Linderiella baetica, Alonso & Garcia-De-Lomas, 2009
|
publication ID |
https://doi.org/ 10.5252/z2009n4a4 |
|
DOI |
https://doi.org/10.5281/zenodo.4548324 |
|
persistent identifier |
https://treatment.plazi.org/id/2A1587F8-8503-FFA2-FF37-77286636F962 |
|
treatment provided by |
Felipe |
|
scientific name |
Linderiella baetica |
| status |
sp. nov. |
Linderiella baetica View in CoL n. sp.
( Figs 2-9 View FIG View FIG View FIG View FIG View FIG View FIG View FIG View FIG )
Linderiella occidentalis View in CoL – Alonso 1985: 191, 192, fig. 5.
“Spanish species of Linderiella View in CoL ” – Thiéry & Champeau 1988: 70, 75, 77. — Thiéry & Fugate 1994: 654.
Linderiella View in CoL sp. – Alonso 1996: 55, 56, figs 21, 22.
MATERIAL EXAMINED. — Puerto Real, Cádiz, Charco Carretones, II.2007, holotype mature ♂ 6 mm in length, preserved in 4% formalin with glycerol ( MNCN 20.04/7963).
Allotype: same data as holotype, mature ♀ 7.5 mm in length, preserved in 4% formalin with glycerol ( MNCN 20.04 View Materials /7964).
Paratypes: same data as holotype, 1 mature ♀ paratype 7 mm, 1 mature ♂ paratype 5.5 mm (MNHN-Bp817) ; 5 mature ♂♂ and 5 mature ♀♀ preserved in 4% formalin with glycerol ( MNCN 20.04 View Materials /7965) ; 20 mature ♂♂ and 20 mature ♀♀ preserved in 4% formalin, and 10 mature males and females preserved in 100% ethanol (UCA-0023-00).
ETYMOLOGY. — The name of this species corresponds to “ Hispania Baetica ”, the name of the old Roman province comprising the south of the Iberian Peninsula (present Andalusia), where it was discovered.
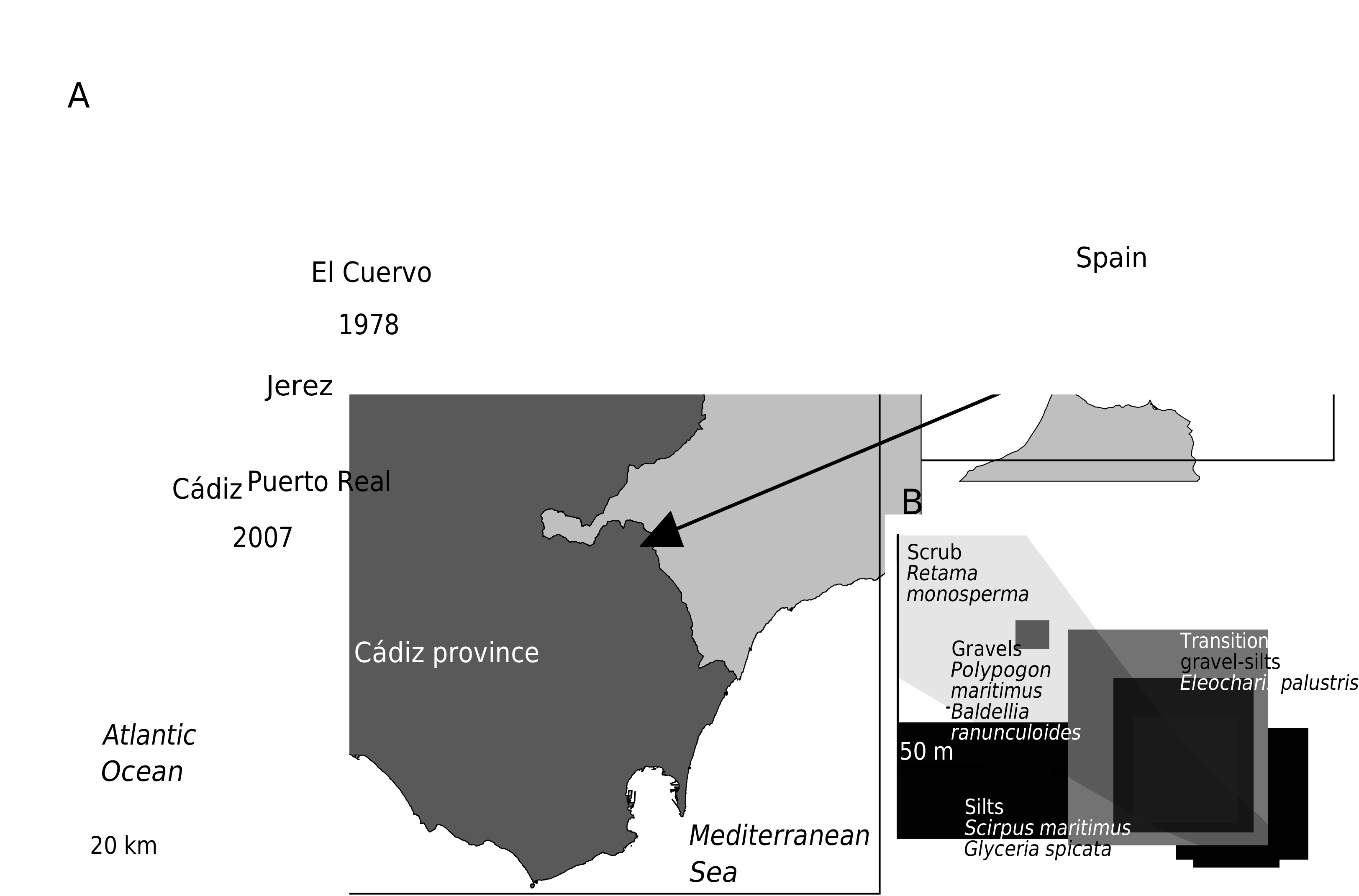
HABITAT. — Charco Carretones in Puerto Real, Cádiz (36°32’N; 6°11’W; elevation = 9 m), is a shallow temporary water body with a surface of about 0.8 ha and a maximum depth of 0.4 m surrounded by bush ( Retama monosperma (L.) Boiss.). Flooding of this pond is directly dependent on the rainfall in autumn and winter. The water body lies within an abandoned quarry with bedrock composed of silty soils in the deepest area and with gravel around the margins ( Fig. 1B View FIG ). During the last decade, this pond was filled for 3-6 months in four winters between 1996 and 2007, coinciding with exceptional high autumn or winter rainfall. Water mineralization ranged from 300 to 600 µS· cm-1, water colour is slightly yellow and turbidity is low (subjective visual appreciation). Macrophytic vegetation and accompanying aquatic fauna is summarized in Table 1.
GEOGRAPHIC DISTRIBUTION. — Up to now the species has been found only in Cádiz, the southernmost Spanish province.
DESCRIPTION
Male
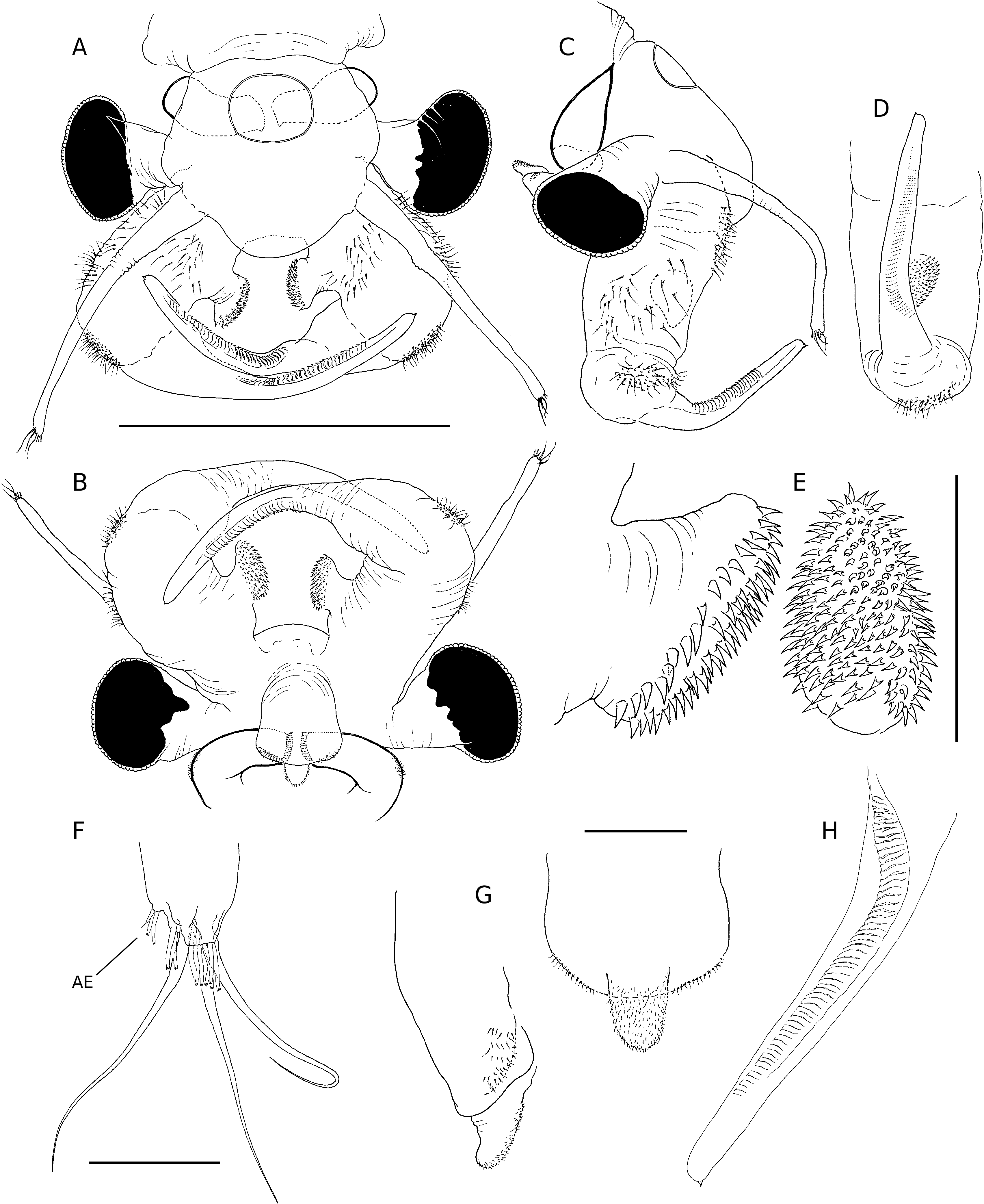
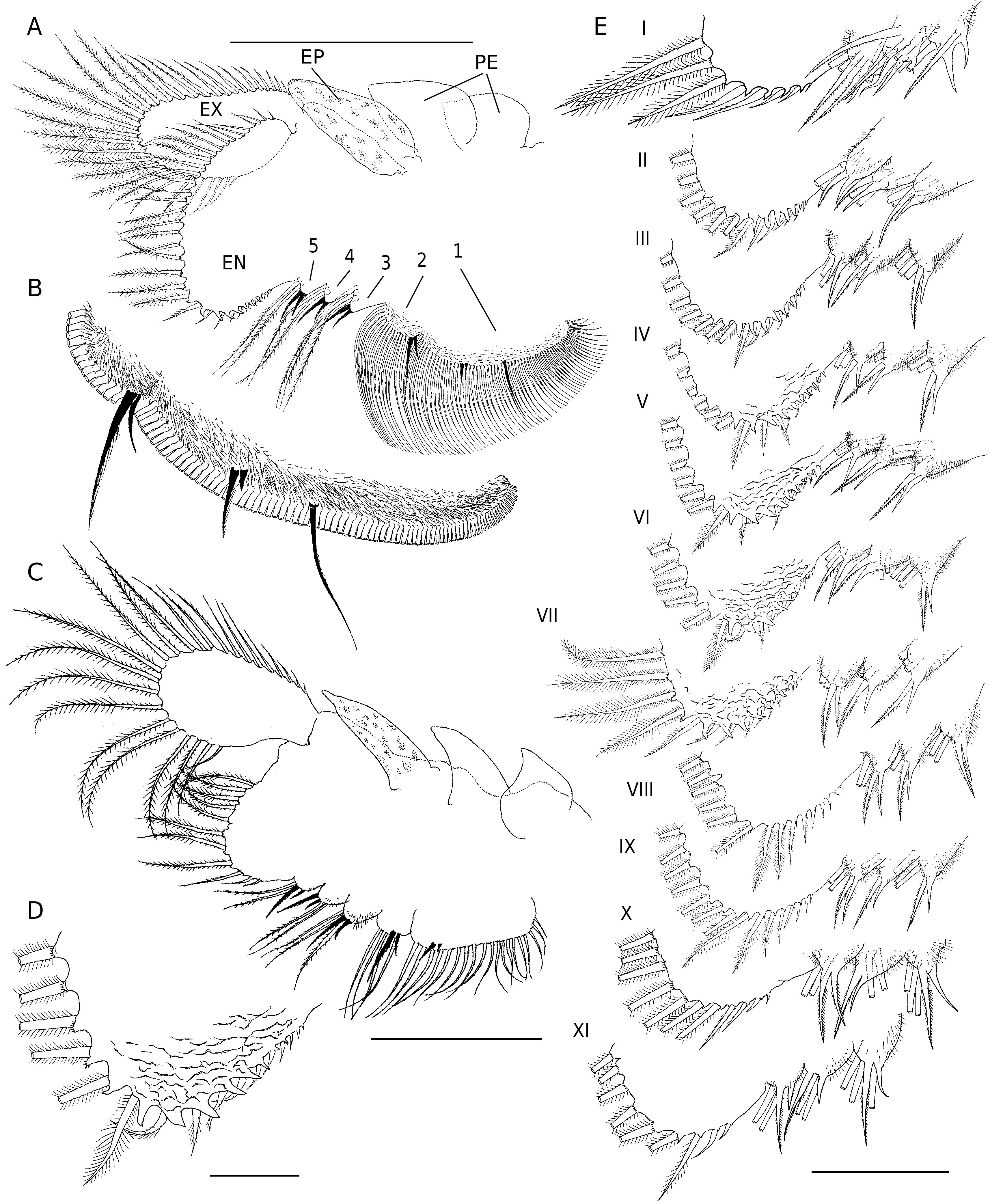
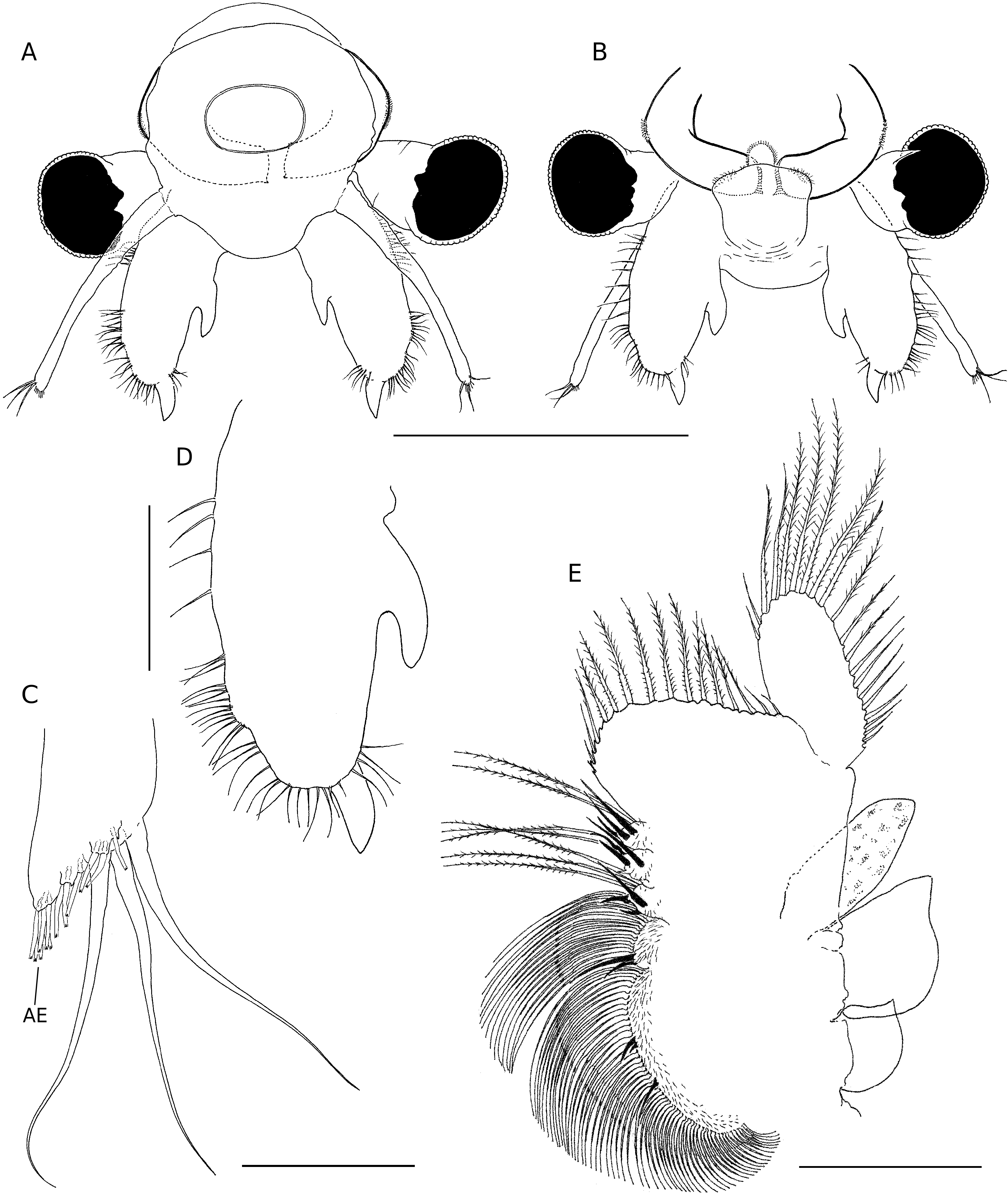
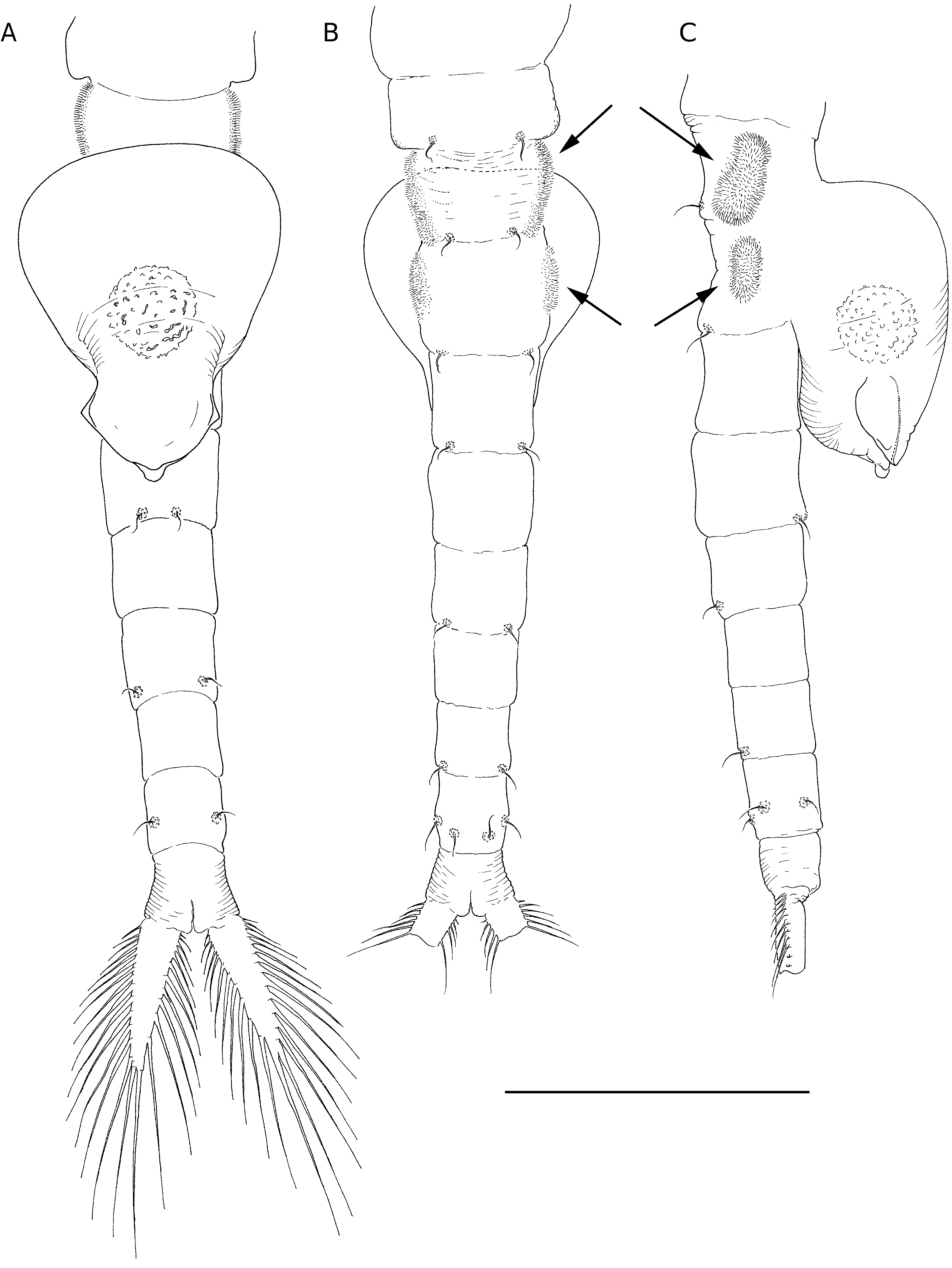
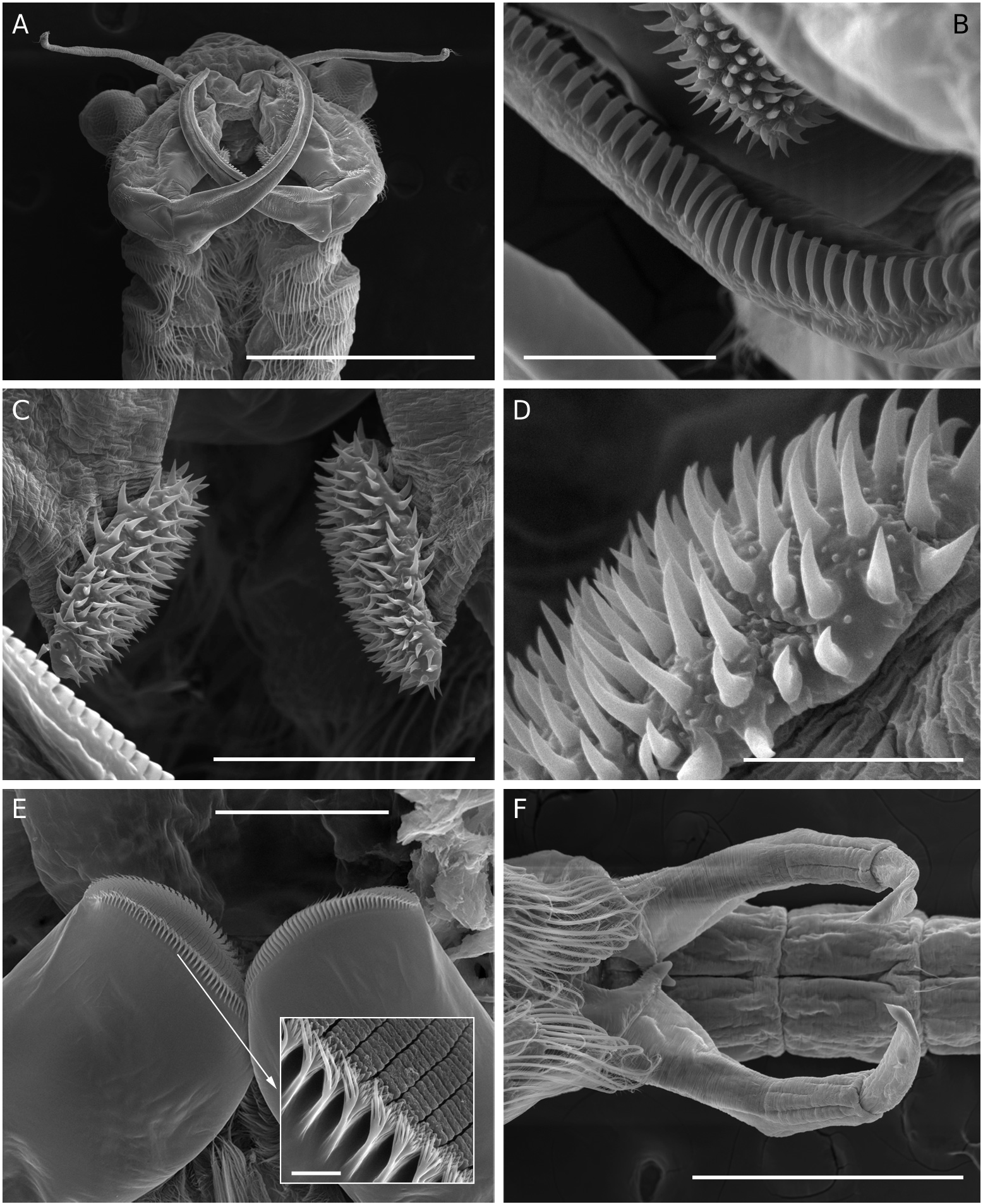
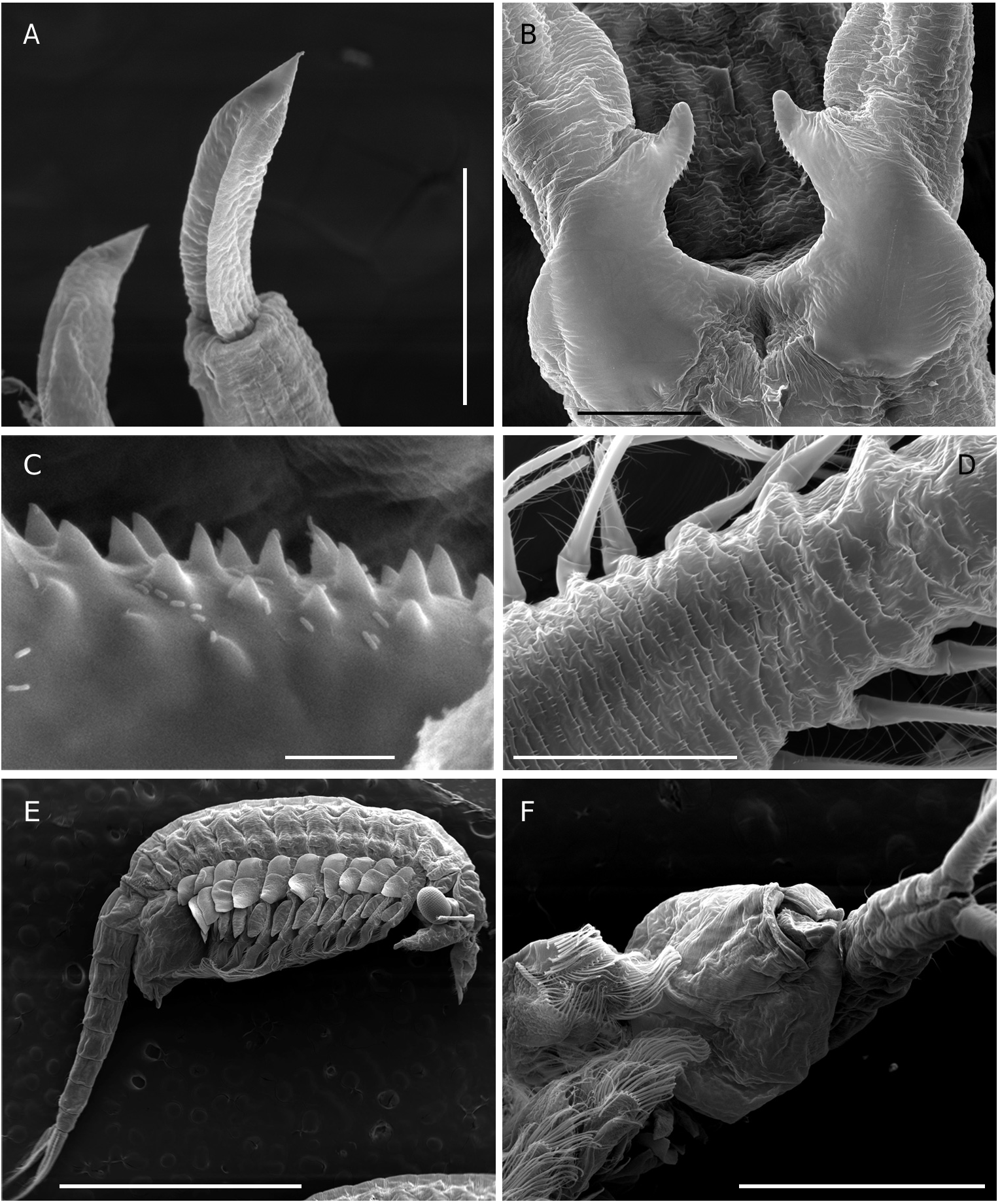
Total length of mature individuals (cercopod setae included) 5.2-7.8 mm (average = 6.6 mm, n = 30). Body unpigmented or slightly red. Head rounded with elliptic nuchal organ ( Fig. 2A View FIG ). Antennule longer than proximal segment of antenna with three long subdistal setae and 12 aesthetascs ( Fig. 2A, B, F View FIG ). Antenna ( Figs 2 View FIG A-D; 7A) two-segmented, proximal segment soft, corrugated, with patches of microtuberculate integument and sensillae distributed on dorsal and dorsolateral surface; basomedial sub-elliptical outgrowth with free face covered with smooth, slightly curved short spinules ( Figs 2E View FIG ; 7C, D View FIG ). Distal segment longer than proximal counterpart, slender, slightly curved inward and tapering distally, with row of transverse ridges on inner surface ( Figs 2 View FIG A-D; 8B); distinctive acute small tooth terminally on segment. Labrum ( Fig. 2G View FIG ) subquadrangular lacking distal protuberances and with setulose lateral margins; short, fleshy setulose tongue-like process subdistally on posterior surface. Mandibles as figured in Figure 7E View FIG . Phyllopodia with gross structure typical for genus ( Figs 3A View FIG ; 5E View FIG ). Praepipod (PE) subdivided into two leaf-like acuminated portions with smooth margins. Epipod (EP) with smooth margin and blunt end except on 11th thoracic limb where it is acute ( Fig. 3C View FIG ). Exopod (EX) broad, provided with short proximal spine-like setae on outer margin; pectinate scales present basally to feathered setae. Endopod (EN) expanded distally; distinct acute projections at base of proximal marginal setae ( Fig. 3 View FIG : I-VII), reaching maximum size at fifth to seventh limbs; integument on medial portion of endopod of latter limbs wrinkle-patterned. First endite of first 10 thoracic limbs with three submarginal setae on anterior surface, two most distal reduced and spinelike, placed close to each other ( Fig. 3B View FIG ). Eleventh thoracic limb lacking of proximal seta on anterior surface ( Fig. 3C View FIG ). Second endite ( Fig. 3B View FIG ) with two unequal submarginal setae on anterior surface close to proximal angle. Third to fifth endites of second to 11th thoracic limbs ( Fig. 3 View FIG : II-XI) each with two short anterior setae and 3, 2, 2 long, plumose posterior setae respectively; endites of first thoracic limb with 3, 5 and 3 anterior setae. Posterior setae as in other limbs ( Figs 3I View FIG ; 5E View FIG ). Genital somites (= abdominal somites 1-2; Fig. 4A, B View FIG ) swollen and partially fused; posterior somite with posterodorsal pair of warty outgrowths each provided with sensilla; non-retractile portion of penis elongated and cylindrical, extending to fourth abdominal somite ( Figs 4C View FIG ; 7F View FIG ), each bearing medio-distal process with medial surface covered with short curved denticles ( Figs 4F View FIG ; 8A View FIG ). Eversible part of penis extending to fifth abdominal somite, conical with lateral integument wrinkle-patterned and with spine-like apex ( Figs 4E View FIG ; 8C View FIG ). Post-genital somites each with a pair of posteroventral warty outgrowths with sensillae ( Fig. 4 View FIG A-C); third, fifth, seventh and eighth abdominal somites each with an additional pair of posterodorsal outgrowths, one pair laterally on eighth somite. Cercopods ( Fig. 4A, D View FIG ) twice length of telson and five times longer than their base width. Surface of cercopods covered with tiny pectinate scales ( Fig. 8D View FIG ). Setae at tip of cercopods implanted as in Figure 4D View FIG , longest being longer than the cercopod itself.
Female ( Fig. 8E View FIG )
Total length (cercopods setae included) 6.4-8.3 mm (average = 7.3 mm, n = 30); mature females (length = 7.3 ±0.5, n=30)were significantly longer (P<0.0001, t-test) than mature males (6.6 ± 0.5, n = 30). Body unpigmented or with dorsal part slightly tinged in blue. Antenna ( Figs 5A, B, D View FIG ; 9A, B View FIG ) slightly shorter than antennule; medial side of proximal segment bearing basal, distally directed horn-like blunt process; lateral margin of segment with several patches of microtuberculate integument, and with sparsely set sensillae distributed as figured. Distal segment of antenna reduced, tongue-like, tapering. Thoracic limbs with acute projections at base of proximal marginal setae of endopod absent or weakly developed. Epipodite wider than in male. Genital somites (= abdominal somites1-2) completely fused,but retaining respective dorsolateral warty outgrowths ( Fig. 6B View FIG ). Lateral sides of each somite displaying field of tiny hair-like setules ( Figs 6B, C View FIG ; 9C, D View FIG ). Brood pouch globose ( Fig. 6 View FIG ) extending to middle of fourth abdominal somite,bright blue in living specimens.Anterior part of brood pouch sub-spherical; posterior third laterally constricted but not depressed. Pore opening subdistally with ventral lip provided with pointed lateral expansions ( Fig.6A View FIG ). Resting eggs 259-318 µm in diameter (average = 290 ± 16 µm, n = 20); egg shell covered with numerous trumpet-shaped spines 26-33 µm long (average = 30 ± 2 µm, n = 20) combined with few acute spines ( Fig. 9E, F View FIG ). A maximum number of 14- 18 eggs per brood pouch was observed.
SPECIES ECOLOGY
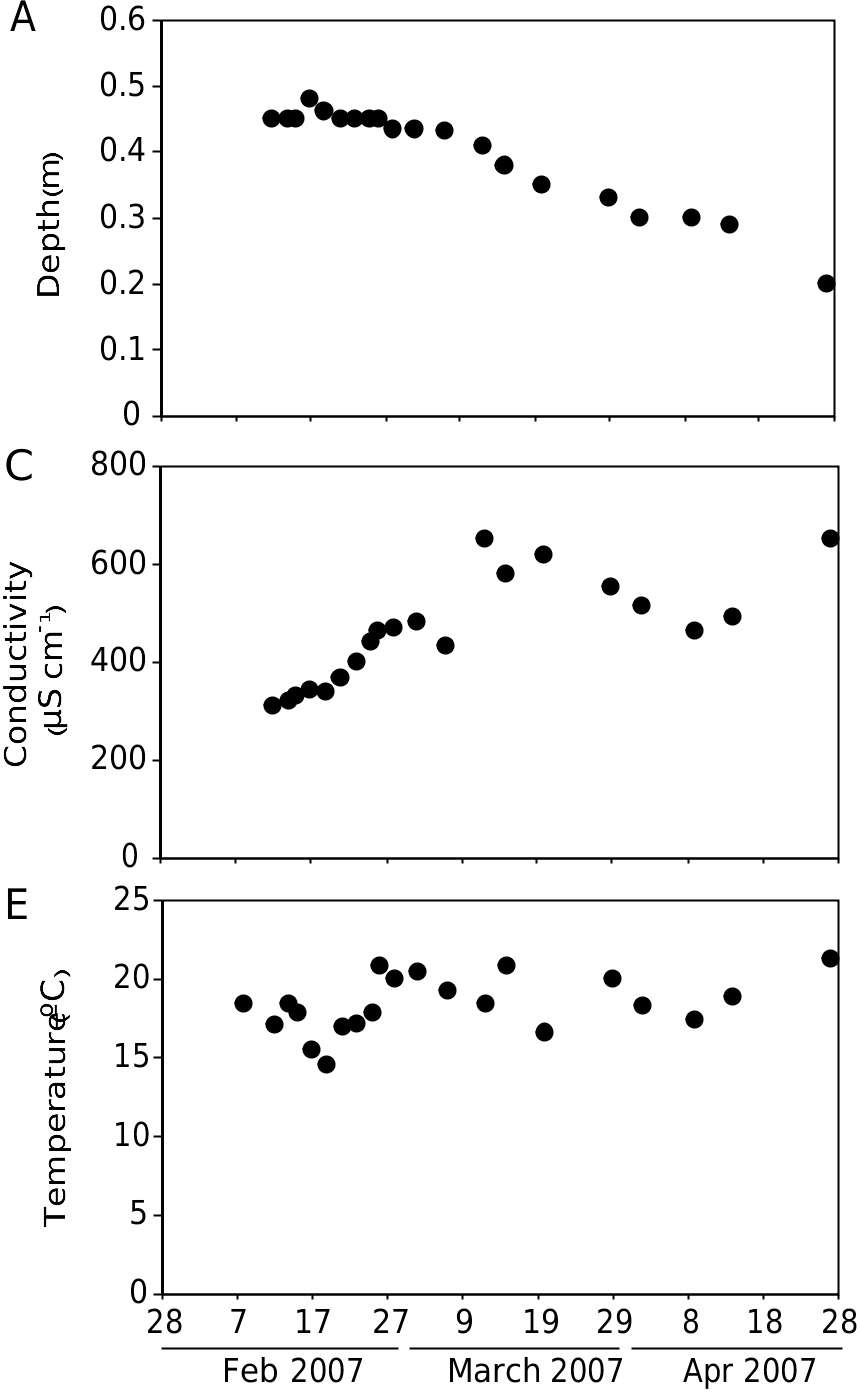
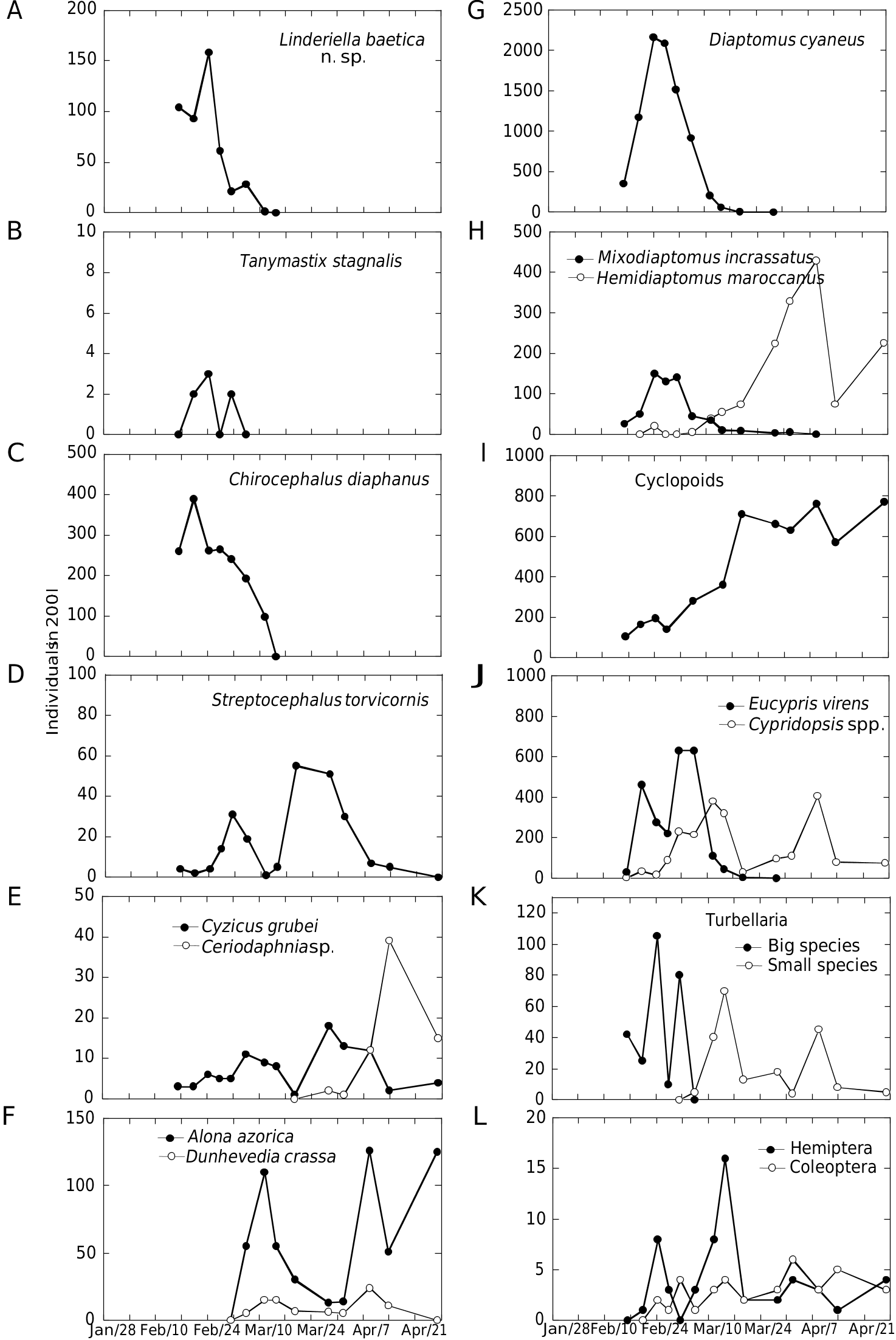
Linderiella baetica n. sp. colonizes a shallow (maximum depth = 0.5 m, Fig. 10A View FIG ), episodic pond. During the last decade, this pond was filled for c. 3-6 months in the winters 1996-1997, 2002- 2003, 2003-2004, and 2006-2007, coinciding with exceptionally high autumn or winter rainfall ( Fig. 10B View FIG ). Maximum densities of Linderiella baetica n. sp. occurred within the first stage of flooding (12 February-12 March 2007) ( Fig. 11A View FIG ), coinciding with minimum conductivities (mean ± SD = 391 ± 63 µS·cm-1, n =12), and the lowest dissolved oxygen concentrations (59 ± 15%, n = 12) ( Fig. 10C, D View FIG ). Temperature was quite constant during the whole sampling period (18 ± 2°C) ( Fig. 10E View FIG ), whereas pH varied between 7.1-7.8 ( Fig. 10F View FIG ). Linderiella baetica n. sp. coincided with other Anostracan species such as Tanymastix stagnalis Linnaeus, 1758 ( Fig. 11B View FIG ) and Chirocephalus diaphanus Desmarest, 1823 ( Fig. 11C View FIG ), Streptocephalus torvicornis Waga, 1842 ( Fig.11D View FIG ), two calanoid copepods ( Diaptomus cyaneus Gurney, 1909 and Hemidiaptomus maroccanus Kiefer, 1954, Fig. 11G, H View FIG ), one ostracod ( Eucypris virens Jurine, 1820 , Fig. 11J View FIG ) and large (length = 2.9 ± 0.9 mm, n = 35) (undetermined) turbellaria species ( Fig. 11K View FIG ). The decline of L. baetica n. sp. coincided with a clear change in the invertebrate community composition. Small chydorid species ( Alona azorica Frenzel & Alonso, 1988 and Dunhevedia crassa King, 1853 ) and other calanoid copepod ( Mixodiaptomus incrassatus Sars, 1903 ) became dominant ( Fig. 11F, H View FIG ). Small ostracoda species like Cypridopsis hartwigi Müller, 1900 and C. parva Müller, 1900 replaced Eucypris virens ( Fig. 11J View FIG ) and even small turbellaria (length = 0.64 ± 0.09 mm, n = 18) substituted the larger species found during the initial stages ( Fig. 11K View FIG ). At the end of the flooding period, Ceriodaphnia sp. (undescribed species of Ceriodaphnia ) became dominant ( Fig. 11E View FIG ).
Assuming that the resting cysts hatched soon after the flooding of the studied pool (at the end of January), the life span of L. baetica n. sp. was estimated in c. 1.5-2 months. The ratio male/ female in L. baetica n. sp. varied between 2.0- 7.7 (within the period 17 February-3 March), but decreased up to 0.27 on 7 March, coinciding with the population decline. Sexual dimorphism in size for Linderiella baetica n. sp. was found: mature females (length = 7.3 ± 0.5, n = 30) were significantly longer (P <0.0001, t-test) than mature males (6.6 ± 0.5, n = 30).
REMARKS
Linderiella View in CoL was already considered by Thiéry & Champeau (1988) as a genus very uniform morphologically. All species share the basic structure of male and female antennae, male and female genitalia, phillopodia and spiny-patterned resting eggs shell surface.The characteristics used to separate Linderiella baetica View in CoL n. sp. from its congeners are not exclusive but displayed in a unique combination in this species. Only its resting eggs morphology is unique within the Anostraca View in CoL . The here so-called trumpet-shaped spines were already considered as a diagnostic character ( Thiéry & Champeau 1988; Thiéry & Fugate 1994; Alonso 1996) for this species before the present description, since resting egg morphology is a valuable taxonomical character for anostracans ( Thiéry & Gasc 1991; Mura 1991, 1992a, b). Resting eggs of L. santarosae View in CoL resemble those of L. baetica View in CoL n. sp. in having distally expanded spines, but they end in several acute cusps (tulip-shaped) whereas have coronate flattened rim (trumpet-shaped) in L. baetica View in CoL n. sp. Also, spine density in L. santarosae View in CoL (15-44 per 0,1 mm2) is higher than in L. baetica View in CoL n. sp. (10 per 0,1 mm2). Combination of flat top and acute spines also has been described for L. occidentalis ( Thiéry & Fugate 1994) View in CoL although in L. baetica View in CoL n. sp. the proportion of trumpet-shaped spines is significantly higher. The lateral fields of hair-like setulae in female genital somites are an exclusive characteristic of L. africana View in CoL , L. massaliensis View in CoL and L. baetica View in CoL n. sp. among anostracans. These hairy surfaces likely facilitate the clutching of the female by the male antennae, as occurs with similar structures in other anostracans, namely the pre-genital dorsal outgrowths in some Galaziella Naganawa & Orgiljanova, 2000 species ( Naganawa & Zagas 2003) or the expanded and warty pre-genital somites in Parartemia Sayce, 1903 View in CoL . The proximal medial margin of the endopod of the phyllopodia is frequently reinforced by short robust spine-like marginal setae (i.e. Branchinecta Verrill, 1869 View in CoL , Branchipus Schaeffer, 1766 View in CoL , Tanymastix Simon, 1886 , Branchinella Sayce, 1903 View in CoL ) or integument acute projections in the base of marginal setae ( Chirocephalus Prévost, 1820 View in CoL , Linderiella Brtek, 1964 View in CoL ). Such projections are well developed in the Mediterranean species L. massaliensis View in CoL , L. baetica View in CoL n. sp. and L. africana View in CoL . In North American species these acute projections are lacking or are weakly developed. The aforementioned and other differential characteristics are summarized in Table 2.
Following this differential diagnosis, a dichotomous key to Linderiella View in CoL species is proposed. Considering that only one sex may be catched during samplings, dichotomous keys for males, females and resting eggs are proposed.
KEY TO THE MALES OF LINDERIELLA BRTEK, 1964 View in CoL 1. Outgrowth on inner side of basal segment of antenna with smooth elongated slender tip ............................................................................................................. L. santarosae View in CoL — Outgrowth on inner side of basal segment of antenna without such distal extension ... 2
2. Outgrowth on inner side of basal segment of antenna acute triangular-shaped ..............
............................................................................................................... L. occidentalis . — Outgrowth on inner side of basal segment of antenna sub-ellipsoidal or subcircular ... 3
3. Margin of praeepipods serrate ................................................................ L. massaliensis — Margin of praeepipods smooth ................................................................................... 4
4. Penis basal projections sharp, with small tubercles ....................................... L. africana — Penis basal projections blunt, with many denticles ............................... L. baetica n. sp.
KEY TO THE FEMALES OF LINDERIELLA BRTEK, 1964 View in CoL
1. Female genital somites displaying lateral fields of tiny hair-like setules ........................ 2
— Female genital somites smooth .................................................................................... 4
2. Pointed lateral expansions in ventral lip of brood pouch pore opening ... L. baetica n. sp. — Ventral lip of brood pouch without such expansions ................................................... 3
3. Inner horn-like process on basal segment of antenna very short and sharp ... L. africana — Inner horn-like process on basal segment of antenna long sharp .............. L massaliensis
4. Inner horn-like process on basal segment of antenna short blunt ................ L santarosae — Inner horn-like process on basal segment of antenna long sharp .............. L. occidentalis
KEY TO THE RESTING EGGS OF LINDERIELLA BRTEK, 1964 View in CoL
1. Acute resting egg spines .............................................................................................. 2
— Coronate resting egg spines ........................................................................................ 4
2. Short (14-23 µm long) twisted resting egg spines ................................... L. massaliensis — Longer straight conical resting egg spines ................................................................... 3
3. Long spines (average longitude: 38 µm) ..................................................... L. africana — Shorter spines (27-33 µm) ....................................................................... L. occidentalis
4. Tulip-shaped spines ................................................................................... L. santarosae — Trumpet-shaped spines ........................................................................ L.. baetica n. sp.
| MNCN |
Museo Nacional de Ciencias Naturales |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Linderiella baetica
| Alonso, Miguel & Garcia-De-Lomas, Juan 2009 |
Linderiella
| ALONSO M. 1996: 55 |
Linderiella occidentalis
| ALONSO M. 1985: 191 |