Leydigia (Neoleydigia) lourdesae, Kotov, Alexey A. & Fuentes-Reinés, Juan Manuel, 2014
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3814.3.7 |
|
publication LSID |
lsid:zoobank.org:pub:CA85230A-29A1-4687-AF97-39EFA9E6E5A6 |
|
DOI |
https://doi.org/10.5281/zenodo.5661774 |
|
persistent identifier |
https://treatment.plazi.org/id/C062527E-D661-FFDD-FF46-A6A5FECDFD40 |
|
treatment provided by |
Plazi |
|
scientific name |
Leydigia (Neoleydigia) lourdesae |
| status |
sp. nov. |
Leydigia (Neoleydigia) lourdesae sp. nov.
( Figs. 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 )
Etymology. The species is named in honor of Dr Lourdes Maria Elmoor-Loureiro, renowned investigator of the Cladocera , a leader of a new generation of the South American cladocerologists.
Type locality. Ciénaga Grande de Santa Marta, Fauna and Flora Sanctuary, Puebloviejo, Magdalena Department, Colombia (10º52'11.25''N, 74º19'31.64''W). The type series contains only three females, which were collected on March and November of 2009 by J. M. Fuentes Reinés.
Type material. Holotype. Parthenogenetic female deposited at the Museo de Colecciones Biológicas de la Universidad del Atlántico – Colombia, UARC 172M.
Paratypes. Undisected female preserved in alcohol ( UARC 173M), and dissected female ( UARC 174M- 178M).
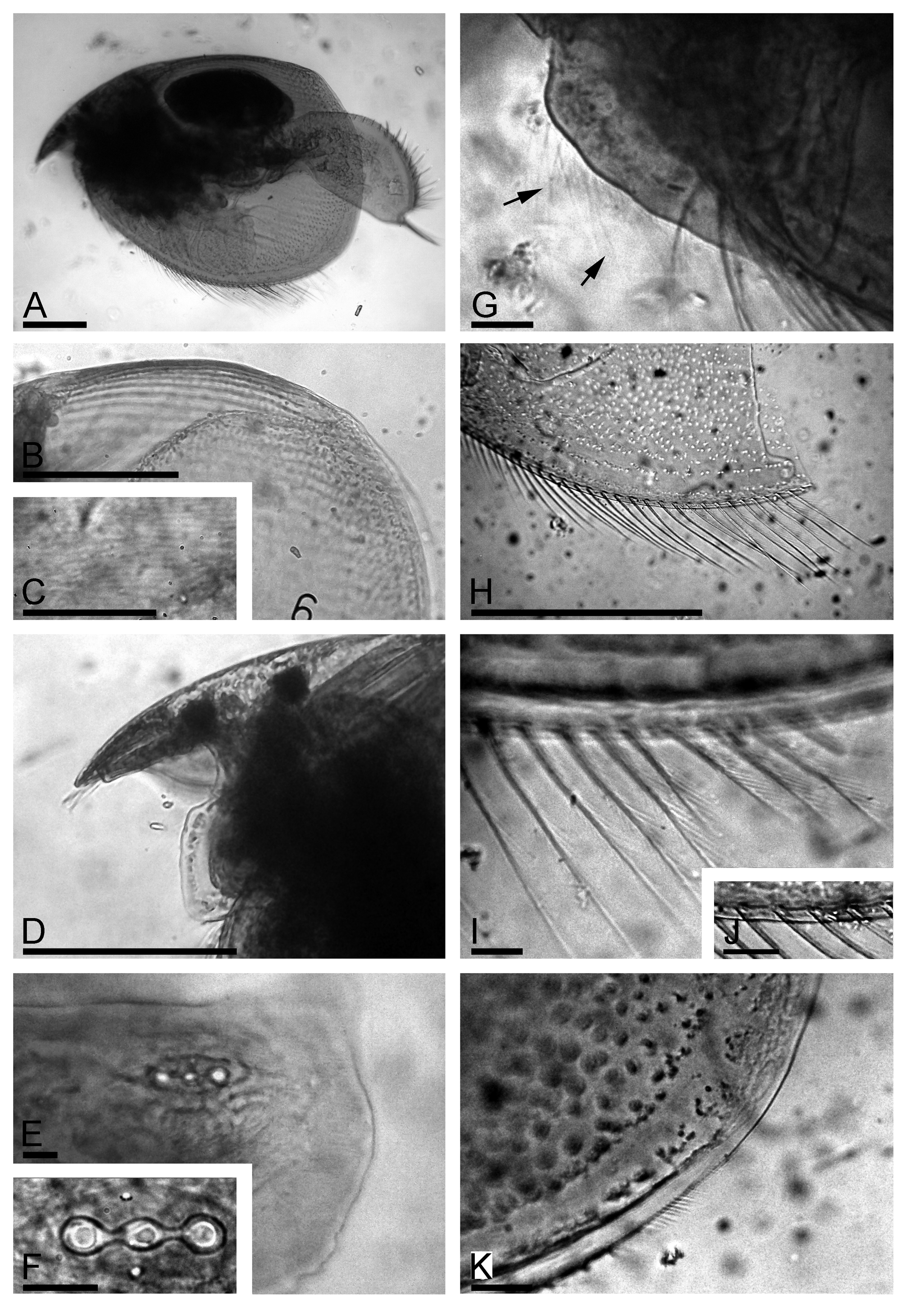
Description. Parthenogenetic female. General. In lateral view, body triangular-ovoid, maximum height in posterior half, height/length = 0.66–0.71 ( Fig. 1 View FIGURE 1 A). Dorsal margin slightly and regularly convex, postero-dorsal angle rounded although expressed. Postero-ventral angle broadly rounded; ventral margin regularly convex. Coarse striation on dorsal and posterior portions of valves ( Fig. 1 View FIGURE 1 B), valves and head shield with a well-expressed fine striation within the lines of coarse reticulation ( Fig. 1 View FIGURE 1 C).
Head relatively small, triangle-round in lateral view, with a relatively short, downward pointing rostrum ( Fig. 1 View FIGURE 1 D). Compound eye and ocellus subequal in size, distance from tip of rostrum to ocellus somewhat larger than between ocellus and eye. Head shield wide, in posterior portion three closely located major head pores of subequal size, with connection between them, post-pore distance about 3–4 inter-pore distance ( Fig. 1 View FIGURE 1 E). Lateral head pores about 0.4 inter-pore distance from midline, slightly anterior to level of central major head pore, all head pores located on a plate without striation ( Fig. 1 View FIGURE 1 E–F). Labrum with large medial labral keel, triangular-ovoid, with welldefined apex; posterior margin straight, anterior margin undulated, with a fringe of long setules from base to apex ( Fig. 1 View FIGURE 1 G, arrows); on sides of keel there are few lateral groups of long setules.
Valves large, subovoid, with numerous setae on ventral margin, longest in posterior half ( Fig. 1 View FIGURE 1 H). In middle of margin bases located slightly submarginally, no setules between them were seen ( Fig. 1 View FIGURE 1 H–J). Posteriorly to the last marginal seta, a row of submarginal setules starts on inner valve face of valve posterior margin ( Fig. 1 View FIGURE 1 K). Marginal membrane (see Kotov 2009) not found.
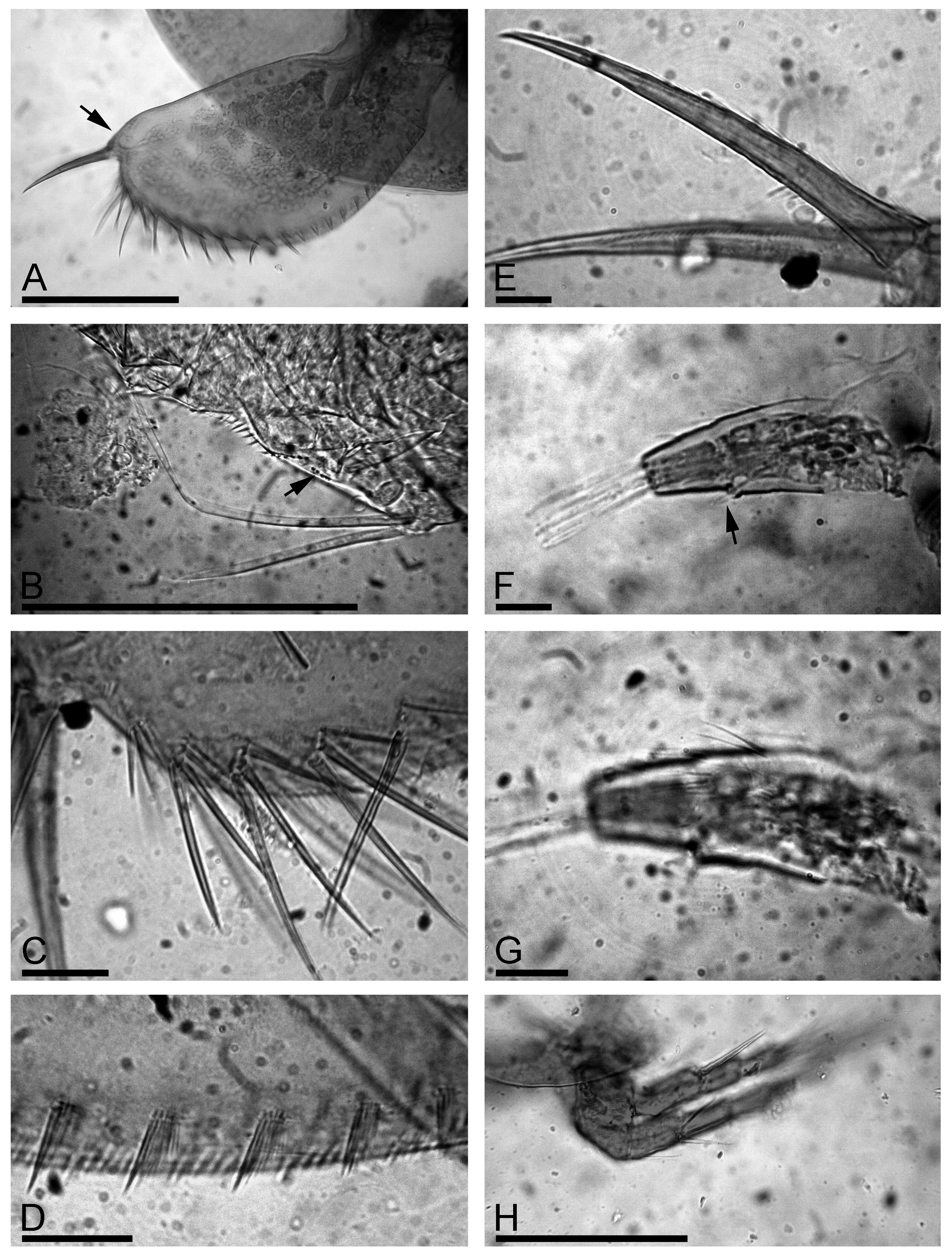
Postabdomen robust, with maximum height in middle ( Fig. 2 View FIGURE 2 A). Ventral margin straight, but its distal portion sloping towards the postabdominal claw ( Fig. 2 View FIGURE 2 A, arrow). Preanal margin ( Fig. 2 View FIGURE 2 B, arrow) somewhat shorter than anus, somewhat undulated, but lacking distinct hillocks; preanal and postanal angles expressed, dorso-distal angle of postabdomen smooth, distal margin well-expressed. Postanal marginal denticles in numerous clusters. On postanal margin, about 9 or 10 fascicles of stout lateral setae, 3 or 4 setae in each fascicle on distal portion, size of setae regularly decreasing from marginalmost to medialmost ones ( Fig. 2 View FIGURE 2 C). Numerous fascicles of lateral setules on basal half of postanal and anal margin ( Fig. 2 View FIGURE 2 D). Postabdominal seta ( Fig. 2 View FIGURE 2 B) about two times longer than anal plus preanal margin; its distal segment somewhat shorter than basal one. Postabdominal claw almost straight, approximately as long as preanal plus anal portion of postabdomen. On lateral side, two successive series of slender setules along the dorsal margin. Basal spine fully absent ( Fig. 2 View FIGURE 2 E).
Antenna I not reaching tip of rostrum, with 4 transverse rows of long setules on anterior face ( Fig. 2 View FIGURE 2 F–G). Sensory seta ( Fig. 2 View FIGURE 2 F, arrow) slender, arising at distance of about 1/3 of appendage length from distal end. Nine aesthetascs of slightly varied size, largest more than half as long as appendage, reaching tip of rostrum.
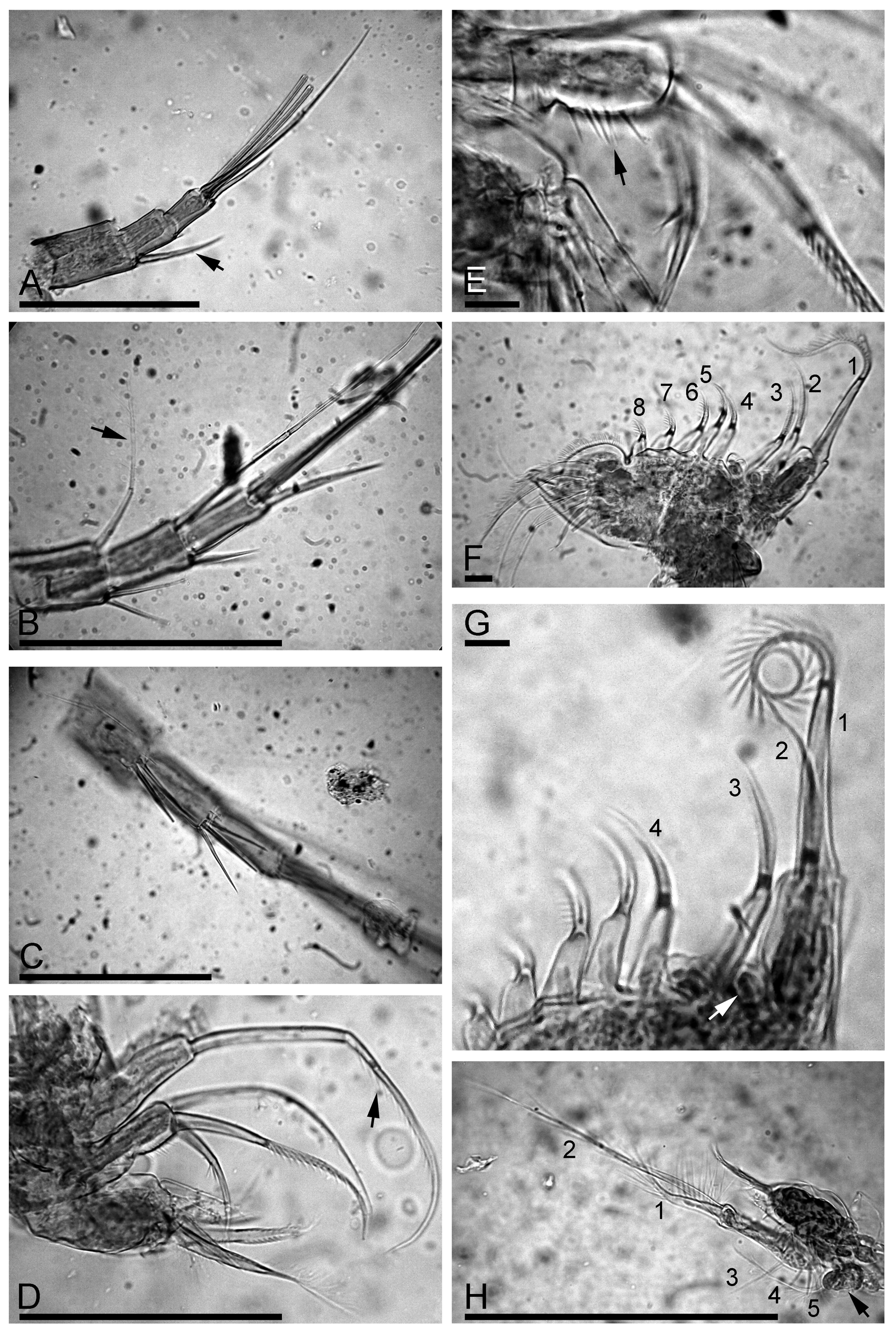
Antenna II relatively short, antennal branches elongated ( Fig. 2 View FIGURE 2 H). Antennal formula, setae 0–0–3/1–1–3, spines 1–0–1/0–0–1 (see discussion of true spines and spine-like setules by Kotov (2009)). On both exopod and endopod three long apical swimming setae different in size ( Fig. 3 View FIGURE 3 A), distal lateral seta shorter than apical setae, proximal lateral seta reaching distal end of exopod ( Fig. 3 View FIGURE 3 B, arrow). Spine on proximal segment of exopod very long, projecting beyond middle of apical segment ( Fig. 3 View FIGURE 3 A, arrow); 2–3 very long (almost reaching distal end of segment) and stout spine-like setules on first and second endopod segment ( Fig. 3 View FIGURE 3 C). Apical spines of exopod and endopod of subequal length, obviously longer than apical segments.
Trunk limb I typical for the genus, without accessory seta, outer distal lobe large, elongate, somewhat narrowing distally, bears a long seta with distal segment unilaterally armed with strong setules ( Fig. 3 View FIGURE 3 D, arrow); inner distal lobe with few long and strong marginal setules ( Fig. 3 View FIGURE 3 E, arrow) and three bisegmented setae, different in size but similar in setulation. A maxillar process not found.
Trunk limb II ( Fig. 3 View FIGURE 3 F) with a globular epipodite and small exopodite lacking setae ( Fig. 3 View FIGURE 3 G, arrow). Inner portion of limb with eight scrapers ( Fig. 3 View FIGURE 3 F-G, 1–8). Distalmost scraper 1 very large, with naked basal segment. Few small projections posterior to scrapers 2–4. Distal armature of gnathobase II with four setae; filter plate with seven setae (some of them were occasionally removed during our dissecting of specimen, represented in Fig. 3 View FIGURE 3 F).
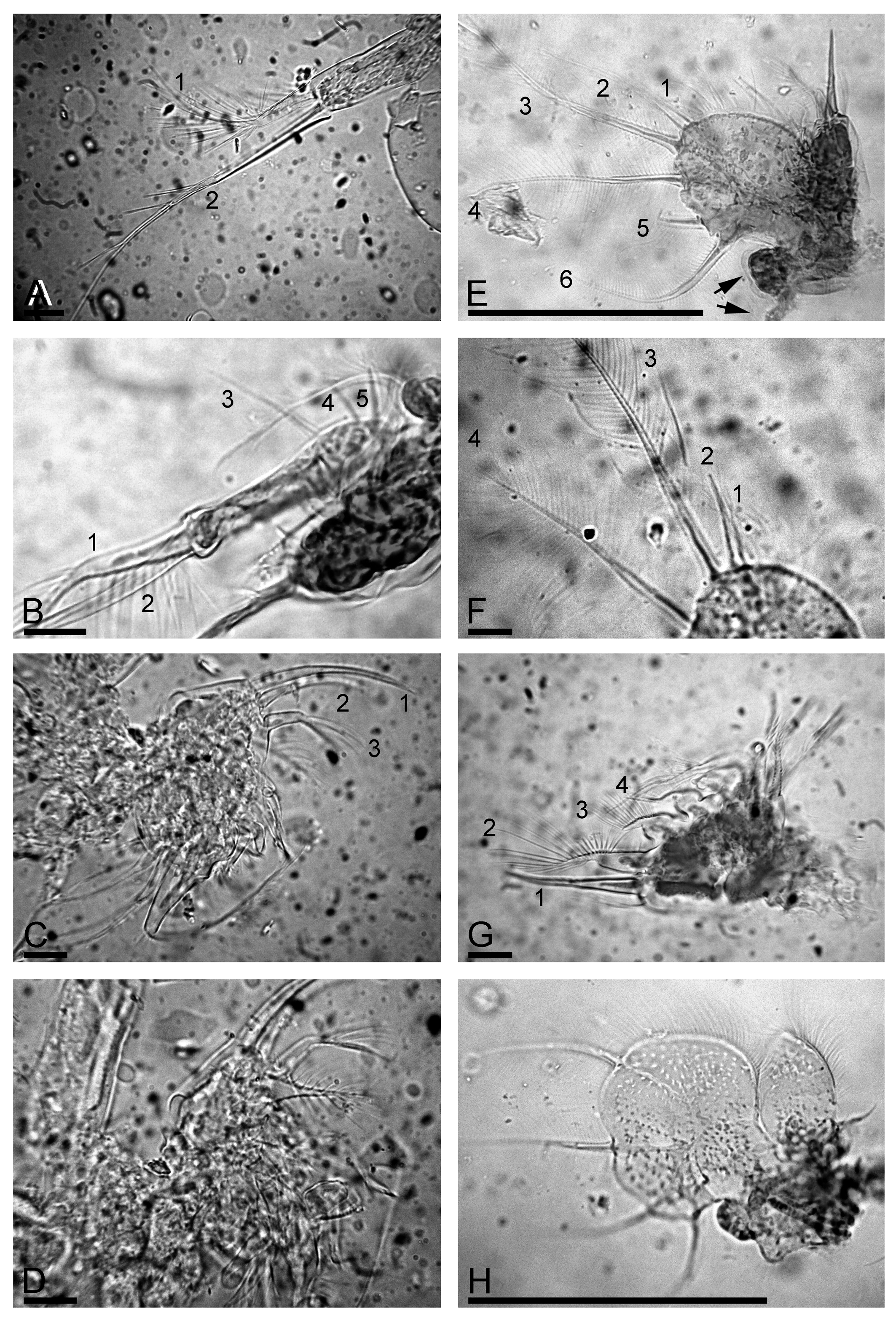
Trunk limb III with sub-globular epipodite ( Fig. 3 View FIGURE 3 H, arrow); exopodite rectangular, elongated, with two distal ( Fig. 3 View FIGURE 3 H, 1–2) and three lateral setae ( Fig. 3 View FIGURE 3 H, 3–5). Seta 1 with proximal segment unilaterally armed by long setules and distal segment bearing long setules, seta 2 specially long, with naked proximal segment and distal segment armed by few long, robust setules ( Fig. 4 View FIGURE 4 A). Size of lateral setae 3–5 regularly decreasing proximally ( Fig. 4 View FIGURE 4 B). Distal endite with three stiff setae ( Fig. 4 View FIGURE 4 C, 1–3), seta 1 specially long and stiff, seta 3 armed distally by longer setules ( Fig. 4 View FIGURE 4 D). The rest of limb as in L. iberica ( Fig. 4 View FIGURE 4 D).
Trunk limb IV with a small pre-epipodite and globular epipodite ( Fig. 4 View FIGURE 4 E, arrows). Exopodite wide, subovoid, with six setae ( Fig. 4 View FIGURE 4 E–F, 1–6), seta 1 shortest, all setae (including 1 and 2) with a similar armature of long, fine setules. Inner portion of limb IV with four marginal setae ( Fig. 4 View FIGURE 4 G, 1–4), seta 1 stout, setae 2–4 with inflated proximal segments and slender, unilaterally setulated distal segments, in seta 2 proximal segment also setulated. Filter plate with five setae, distalmost with inflated basal segment and fully setulated.
Trunk limb V with a setose pre-epipodite, and globular epipodite of similar size ( Fig. 4 View FIGURE 4 H). Exopodite large, with four setae. Inner limb portion as a flat lobe, with setose inner margin. On inner face, two setulated setae of unequal length. Distal armature of gnathobase a setulated lobe, two setae in “filter plate”.
Ephippium, male. Unknown.
Size. Holotype 0.55 mm; parthenogenetic females from type locality 0.49–0.55 mm (n=3).
Differential diagnosis. L. lourdesae sp. nov. is a member of the subgenus Leydigia (Neoleydigia) Kotov, 2009 , having all diagnostic characters of the latter. It could be the closest relative of L. iberica Kotov & Alonso, 2010 , having a unique synapomorphy with the latter, five setae on exopodite III. Differences between two these taxa are listed in Table 1 View TABLE 1 .
Distribution and ecology. The species is known only from a single locality, Ciénaga Grande de Santa Marta ( Colombia). This large (surface area of 450 km 2) lagoon system is a shallow water body (depth 90–110 cm only), with temperature ranging over the season between 25.3–31.1ºC; dissolved oxygen 2.4–7.6 mg /L; salinity 0–0.4 PSU. Submerged and floating aquatic vegetation is abundant, represented by patches of Eichornia crassipes and Nymphaea cf. pulchella surrounding the mouth of Sevilla River. Totally, 45 species of Cladocera were found in this water body ( Fuentes-Reinés et al. 2012; Fuentes-Reinés & Zoppi de Roa 2013).
Leydigia View in CoL is a genus unique for the Aloninae and for all the Cladocera having a number of setae on the exopodite III variable between different species from 3 to 7 ( Kotov 2009). The cause of two presumably related species, L. lourdesae sp. nov. and L. iberica View in CoL , shows that not only number, but also size and armature of the setae on exopodite III is very important for the discrimination of the species in Leydigia View in CoL (see Table 1 View TABLE 1 ). Fryer (1968, P. 369) concluded that the seta 2 on exopodite III "serves as a cleaner of the more posterior exopods". In general, just distalmost portions of the limbs (large seta of outer distal lobe of limb I, marginal setules on inner distal lobe of limb I, seta 1 on limb II, setae 1–2 on exopodite III, seta 1 on inner portion of limb III, setae 1–2 on exopodite IV) — presumably cleaning structures — bear most valuable characters for the Leydigia View in CoL taxonomy. Peculiarities of their morphology could be explained as adaptations to the life in a particular substratum, where the species lives. We need to underline that such "fine" differences in the troracic limbs are more important for the taxonomy of many anomopod groups than, for example, body shape: very variable, ecologically plastic trait. Unfortunately, even recently, many hydrobiologists dealing with the Cladocera ignore their troracic limbs and newer dissect the specimens for their determination.
Differences in shape and spinulation of the postabdomen and size of spines on antenna II could be explained in terms of functional morphology. Postabdomen, the main pushing organ of benthic (and some vegetation-living) taxa of the Anomopoda View in CoL , is traditionally regarded as the main sourse of their diagnostic characters ( Smirnov 1996; Kotov 2009; Van Damme et al. 2011; Van Damme & Sinev 2013). The spines on antenna II are also very characteristic for truly benthic animals ( Fryer 1968; Kotov 2006), and their size has a great significance for the taxonomy of different groups of the Anomopoda View in CoL .
Kotov (2009, P. 53) concluded that within the subgenus Leydigia (Neoleydigia) View in CoL there are (1) a compact acanthocercoides -group, and (2) a series of basal taxa, which are endemics of the Iberian Peninsula, South Africa (especially, Cape Area) and Australia. Subsequently, even two more taxa, endemics of the Iberian Peninsula, were described ( Kotov & Alonso 2010). Now we found one more basal taxon of the Leydigia (Neoleydigia) View in CoL , L. lourdesae sp. nov., and added one more region where such taxa are present. In addition, it is the first record of the basal neoleydigias from the South American continent.
L. lourdesae sp. nov. was found only in a single locality of Colombia in the course of a special program to investigate numerous water bodies in this country. Therefore at this level of study it seems to be a locally distributed species. Also this is a rare species even in its type locality since only 3 specimens were found as a result of several sampling campaigns. Endemism and rarity are characteristic signs of the relict taxa.
Cladoceran taxa of generic rank could be very old, of Mesozoic origin before the disruption of recent continents ( Kotov & Taylor 2011). Korovchinsky (2006) proposed that many cladoceran taxa passed through a mass extinction in Caenozoic due to strong climate changes, first of all, aridization of large territories on the planet. We believe that the basal taxa of L. (Neoleydigia) are remains of an antique pan-continental group, probably of Mesozoic age, partly surviving after the mass extinction and represented now by a series of locally distributed taxa in very distant localities of the planet ( Kotov & Alonso 2010).
L. lourdesae sp. nov. is morphologically similar to L. iberica View in CoL . We cannot understand now, whether L. iberica View in CoL species a is monophyletic group? The final conclusion could be done only as a result of a molecular genetic study of the genus Leydigia View in CoL . But it is a very difficult task as most hydrobiologists deal with the plankton only, and benthic species are collected mainly occasionally. Apparently the hydrobiologists around the world need to pay more attention to benthic cladocerans, which are a source of unrecorded taxa, as it was demonstrated once more in present paper.
TABLE 1. Differences between Leydigia (N.) lourdesae sp. nov. and L. (N.) iberica.
| Character | L. (N.) iberica | L. (N.) lourdesae |
|---|---|---|
| Post-pore distance/inter-pore distance Setules between setae on ventral margin | 7–8 present | 3–4 absent |
| Postabdomen ventral margin | slightly, regularly convex | distal portion sloping towards the postabdominal claw |
| Preanal margin | with distinct hillocks | undulated |
| Antennular sensory seta arises | at 1/5–1/6 of antenna I length | at 1/3 of antenna I length |
| Spine at proximal segment of exopod of antenna II almost reaching tip of exopod | no | yes |
| Spine-like setules on endopod of antenna II projecting beyond middle of next segment | no | yes |
| Marginal setules on inner-distal lobe of limb I | fine and numerous (15–20) | strong, about 5–6 in number |
| Proximal segment of seta 1 on limb II | with long hairs | naked |
| Seta 2 on exopodite III | with fine setules | with few robust setules |
| Setae 3–4 on exopodite III | seta 4 longest | descreasing in size proximally |
| Discussion |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.