Janthina chavani ( Ludbrook, 1978 )
|
publication ID |
https://doi.org/ 10.3853/j.2201-4349.69.2017.1666 |
|
publication LSID |
lsid:zoobank.org:pub:08B086EB-8D24-4FD0-975A-E045E2596BF1 |
|
DOI |
https://doi.org/10.5281/zenodo.7551517 |
|
persistent identifier |
https://treatment.plazi.org/id/03EF87AB-FFC0-FFF4-CED3-FE303804F837 |
|
treatment provided by |
Carolina |
|
scientific name |
Janthina chavani ( Ludbrook, 1978 ) |
| status |
|
Janthina chavani ( Ludbrook, 1978)
Figs 27–28 View Figure 27 View Figure 28
Heligmope postulatus (Bartrum) .– Fleming, 1953b: 139 (misidentification).
Hartungia postulata (Bartrum) .– Carter, 1972: 306, 321 (misidentification).
Hartungia typica typica (Bronn) View in CoL .– Johnstone et al., 1973: 14; Quilty, 1974a: 308; Quilty, 1974b: 29 (misidentification by A. Beu & G. Kendrick pers. comm.).
Hartungia dennanti chavani Ludbrook, 1978: 119 View in CoL , pl. 12, figs 1–14; Ludbrook, 1983: 45, figs 3h‒j; Ludbrook, 1984: 232, figs 57o–p; Kendrick et al., 1991: 424, 436.
Parajanthina japonica Tomida & Itoigawa, 1982: 60 View in CoL , pl. 19, figs 1a–c; Ogasawara, 2002: 545 (in part).
Hartungia japonica (Tomida & Itoigawa) . – Tomida & Itoigawa, 1984: 112, pl. 31, figs 1a–2b; Tomida & Itoigawa, 1989: 126, pl. 23, figs 1a–2d; Noda et al., 1995: 83, figs 11.7a–d; Nobuhara et al., 1995: 39, figs 3.2a–b; Tomida & Kitao, 2002: 158, figs 2.1a–2c; Ogasawara, 2002: 394, 545.
Hartungia chavani (Ludbrook) View in CoL . – Kendrick in Tomida & Itoigawa, 1984: 112, pl. 31, figs 3a–5b; Beu & Maxwell, 1990: 411; Maxwell in Spencer et al., 2009: 245.
Hartungia sp.– Nobuhara et al., 1995: 38, figs 3.1a–b.
Kaneconcha knorri Kaim, Tucholke & Warén, 2012: 247 View in CoL , figs 3A–E.
Janthina (Hartungia) typica (Bronn) . – Tomida et al., 2013: 60, figs 3E–L only (in part misidentified).
Type material. Hartungia dennanti chavani , holotype WAM 69.300c, with six figured and numerous unfigured paratypes in WAM, Geological Survey of Western Australia, and Geological Survey of South Australia (listed by Ludbrook , 1978: 120; WAM and GSSA material observed); from Roe Calcarenite (late Pliocene ; Beu & Darragh , 2001: 31, fig. 6), pits c. 50–125 km W of Eucla , Roe Plain , southeastern Western Australia. As noted above, although Beu & Darragh (2001: fig. 6) suggested an early Piacenzian age, correlation with successions in New Zealand now indicates a late Piacenzian age for Roe Calcarenite. Ludbrook (1978: 120) also recorded specimens from water wells beneath Perth, Western Australia, from Plio-Pleistocene Bridgewater Limestone in the region around Naracoorte, South Australia, and from Reedy Wells, Culburra, South Australia (the last not seen; from Bridgewater Limestone near Mount Gambier).
Parajanthina japonica , holotype MFM 110004 , from Dainichi Sand (late Pliocene, Piacenzian, upper part of planktonic foraminiferal zone N 21), Higashigumi, Iida, Shizuoka Prefecture, Honshu, Japan (Tomida & Itoigawa, 1982: 61); not seen .
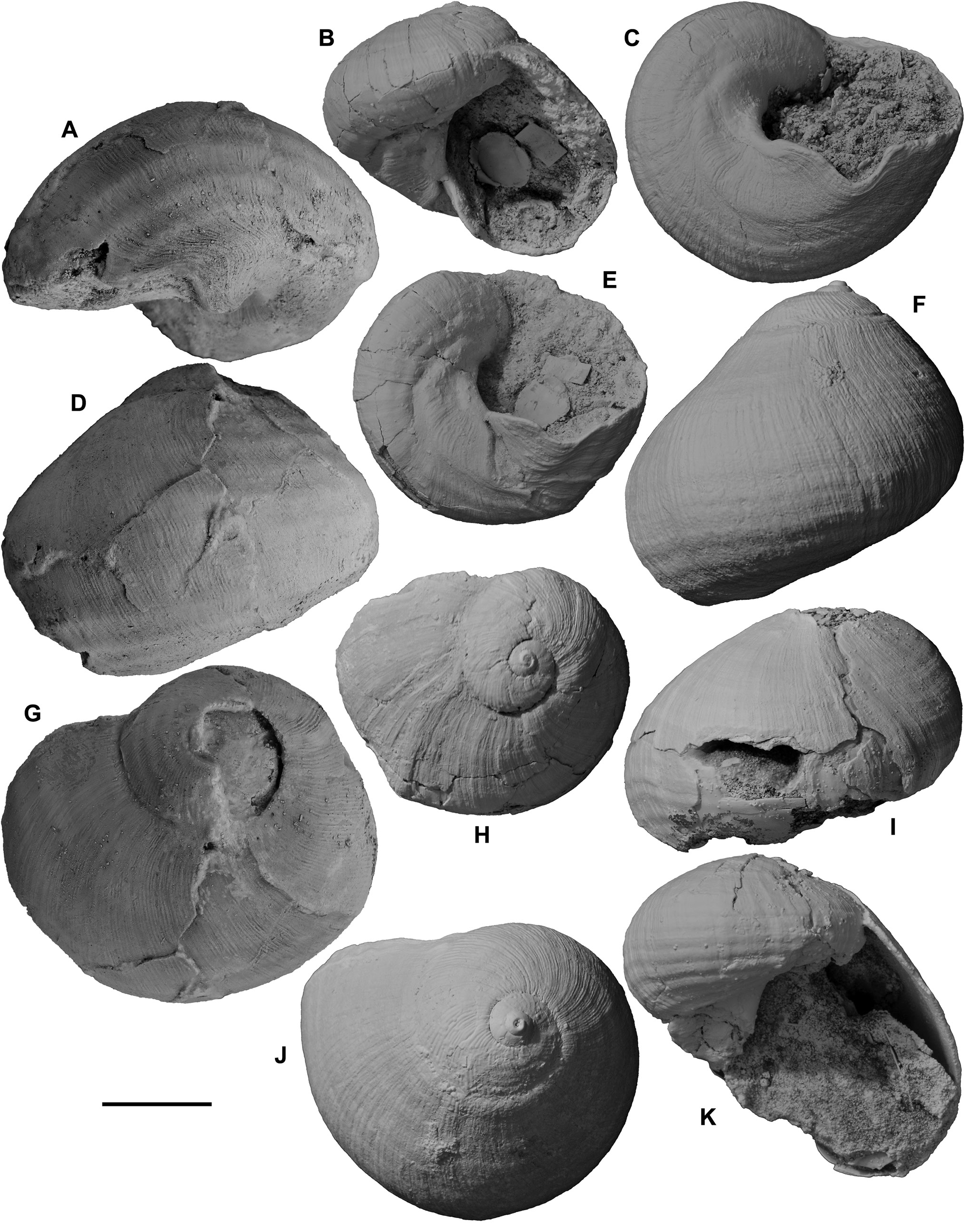
Kaneconcha knorri , holotype ( Figs 28A, D, G View Figure 28 ) in Institute of Paleobiology , PolishAcademy of Sciences , Warsaw , ZPAL Ga.16/1, GoogleMaps with six incomplete paratypes, ZPAL Ga.16/2–7, from Knorr dredge station 180-2-26, 23°23'N 45°23'W, Kane Megamullion , east flank of Adam Dome , mid-Atlantic ridge, 3293–2827 m (mapped by Kaim et al., 2012: fig. 1) GoogleMaps . The writer has seen photographs of only the holotype, which is a slightly crushed specimen of Janthina chavani . Although it has weak spiral folds on the weakly convex sutural ramp, it is identified as J. chavani partly because the matrix was dated by calcareous nannofossils by M.-P. Aubry (the leading expert on nannofossil biostratigraphy; in Kaim et al., 2012) as zone NN16B, 2.5–2.8 Ma (late Piacenzian–earliest Gelasian, latest Pliocene–earliest Pleistocene). Kaim et al. (2012: 427–429, figs 5C–D) mentioned that although six other specimens were collected, they are all fragmentary. They described two shell layers (both now replaced by calcite) in a paratype fragment, and suggested that the outer, dark brown later 25 µm thick is a periostracum preserved by replacement with calcite. The inner layer, 175 µm thick over the columella, is milky white homogeneous calcite. However, reidentification of this shell as J. chavani demonstrates that the outer layer is the brown calcite outer shell layer (violet in life), only 25 µm thick, whereas the inner white layer is the original aragonite inner layer. In most specimens from other localities the inner layer apparently is not so thick.
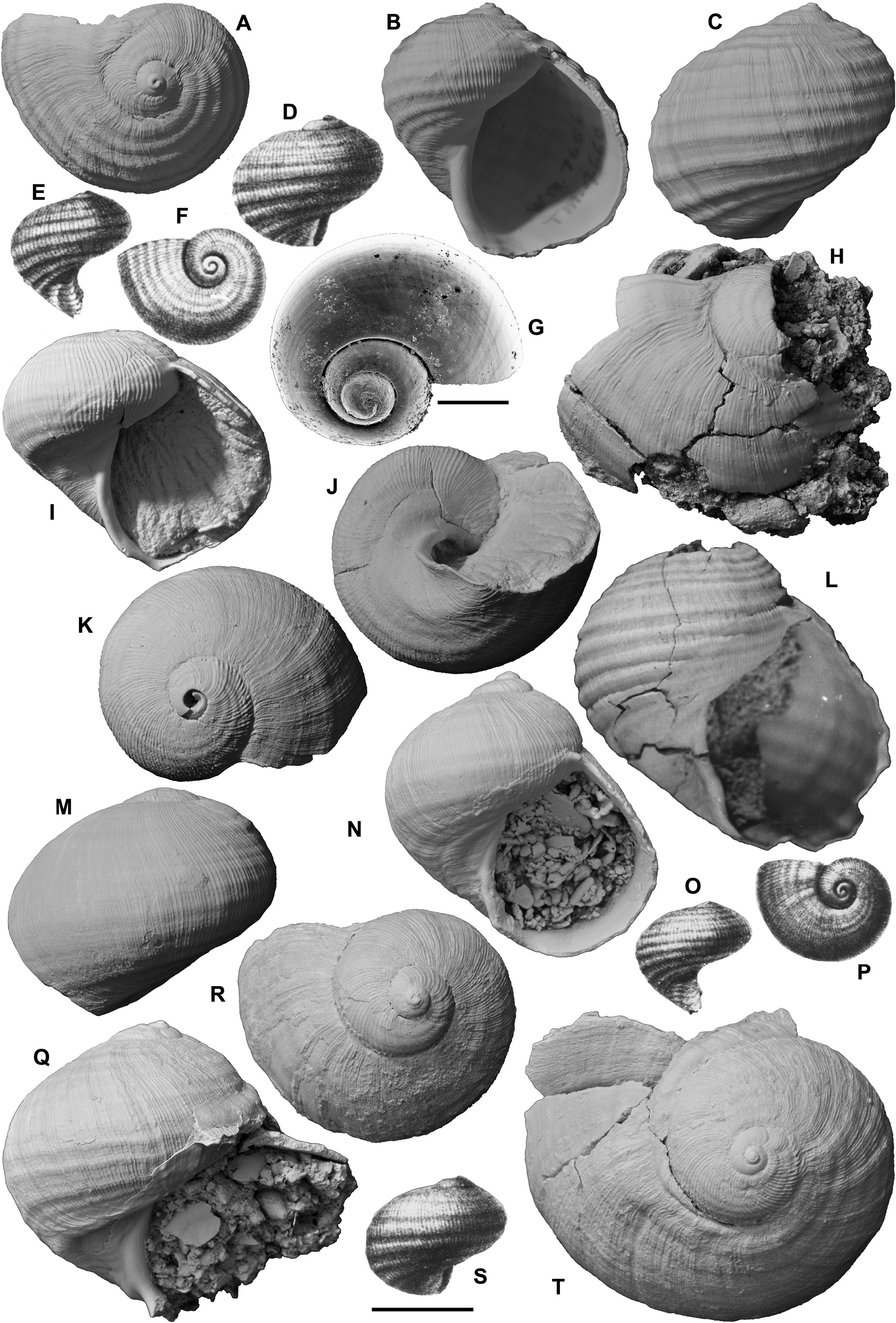
Other material examined. Australia: South Australia: Two paralectotypes of Heligmope dennanti , from Hallett Cove Sandstone, coast east of Hallett Cove, south of Adelaide (see above under H. dennanti ; SAMA T1515A, B); Geological Survey of South Australia, Bridgewater Limestone (Piacenzian–Calabrian?), Kanawinka Fault scarp, Naracoorte, SE South Australia ( GSSA F87/65, 2; photographs sent by N. H. Ludbrook); cliffs S of car park, Point Ellen, Vivonne, Kangaroo Island, South Australia, grid ref. Vivonne 970138 (locality PL3173, NMV P316448, 22); thin cemented beds in Bridgewater Limestone (late Piacenzian–Calabrian?) at top of Henske’s Quarry, Elderslie Road, 2.6 km SE of Naracoorte, SE South Australia, grid reference Hynam 793085 (locality PL3249, NMV P318105, 20, in small limestone blocks, collected by T. A. Darragh and A. G. Beu; also block of specimens c. 1 m 2 observed at quarry office); Naracoorte quarries, SE of Naracoorte, SouthAustralia (clearly from Henske’s Quarry; NMV P316446, 1). Western Australia: Roe Plain: Roe Calcarenite (late Piacenzian), district around Hampton microwave repeater tower, Roe Plain, 48–126 km W of Eucla Motel, SE Western Australia, suites in several museums ( WAM.71-1438a–g, 7; WAM.69-298, 1; WAM.69-300a–z, 26; WAM.69- 301a–d, 4; WAM.69-299a–d, 4; WAM.59-305, 1; WAM.69-302a–c, 3; WAM.69-303, 1; WAM.70-2156a–c, 3; WAM.69-297a–f, 6; WAM.67-778a, b, 2; WAM.69-306, 1; WAM.69-304, 1; NMV P26917, 2; P316447, 18; P322322, 1, Fig.27I View Figure 27 ; GNS WM14468, 10, Figs 27A–H View Figure 27 , 28C, F, J View Figure 28 ); Madura Cave, Roe Plain ( WAM.62-50, 1; WAM.63.44, 1). Perth Basin: “lower” Ascot Formation, water wells in Perth Basin, collected over many years by G.W. Kendrick (Kendrick in Quilty, 1974b: 29; Kendrick et al., 1991: 424, 436), all in WAM; most lots consist of one specimen or fragment: 30 m, Vale’s bore, Evelyn St, Gosnell’s, Perth ( WAM.70-2615); Redcliffe primary school bore, Perth ( WAM.69-292, WAM.69-293, WAM.69-294); Geological Survey of Western Australia bore Gnangara no. 21, W Bullsbrook, Perth ( WAM.68-179, many fragments); Kowalski’s bore, corner Bullfinch & Balfour Streets, Gosnell’s, Perth ( WAM.69-296).
New Zealand: Mangapanian (late Piacenzian–earliest Gelasian): Hawke’s Bay: Cricklewood Road ( GS 12515, W19/f020, grid ref. W19/798357; 4 fragments); brown sandstone 3 km upstream from road bridge, Mohaka River ( GS 13079, W19/f031, grid ref. W19/634313; 1, now fragmentary); Matahorua Road, Tutira ( GS 12508, V19/f011, grid ref. V19/481224; 1); sandstone between conglomerate beds, Pohokura Road, Tutira ( GS 12507, V20/f018, grid ref. V20/444168; 2 fragments). Whanganui Basin: basal conglomerate of Komako Formation, Te Ekaou Stream, Pohangina Valley ( OUGD, OU 8037 , T23/f6565, grid ref. T23/576173; 1; Carter, 1972).
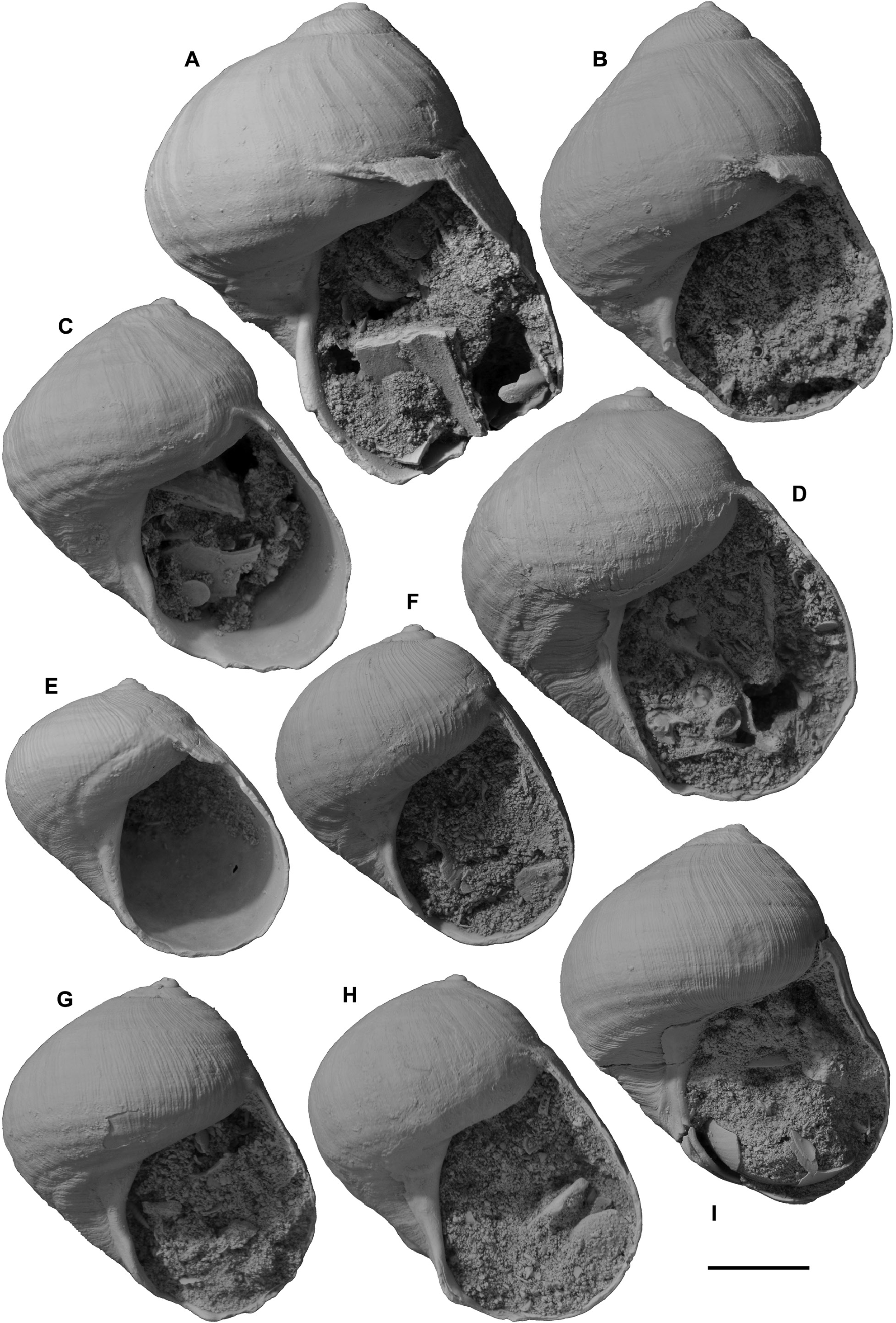
Nukumaruan (Gelasian–earliest Calabrian): Hawke’s Bay: Darkies Spur Formation, road cut, Darkies Spur Road, Arapaoanui Valley, c. 30 km N of Napier ( GS 11225 , V20/f8002, grid ref. V20/407104; 1 + fragment; Figs 28I, K View Figure 28 ). Whanganui Basin: Komako Formation, Pohangina Valley ( Carter, 1972: 306, 321), Makawakawa Stream ( OUGD, OU7597 , T23/ f6516, grid ref. T23/594099; 1); Konewa Stream ( OUGD, OU7668 , T23/ f6548, grid ref. T23/595201; 1); Te Ekaou Stream ( OUGD, OU8125 , T23/ f6563, grid ref.T23/574175; 1); Hautawa Shellbed, Te Ekaou Stream,Dept. of Earth Sciences, University of Waikato (T23/f060, grid ref. T23/577174; 1); lowest lens of Nukumaru Limestone, Waitotara “desert”, coast W of Whanganui ( GS 4258 , R22/f6488, grid ref.R22/581489; 1; Fleming, 1953b: 139); 0.1 m-thick sandy shell lens in massive mudstone, “undifferentiated Upper Okiwa Group” between Tuha Sand and Ohingaiti Sand ( Fleming, 1953b: 133, 136), road cut on hillside 200 m E of Makohine Stream , 2 km S of Ohingaiti, Rangitikei Valley (T22/f8506, grid ref. T22/550356; GNS TM4495, 1, Figs 28B, E, H; D View Figure 28 . Cowe collection, 1967, several); Tewkesbury Formation (late Nukumaruan),shellbed enclosing Vinegar Hill Tephra ( MIS 61, 1.75 Ma; Pillans et al., 2005: 79, figs 5A, 11; Townsend et al., 2008: fig. 35), Brunswick Road, SE side Kai Iwi Valley, W of Whanganui ( GS 15348 , R22/f6542A, grid ref. R22/773506; 1).
The only other material observed in world museums is the type material of Hartungia dennanti chavani , listed above.
Distribution. Janthina chavani is particularly abundant in the type area, in Roe Calcarenite (late Piacenzian) on the Roe Plain, southeastern Western Australia. It is also abundant at a few quarries in Bridgewater Limestone (late Piacenzian–Calabrian?) around Naracoorte, SouthAustralia, and Tate’s two paralectotypes of Heligmope dennanti from the upper part of Hallett Cove Sandstone near Hallett Cove in South Australia are also J. chavani . Ludbrook (1978) also recorded it from a few other localities in South Australia. Ludbrook (1983, 1984) also recorded J. chavani from Point Ellen Formation at Cape Jervis, Fleurieu Peninsula, mainland South Australia, and at Point Ellen, Vivonne, Kangaroo Island, South Australia. Ludbrook (1983: 45, figs 3h‒j; Geological Survey of South Australia GSSA10025a‒c, three illustrated) recorded 17 specimens from Point Ellen and four from Cape Jervis; further specimens have since been collected by T.A. Darragh at Point Ellen (listed above). Many fragments and a few complete specimens have also been seen from “lower” Ascot Formation in water wells in the Perth Basin, WesternAustralia (material in WAM). In New Zealand it is much less common and widespread than J. typica , and is recorded from only 13 localities in Mangapanian and Nukumaruan (late Piacenzian–early Calabrian) rocks in Hawke’s Bay and Whanganui Basin. In Japan, a few specimens have been collected from near the Pacific coast of SE Honshu Island, and two were reported by Tomida et al. (2013) from Hioki, Miyazaki Prefecture, near the east coast of Kyushu. The single Atlantic record is from Kane Megamullion, on the mid-Atlantic ridge ( Kaim et al., 2012), where specimens apparently were dredged from “normal” seabed, and not from a hydrothermal seep site as Kaim et al. (2012) thought. Late Pliocene–early Pleistocene rocks of suitable facies for the preservation of Janthina apparently are not exposed on the Atlantic islands where J. typica occurs, although the records from São Vicente, Madeira, and from Selvagem Grande Island require re-collection to be certain of their identities and are possibly J. chavani .
Dimensions. See Table 5.
Diagnosis. Teleoconch moderate-sized to very large for Janthina (up to H 48, D 40 mm), covered with fine, straight, closely spaced axial ridges; axial ridges tending to fade out over last whorl of large specimens; 8–11 spiral folds per whorl (9 or 10 on most specimens) but most specimens with spiral folds significantly less obvious than on J. typica ; at least two spiral folds suppressed on upper sutural ramp. Outer lip sinus small, basal, as in J. typica , but slightly wider, narrowly V-shaped in some specimens. Teleoconch increasing in height with weak allometry; most juvenile specimens with low spires, most large specimens taller and narrower than all other large Janthina species, although much shorter and wider than Recluzia species.
Remarks. Janthina chavani resembles J. typica closely, but can be distinguished by three characters: (1) the spiral folds are weaker, particularly over the sutural ramp, than on J. typica . The two uppermost spiral cords, at least, are suppressed, so that the ramp is smooth (apart from the fine axial ridgelets) in almost all specimens. Many Roe Calcarenite specimens have very weak, almost uncountable spiral folds, and resemble J. janthina quite closely ( Figs 27A–I View Figure 27 , 28C, F, J View Figure 28 ). Spiral cords are not visible at all on spire whorls of most specimens, and only around the outer edges of the spire whorls of others. (2) The fine axial ridgelets tend to fade out after the spire whorls and, on many specimens, particularly the (slightly abraded?) Roe Calcarenite population, the axial ridgelets are very weak on or absent from the last whorl. (3) Teleoconch spire height exhibits a much greater range of variation than in any other Janthina species, tending to produce an allometrical change with growth from very low-spired juvenile specimens to tall-spired adults. Obviously, this third character is visible only in large collections, and it is doubtful whether it would have been recognized without observing the large number of beautifully preserved specimens from Roe Calcarenite in southeastern Western Australia. Once it became evident in Roe Calcarenite collections, the great shape variability of other populations in New Zealand, southern Australia and Japan became more comprehensible. Many specimens have an obviously convex (cyrtoconoid) spire outline, resulting from the change in shape during growth, but others have straight outlines. Still others have an unusually rapid whorl translation, so they accommodate the change in shape with a stepped spire, each succeeding whorl descending below the periphery of the preceding one.
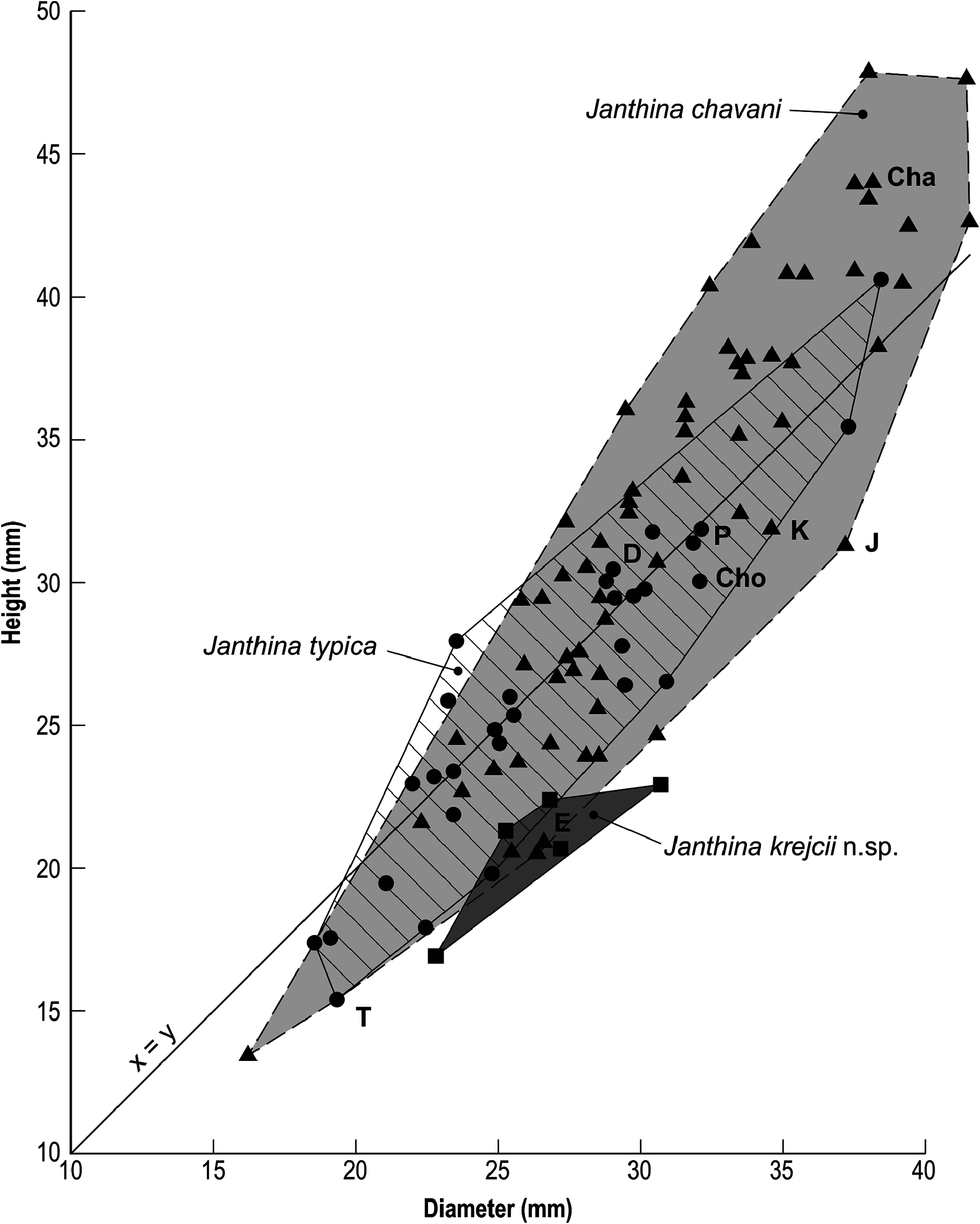
Shape differences are shown in Fig. 29 View Figure 29 , a scatter diagram comparing height with width in Janthina typica , J. chavani and J. krejcii sp. nov. The field occupied by J. typica in Fig. 29 View Figure 29 is aligned more nearly along the x = y diagonal axis than that of J. chavani . This demonstrates that on average, height is almost equal to diameter in J. typica , and there is little change in shape with growth, although most small specimens have shorter spires than large specimens; height increases regularly throughout growth. The field of J. krejcii sp. nov. lies well below but parallel to the x = y axis, confirming the very low, wide shape of this species. All specimens are wider than they are high, and again there is no obvious change of shape with growth, although only five specimens were complete enough to include in this diagram. However, the field occupied by J. chavani is aligned up a steeper axis than x = y. On average, small (juvenile) specimens are wider than they are high, large adults are markedly taller than they are wide, and there is a weak allometrical increase in height as the shell grows in J. chavani . Although the allometry is weak, 22 of the 50 plotted specimens of J. chavani (44%) are taller than all 29 specimens of J. typica plotted in Fig. 29 View Figure 29 , confirming the visual estimation of shape and size differences.
The sinus in the outer lip in Janthina chavani is basal, as in J. typica , but while in many specimens it is semicircular ( Figs 28C, E View Figure 28 ), others have a wider, narrowly V-shaped sinus, and in still others it is intermediate in shape ( Ludbrook, 1978: pl. 12, figs 3, 5–6, 10). Specimens of J. chavani reach 48 mm in height and 40 mm in diameter, with 8–11 spiral folds on the last whorl. Most specimens have 9 or 10 folds, as in J. typica . Presumably the allometrical increase in spire height in J. chavani produces a taller sutural ramp in adults that allows almost the same number of spiral folds to be present in both species, bordered above by an unfolded area in J. chavani that is absent from J. typica . The spiral folds of J. chavani also possibly are slightly narrower than those of J. typica , although any difference is not obvious. A high proportion of Roe Calcarenite specimens is conspicuously large, robust, thick-shelled, weakly sculptured and tall-spired for a fossil Janthina species, but this is presumably partly because of the large population available to select from. The weakly consolidated nature of the formation allows the excellent preservation and easy collection of fragile shells. Ludbrook (1978: pl. 12, figs 13–14) illustrated a specimen with all sculpture abraded off the earliest 1.5 teleoconch whorls, which are weakly inflated in this specimen, and labelled it as “showing smooth protoconch”, but this is misidentified and does not resemble the very small, tall, pupiform, planktotrophic Epitonium protoconch of all living Janthina species. It is quite similar in appearance to the smooth apex of the holotype of Eunaticina abyssalis ( Fig. 25G View Figure 25 ), although the outer layer is present and the smoothness results from surface abrasion rather than corrosion in this case. The protoconch of J. chavani has not been observed.
The younger Japanese specimens are referred to planktonic foraminiferal zones N21 and N22 and were identified by Japanese authors as Janthina (or Hartungia ) japonica . They are relatively small and, consequently, have low spires (Tomida & Itoigawa, 1982: pl. 19, figs 1a–c, holotype of Parajanthina japonica ; Tomida & Itoigawa, 1984: pl. 31, figs 1a–2b; 1989: pl. 23, figs 1a–2d; Noda et al., 1995: figs 11.7a–d; Tomida & Kitao, 2002: figs 2.1a–2c; Tomida et al., 2013: figs 3E–L). However, they agree with J. chavani in having weaker spiral sculpture than older specimens, they closely resemble most New Zealand specimens identified as J. chavani , and they overlap with the range of variation in spire height of New Zealand and southern Australian specimens. The apparent difference in spire height results from the scarcity of large adults in Japanese samples, and their abundance in Roe Calcarenite collections. Like New Zealand specimens, many Japanese ones also have been distorted and crushed to varying extents by compaction. In the writer’s estimation, these youngest Japanese specimens fall within the range of variation of J. chavani .
The overall impression of the characters and range of variation of Janthina chavani is that this species is closely similar to J. janthina as well as to J. typica . It is feasible that it was the immediate ancestor of J. janthina . This closely similar appearance provides the main evidence that fossils allow for the evolutionary history of the entire group, and so must be given strong weight when evaluating the phylogeny of Janthina . The very weak spiral folds on some specimens of J. chavani from Roe Calcarenite are only slightly more obvious than the faint spiral folds and grooves on the base of some Recent specimens of J. janthina , and the most fundamental differences between them are the narrow basal sinus in the outer lip of J. chavani and its wider shape and more nearly central apex in J. janthina , the weakly trochiform shape of J. janthina compared with an evenly convex shape (although with a flattened sutural ramp) in J. chavani , and the presence of axial ridgelets over most of the spire whorls in J. chavani , whereas they are limited to the first c. 1–1.5 spire whorls in J. janthina . Weakening of the axial ridgelets and spiral folds and flattening of the sutural ramp in J. chavani compared with their state in J. typica are interpreted as precursors to the more marked state of these characters in J. janthina . The much smaller sinus in the outer lip in J. typica , J. krejcii and J. chavani than in all living Janthina species seems to indicate that the function of the sinus has changed during the evolution of the genus. Wilson & Wilson (1956: 302) described the extrusion of capsules from between the gills and the bottom of the foot in J. janthina , so possibly the small early sinus aided this capsule extrusion, and the sinus only later came to be adapted to be used more continuously to accommodate the protruding head.
Time range. Late Piacenzian–early Calabrian; 3.0–c. 1.7 Ma (Mangapanian–Nukumaruan New Zealand Stages; latest Pliocene–early Pleistocene; Cooper, 2004: fig. 13.1, modified by inclusion of the Gelasian Stage in the Pleistocene); probably considerably younger (late Calabrian, 1.0 Ma, or even younger) in Bridgewater Limestone in SE South Australia. The equally meagre record in Japan and southern Australia confirms the mid-Piacenzian origination observed in New Zealand, but the upper limit is not constrained in any stratigraphical succession the writer is aware of.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Janthina chavani ( Ludbrook, 1978 )
| Beu, Alan G. 2017 |
Janthina (Hartungia) typica (Bronn)
| Tomida, S 2013: 60 |
Hartungia chavani (Ludbrook)
| Spencer, H & Marshall, P & Maxwell, J & Grant-Mackie, J & Stilwell, R & Willan, H & Campbell, J & Crampton, R & Henderson, M & Bradshaw, J 2009: 245 |
Parajanthina japonica
| Ogasawara, K 2002: 545 |
Hartungia japonica (Tomida & Itoigawa)
| Ogasawara, K 2002: 394 |
| Noda, H 1995: 83 |
| Nobuhara, T 1995: 39 |
Hartungia
| Nobuhara, T 1995: 38 |
Hartungia dennanti chavani
| Kendrick, G 1991: 424 |
| Ludbrook, N 1984: 232 |
| Ludbrook, N 1983: 45 |
| Ludbrook, N 1978: 119 |
Hartungia typica typica (Bronn)
| Quilty, P 1974: 308 |
| Quilty, P 1974: 29 |
| Johnstone, M 1973: 14 |
Hartungia postulata (Bartrum)
| Carter, R 1972: 306 |
Heligmope postulatus (Bartrum)
| Fleming, C 1953: 139 |