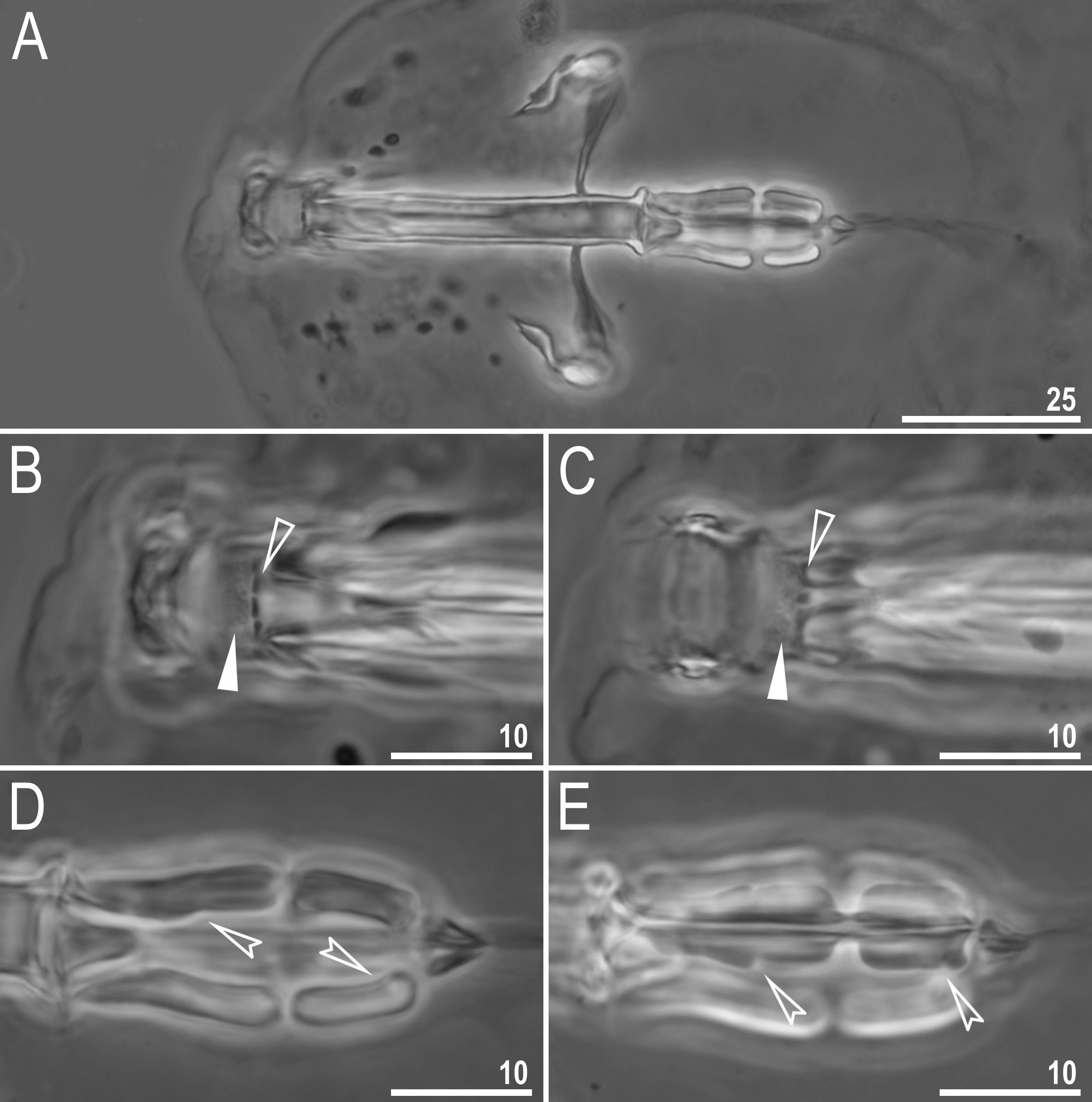
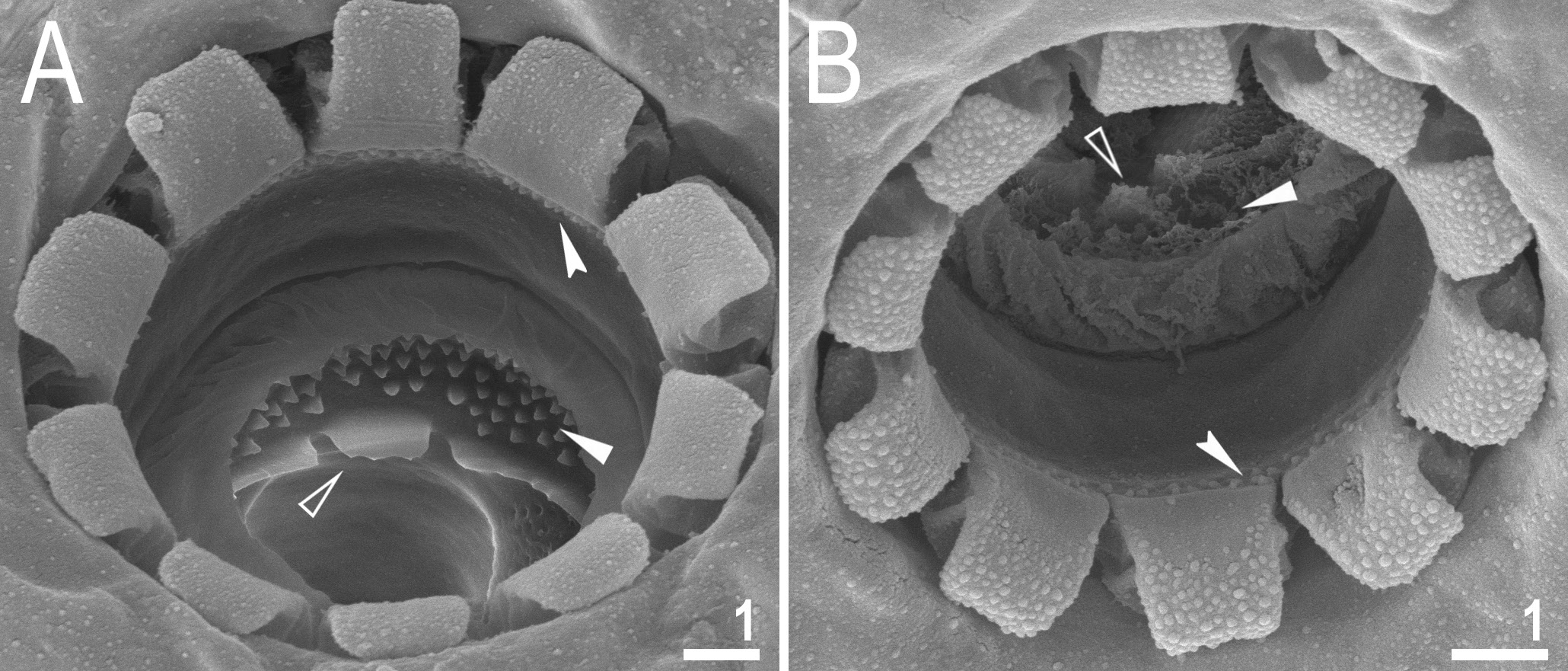
Mouth antero-ventral, followed by ten peribuccal lamellae and a circular sensory lobe, surrounded by the ring of large pores ( Figs 2A View Fig , 5A View Fig , 6 View Fig ). Bucco-pharyngeal apparatus of the Macrobiotus type ( Fig. 5A View Fig ). Under LCM, the oral cavity armature is of the patagonicus type, i.e., with only the 2 nd and 3 rd bands of teeth visible ( Fig. 5 View Fig B–C). However, in SEM all three bands of teeth are visible, with the first band situated at the base of peribuccal lamellae and composed of a 1–2 rows of small, cone-shaped teeth arranged around the oral cavity ( Fig. 6 View Fig ). The second band of teeth is situated between the ring fold and the third band of teeth, and comprises 3–6 rows of small cone-shaped teeth ( Figs 5 View Fig B–C, 6). The teeth of the third band are located within the posterior portion of the oral cavity, between the second band of teeth and the buccal tube opening ( Figs 5 View Fig B–C, 6). The third band of teeth is discontinuous and divided into dorsal and ventral portions. Under LCM, the dorsal teeth are seen as three distinct transversal ridges, whereas the ventral teeth appear as two separate lateral transverse ridges and a median roundish tooth ( Fig. 5 View Fig B–C). In SEM, both dorsal and ventral teeth are also clearly distinct ( Fig. 6 View Fig ). Under SEM, the margins of the dorsal teeth slightly serrated ( Fig. 6A View Fig ). Pharyngeal bulb spherical, with triangular apophyses, two rod-shaped macroplacoids and a small, triangular microplacoid ( Fig. 5A View Fig , D–E). The macroplacoid length sequence 2<1. The first macroplacoid has a central constriction, whereas the second macroplacoid is sub-terminally constricted ( Fig. 5 View Fig D–E).
Eggs (measurements and statistics in Table 3)
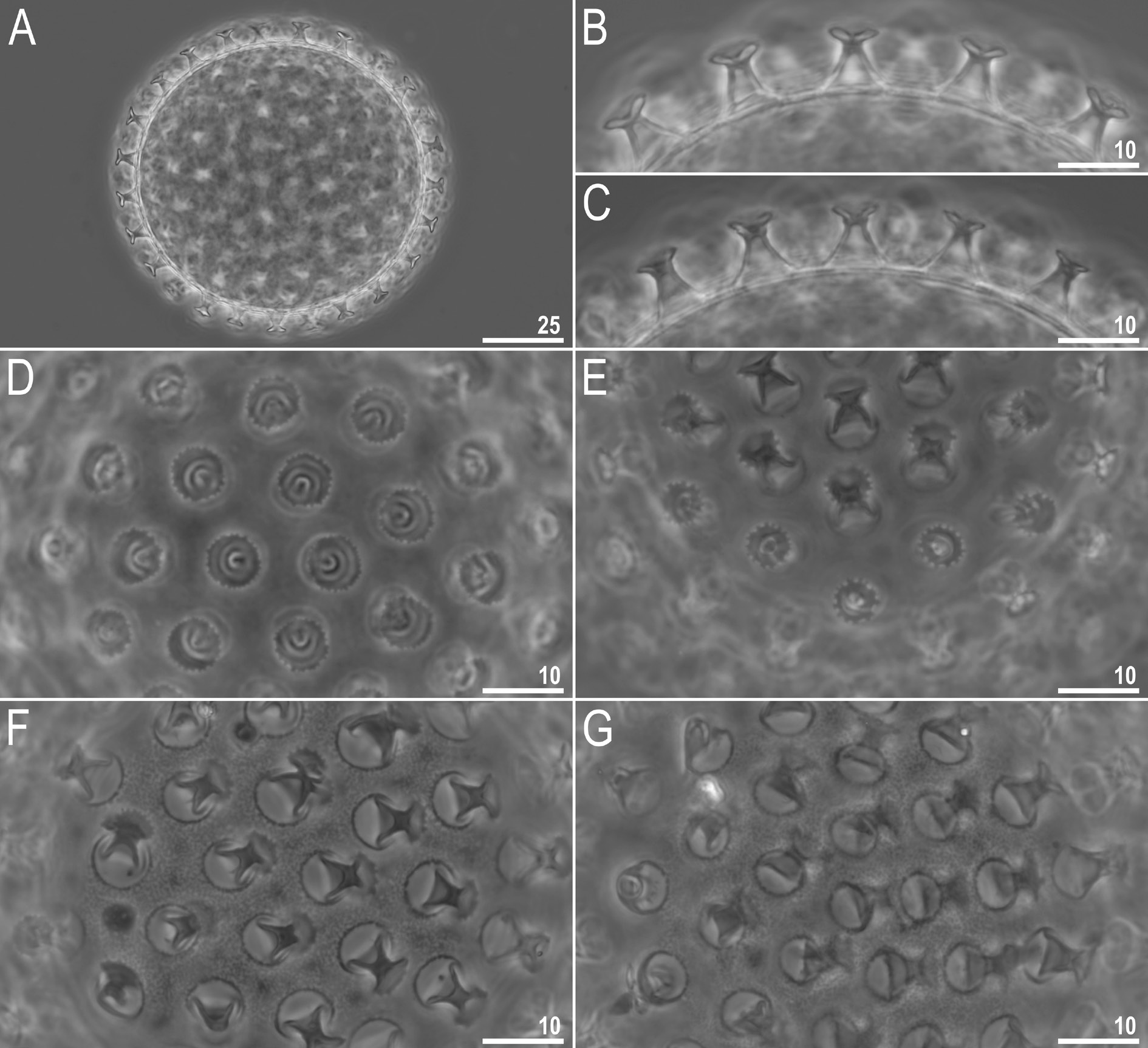
Laid freely, yellowish, spherical ( Figs 7A View Fig , 8A View Fig ). The surface between processes is of the persimilis type, i.e., with the continuous smooth chorion, never with pores or reticulum ( Figs 7 View Fig F–G, 8). Under PCM labyrinthine layer is visible as dark dots/thickenings on the surface between processes, whereas under SEM the surface is smooth ( Figs 7 View Fig F–G and 8, respectively). Processes are of the inverted goblet shape, with slightly concave trunks and concave terminal discs ( Figs 7 View Fig B–C, 8A–C). Terminal discs are round with margins ranging from only serrated to clearly indented ( Figs 7 View Fig D–E, 8). Each terminal disc has a distinct concave central area, which may contain some scattered granulation within, and micro-granulations which are always present on the margins (visible only under SEM; Fig. 8 View Fig C–D).
Reproduction
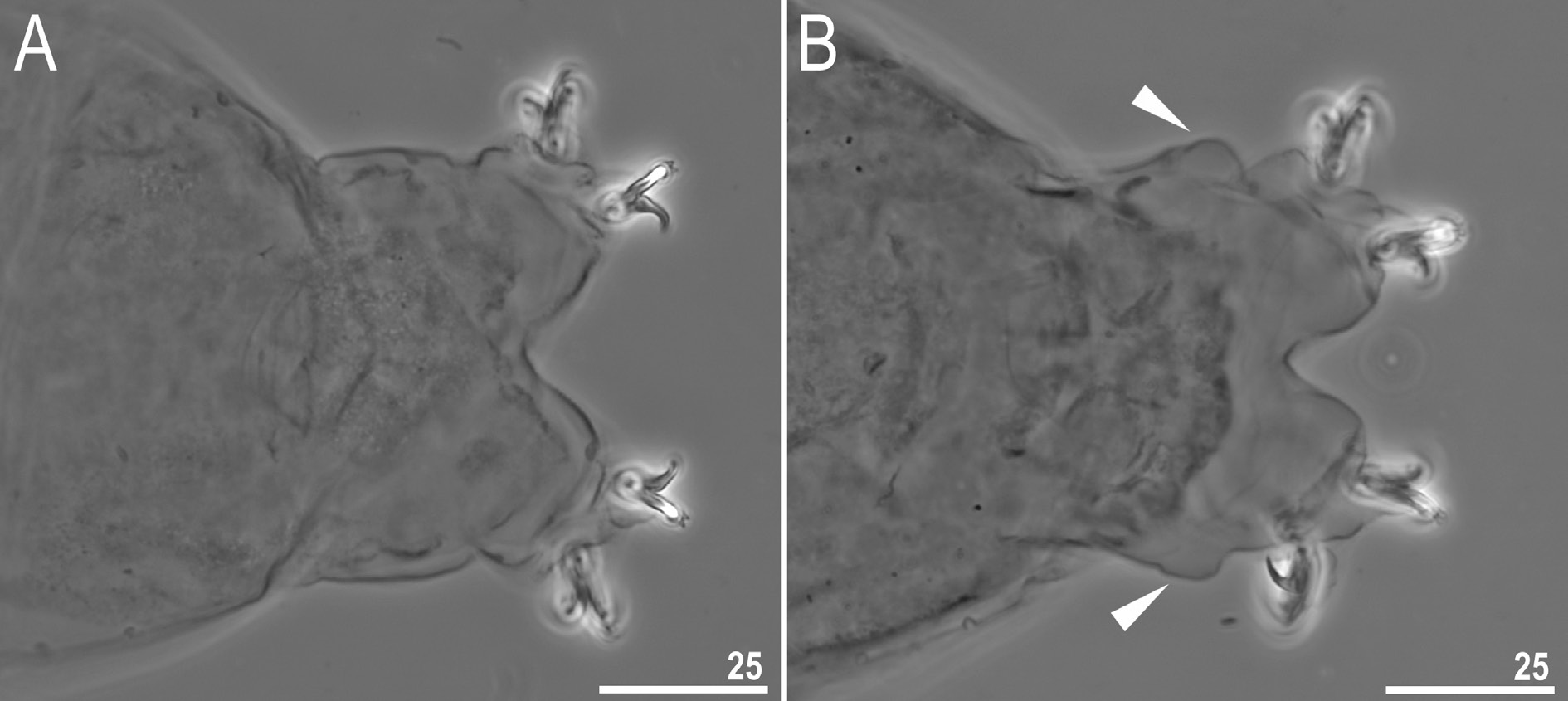
The new species is dioecious. Spermathecae in females as well as testis in males have been found to be filled with spermatozoa, clearly visible under LCM up to 24 hours after mounting in Hoyer’s medium. The new species exhibits a male secondary sexual dimorphism trait in the form of evident lateral gibbosities on legs IV ( Fig. 9 View Fig ).
DNA sequences
We obtained sequences for all four of the above mentioned DNA markers. Sequences of 18S rRNA and 28S rRNA were represented by single haplotypes, whereas sequences of ITS-2 and COI were represented by two (distance: 0.5%) and three (distance: 1.3–1.8%) haplotypes, respectively:
18S rRNA sequence (GenBank: MN443039 View Materials ), 1017 bp long;
28S rRNA sequence (GenBank: MN443034 View Materials ), 783 bp long;
ITS-2 haplotype 1 sequence (GenBank: MN443036 View Materials ), 374 bp long;
ITS-2 haplotype 2 sequence (GenBank: MN443037 View Materials ), 374 bp long;
COI haplotype 1 sequence (GenBank: MN444824 View Materials ), 638 bp long;
COI haplotype 2 sequence (GenBank: MN444825 View Materials ), 638 bp long;
COI haplotype 3 sequence (GenBank: MN444826 View Materials ), 638 bp long.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |