Hornera mediterranea, Harmelin, 2020
|
publication ID |
https://doi.org/ 10.5252/zoosystema2020v42a27 |
|
publication LSID |
urn:lsid:zoobank.org:pub:08634080-E772-41F1-BDA7-D1AAD200A2BF |
|
DOI |
https://doi.org/10.5281/zenodo.4327909 |
|
persistent identifier |
https://treatment.plazi.org/id/8A0CE681-7201-486D-A0E5-1E64C55347A9 |
|
taxon LSID |
lsid:zoobank.org:act:8A0CE681-7201-486D-A0E5-1E64C55347A9 |
|
treatment provided by |
Felipe |
|
scientific name |
Hornera mediterranea |
| status |
sp. nov. |
Hornera mediterranea n. sp.
Figs 5-8 View FIG View FIG View FIG View FIG , tables 2-4
urn:lsid:zoobank.org:act:8A0CE681-7201-486D-A0E5-1E64C55347A9
Hornera mediterranea Waters, 1904: 94 (nomen nudum); 1905: 15 – Smith et al. 2008: 390.
Hornera lichenoides (Linnaeus) View in CoL – Calvet 1931: 43 (part: st. 344 & 633) – Laubier 1966: 223, table (dubious identification) – Zabala 1986: 820, fig. 244, pl. 29-D; 1993: 572 – Saguar & Boronat 1987: 413, table, figs 3-5 – Zabala & Maluquer 1988: 182, figs 620-624 – Rosso 1989: 270, tabs 5, 23, pl. 1b; 1996: 209, table; 2005: 263, table 3 – Costa et al. 1991: 418, table 2 – Harmelin & d’Hondt 1992: 609 – Di Geronimo et al. 1993: 92, table 3; 1997: 200, table 3; 1998: 248, table 1; 2001: 282, table 3; 2005: 73, table 4.
‘ Hornera lichenoides ’ Auctt. View in CoL not ( Linnaeus, 1758) – Rosso & Di Martino 2016: 570.
Hornera frondiculata Lamouroux, 1821 View in CoL – Harmelin 1968: 1187.
Hornera View in CoL sp. - Harmelin 1976: 223, table I, 229, table III; 1978: 137 – Abdelsalam 2014: 272, fig. 3 (dubious identification) – Rosso 2009: 134.
Hornera serrata Meneghini, 1844 View in CoL – Neviani 1939: 70 (dubious identification).
Hornerra violocea var. proboscina Busk, 1875 – O’Donoghue & de Watteville 1939: 8 (dubious identification).
Type locality. — France, Marseille, Riou Island.
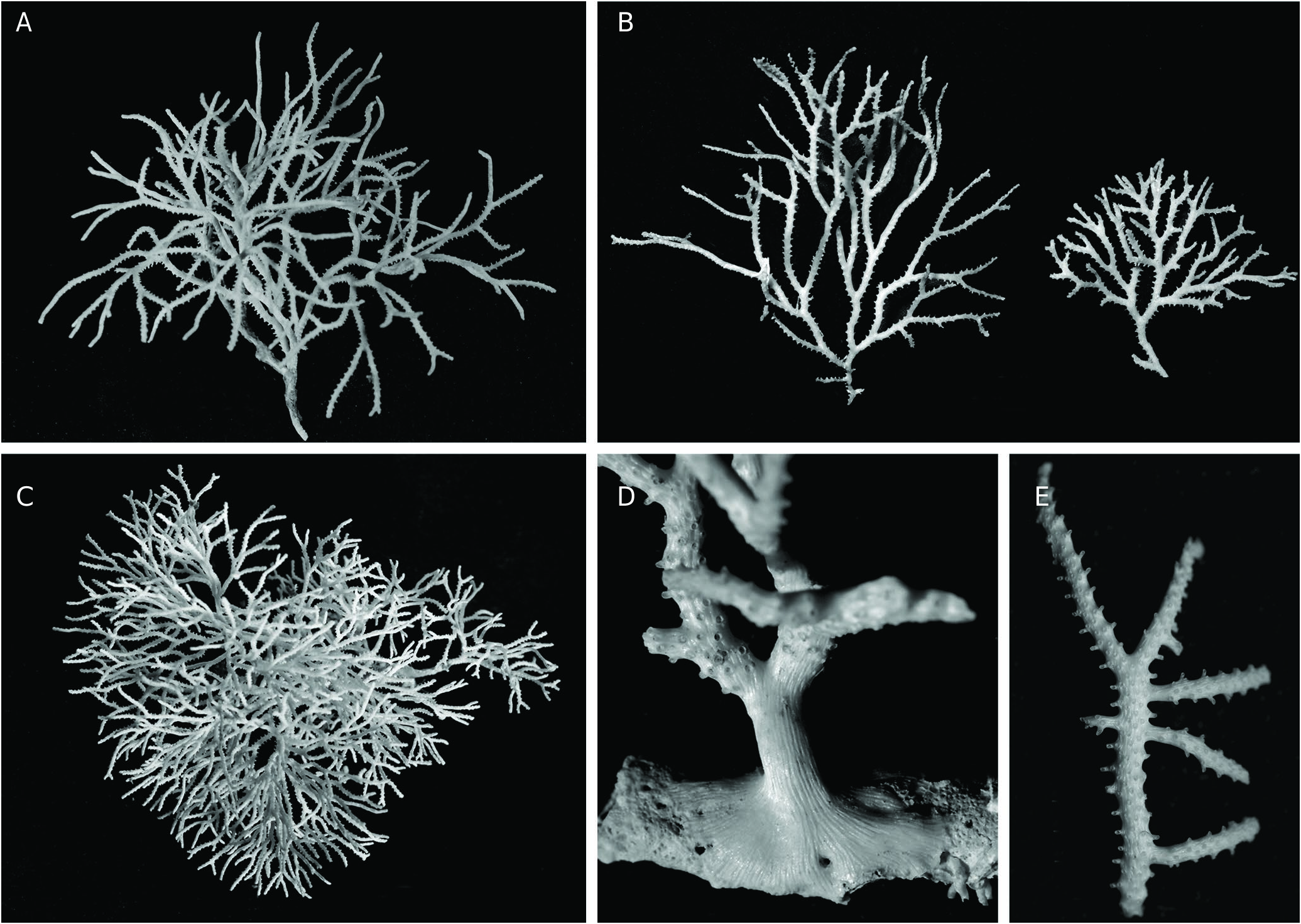
TYPE MATERIAL. — Holotype. France • 1 large fertile colony, ca. 5 cm high, 6.7 cm wide, with long, contorted branches, 58 bifurcations and 6 gonozooids ( Fig. 5A View FIG ); Marseille, SW Riou Island ; 43°10’25”N, 5°22’52’’E; 60-62 m; VII.1982; coarse DC and low rocks; JGH leg.; MNHN-IB-2017-225. GoogleMaps
Paratypes. France • 1 fragmented colony with 10 bifurcations and 5 gonozooids; same data as for holotype; JGH leg.; MNHN- IB-2017-226 GoogleMaps • 2 fragments of colony with gonozooids; same origin as the holotype; JGH leg.; MNHN-IB-2017-227 • 2 fragments of fertile colony with 2 gonozooids; Marseille , South Riou Island; Stn JGH-71.33; 74 m; 5.VII.1971; Div; MNHN-IB-2017-228 • 2 fragments of well-calcified colony; Marseille , South Riou Is.; Stn JGH-71.34; 70 m; 3.VII.1971; Dre; with H. frondiculata ; JGH leg.; MNHN-IB-2017-229 • 1 large colony with lateral branches; Marseille , Riou Island, Impérial du large; 55 m; 2.II.1976; coarse DC; JGH leg.; MNHN-IB-2017-230 .
OTHER MATERIAL EXAMINED. — France • several small colonies, 1 gonozooid; same data as for paratype MNHN-IB-2017-228; JGH leg. • 1 colony; same data as for paratype MNHN-IB-2017-229; JGH leg. • several fragments; Marseille, South Riou Is.; Stn JGH- 71.43; 70 m; 8.XII.1971; coarse DC; Div; JGH leg. • several small colonies; Marseille, South Riou Island; Stn JGH-72.8; 90 m; 2.III.1972; silty DC; Dre; JGH leg. • 1 colony; Marseille, South Riou Is.; Stn JGH-72.22; 100 m; 26.V.1972; silty DC; Dre; JGH leg. • several fragments; Marseille, South Riou Is.; Stn JGH- 73.9; 90-100 m; 6.IV.1973; silty DC; Dre; with H. frondiculata ; JGH leg. • several fragments; Marseille, South Riou Is.; Stn JGH- 73.17; 85 m; 12.VII.1973; DC; Dre; JGH leg. • several colonies; Marseille, South Riou Is., Impérial du large; 55 m; 2.II.1976; coarse DC; Div; JGH leg. • several large colonies, some fertile; same data as for holotype; JGH leg. • several fragments; Marseille, North Mangespin; 65 m; 18.IV.1972; Dre; with H. frondiculata ; JGH leg. • fragments; Marseille, Mangespin, South Veyron; Stn JGH-72.21; 60 m; 25.V.1972; Dre; DC; JGH leg. • fragments; Bay of Cassis; 75 m; XI.1970; DC; Dre; M. Bourcier leg. • frag- ments; Bay of Cassis; 95 m; silty DC; Dre; M. Bourcier leg. • frag- ments; Bay of Cassis; 75 m; IX.1971; DC; Dre; M. Bourcier leg. • fragments; off Cassis, East Cassidaigne canyon; 170-200 m; 30.IX.1969; silty DC; Dre; H. Zibrowius leg. • fragments; off Cassis, West Cassidaigne canyon, Stn JGH-71.13; 140-170 m; 5.V.1971; silty DC, Dre; JGH leg. • fragments; off Cassis, West Cassidaigne canyon, Stn JGH-72.9; 115-130 m; 22.III.1972; silty DC; Dre; JGH leg. • 1 colony; Bay of La Ciotat; 60 m; 27.VII.1992; DC; Dre; M. Bourcier leg. • fragments; Hyères Islands, South Porquerolles Island, 2.2 nautical miles South off Cap d’Armes; Stn JGH-76.24; 130 m; 19.VI.1976; coarse DC; Dre; JGH leg. • Hyères Islands, off Levant Island, Magaud Bank; Stn JGH-71.25; 100 m; 24.VI.1971; DC + rocks; Dre; JGH leg. • fragments; Hyères Islands, off Levant Island, Magaud Bank; Stn JGH-71.27; 100 m; 24.VI.1971; DC + rocks; Dre; JGH leg. • fragments; French Riviera, Bay of Cannes; 75 m; IV.1970; silty DC; Dre; M. Bourcier leg .
Italy • 10 small fragments; South Sicilia, Gulf of Noto ; Stn PS /81 4C; 95- 86 m; A. Rosso leg. • 7 fragments; South Sicilia, Gulf of Noto ; Stn PS /81 4X; 102- 93 m; A. Rosso leg. • 4 fragments; Sicilia Strait ; Stn V 2B; 125 m; A. Rosso leg. • 1 small fragment; Sicilia Strait ; Stn V 3; 120 m; A. Rosso leg. • 1 fragment; Sicilia Strait ; Stn V 4; 120 m; A. Rosso leg. • 2 small fragments; Sicilia Strait ; Stn V 9; 129 m; A. Rosso leg. • 1 small fragment; Sicilia Strait ; Stn V 17; 120 m; A. Rosso leg.
Greece • 1 small fragment; Santorini; R/ V Jean Charcot, Stn 32.MO.67; 36°32.3’N, 25°17.85’E.; 110-128m; 4.IX.1967; coarse DC and biogenic concretions; Dre; JGH leg. (as H. frondiculata in Harmelin 1968 ) GoogleMaps .
ADDITIONAL MATERIAL. — Museum specimens from the Atlantic and boreal seas not belonging to H. mediterranea n. sp., attributed to Hornera lichenoides • 6 SEM photos; Greenland, 70°30’N, 54°44’W, 175 fms, HMS Valourous expedition 1875, NHM 1911.10.1.181; P. Kuklinski leg., sent by P. D. Taylor on 27.V.2005 GoogleMaps • 1 colony; Bay of Biscay; R/V Travailleur 1881; 392 m; Dre. 40, Calvet 1906; MNHN no. 866, examination on 15.II.2005 • 1 colony; Norway, Finnmark, Jarŋord; Pouchet expedition 1891; Dre. 26; L. Calvet, 11 t. 18; MNHN no. 222, examination on 15.II.2005 • 1 colony; Finnmark, Loppen; Smitt, M6 (R) -1867; MNHN no. 177g, examination on 15.II.2005 .
ETYMOLOGY. — mediterranea : from the Mediterranean Sea, the source of all specimens examined for the present description.
DIAGNOSIS. — Zoarium erect, ramified dichotomously with narrow, isodiametric branches oriented in several directions. Frontal side of branches occupied by rows of autozooids pierced by small mural pores, with short, relatively broad peristomes with ellipsoidal apertures. Gonozooid globular or oval, built from a broad basal tube, covered with a stratified network of calcified strings, ooeciostome opening laterally. Secondary calcification of frontal side leading to a uniform cover punctuated by small pustules, with 1-3 small windows per autozooid. Dorsal side convex with longitudinal ridges bearing small pustules aligned transversally.
DESCRIPTION
Zoarium erect, firmly fixed on small, discrete substrata by layers of secondary calcification expanding widely ( Fig. 5D View FIG ), white in colour, bushy, reaching large size (> 10 cm), but often smaller, ramified dichotomously many times without anastomoses, with slender, nearly cylindrical, isodiametric branches, bent in several directions, often with long segments between two bifurcations (up to 1-1.5 cm) ( Fig. 5 View FIG A-C); lateral branches growing at right angle present, but not frequent ( Fig. 5E View FIG ). Frontal side occupied by autozooids opening alternatively along 4-7 longitudinal rows on the frontal side ( Fig. 5A, D View FIG ), with wall pierced by round pores (about 8-11 µm), scattered all around the tube except above the base of the peristomes ( Fig. 6B View FIG ); peristomes short, longer on branch sides (180-210 µm), with aperture entire, ellipsoidal, slightly broader distally, with long axis oriented longitudinally in medial rows, more obliquely on lateral rows ( Fig. 5B View FIG ). External aspect of autozooids varying markedly from the branch tips to the base of the colony according to the increase with age in the amount of secondary calcification; four schematic stages perceivable in this progression ( Fig. 5 View FIG C-F): (stage 1) apical area of branches ( Fig. 6 View FIG A-C), autozooids with raised peristome and primary frontal wall fully exposed, 8-10 mural pores and both sides slightly thickened by a smooth longitudinal ridge, a large empty space (40-60 µm) bordered with the base of the lateral ridges at the proximal end of frontal wall, (stage 2) ( Fig. 6D View FIG ) thickening and broadening of the lateral ridges that tend to cover the whole frontal wall, leaving only few mural pores visible, which can be included within narrow, longitudinally oblong windows, (stage 3) in older parts ( Fig. 6E View FIG ), peristomes emerge from a thick cover of secondary calcification formed by thick, convex, longitudinal ‘mouldings’ covered with transverse lines of pustules, which border 1-2 large, oblong windows, (stage 4) in more basal parts ( Fig. 6F View FIG ) peristomes hardly emerging from a uniform mass of secondary calcification, which is densely punctuated by small pustules distributed transversally, and interrupted by small, irregularly shaped windows, 1 to 3 per zooid. Dorsal side markedly convex, entirely covered by layers of secondary calcification deposited straight from the branch tip, deeply striated with narrow, longitudinal, anastomosed ridges with rounded surface covered with tiny pustules aligned transversally, leaving long, narrow spindle-shaped empty spaces between them, open or closed by a wall pierced by 2-5 small pores (10- 13 µm wide) ( Fig. 7 View FIG A-C). Gonozooid chamber on the dorsal side, globular with ovoidal or roundish outline, broader than the branch on which it is placed ( Fig. 7E View FIG ); basal part made of a tube migrated from the frontal side and markedly widened (W = 220-260 µm) before building the floor of the gonozooid across a large part of the branch width ( Fig. 7F View FIG ); cover of fully grown gonozooid densely reticulated by a complex network made of stratified layers of anastomosed strings of secondary calcification converging towards the top of the gonozooid and forming a low crest towards the ooeciostome ( Fig. 7F, G View FIG ); areas between the strings irregularly shaped and sized, some closed by calcified, porous wall; primary gonozooid wall pierced with rounded pores closed by a diaphragm made of converging pointed processes ( Fig. 7H View FIG ). Ooeciostome a short tube opening laterally, slightly curved downwards, placed just above the basal tube of the gonozooid and seemingly prolonging it, frequently with a low crest on the upper midline, ooeciopore oval, a little broader than the autozooid peristomes (x 1.5 in average) ( Fig. 7F View FIG ). Frequency of fertile colonies and number of gonozooids on them relatively low ( Table 2 View TABLE ); floor of gonozooids sometimes remaining on branches after loss of the upper parts ( Fig. 8 View FIG ). Ancestrula and early astogenetic stages not observed.
REMARKS
Taxonomic issues
The species name mediterranea was introduced by Waters (1904: 94, 1905: 15) for a specimen from Naples, first assigned by him to H. lichenoides (Linnaeus) because of similarities in the gonozooid, but differing from the latter by colony and autozooid features. However, this new species name fails to comply with article 12 of the ICZN as Waters did not give a real description of this taxon, nor a figure, and has not deposited type material, nor specimen bearing this name in the museums known to house his material (NHMUK, Museum of Manchester). Therefore, although there is a strong presumption that Waters designated under the name H. mediterranea a specimen belonging to the species described here, this specific name is considered to be a nomen nudum, and thus available. In tribute to A. W. Waters, the species name mediterranea is given here for the second Hornera species present in the Mediterranean.
Records in the literature of Mediterranean bryozoans that can be referred with some confidence to H. mediterranea n. sp. are the following which were originally cited as (i) H. lichenoides (Linnaeus) ( Calvet 1931; Zabala 1986; Zabala & Maluquer 1988; Harmelin & d’Hondt 1992; Di Geronimo et al. 1993; Rosso & Di Geronimo 1998), (ii) H. lichenoides Auctt. not Linnaeus ( Rosso & Di Martino 2016), (iii) H. ‘lichenoides’ ( Rosso et al. 2010; Rosso & Di Martino 2016), and (iv) Hornera sp. ( Harmelin 1976, 1978; Rosso 2009; Abdelsalam 2014). These records are recent specimens, but there are also fossils from the Plio-Pleistocene ( Zabala 1986; Saguar & Boronat 1987; Zabala & Maluquer 1988; Rosso 1989; Rosso & Di Geronimo 1998; Abdelsalam 2014).
Morphological features
Colonies of H. mediterranea n. sp. are readily distinguishable from those of H. frondiculata . They are typically formed of narrow, often curved, subcylindrical branches, irregularly bifurcating in three dimensions. These colonies are fragile and easily fragmented, but sampling by diving has shown that they can reach a large size (> 10 cm, Fig. 5C View FIG ) in favourable sites, such as that of the holotype. Lateral branching is present, but less common than in H. frondiculata . The autozooids differ from those of H. frondiculata in their larger diameter ( Table 2 View TABLE ), smaller mural pores, and shorter peristomes with an ellipsoidal aperture, slightly broader distally. The development of secondary calcification on the frontal side follows the same succession of stages as in H. frondiculata , with a similar pattern of thickening, that can be divided into 4 stages ( Fig. 6 View FIG ). The main difference concerns the windows (‘lacunes’), which are less numerous and smaller in the last stages of calcification in H. mediterranea n. sp. ( Fig. 6F View FIG ). The thickening of the convex dorsal side is typically achieved by distinct ribs with rounded outline, covered by transverse lines of small pustules, a structure resembling that of H. brancoensis Calvet, 1906 from Cape Verde Islands. Within depressions between these ribs, pores are smaller than in H. frondiculata , in which they are included in spindle-shaped depressions. As in H. frondiculata , the gonozooid is broader than the branch on which it is developed, but its relative size is smaller, its shape is rounded or oval, and it is not carinated. Unlike H. frondiculata , H. mediterranea n. sp. produces few gonozooids ( Table 2 View TABLE ). The occurrence on branches of H. mediterranea n. sp. of vestiges of brood chambers consisting of floors more or less covered by secondary calcification ( Fig. 8A View FIG ), or limited to the basal tube of the chamber ( Fig. 8B View FIG ), can be diversely interpreted. These remains might be signs of aborted growth due as well to strong limitations in time of suitable conditions for the development of gonozooids as to vulnerability to particular adverse conditions. However, according to Batson et al. (2020), similar vestiges of gonozooid floors observed in Hornera colonies from New-Zealand would result from the resorption of brood chamber walls.
Fossil records
Hornera lichenoides Auctt. was recorded together with H. frondiculata in fossil deep coral assemblages from the Early Middle Pleistocene of southern Italy by Di Geronimo et al. (2005) and in other deposits of the same age ( Rosso & Di Geronimo 1998; Rosso 2005). The occurrence of this species in Early Pleistocene deposits in southern Italy was considered by Rosso & Di Geronimo (1998) as an indication that this species was a ‘residual boreal guest’ that had colonized the Mediterranean during the glacial intervals. These Pleistocene specimens attributed to H. lichenoides can be identified as H. mediterranea n. sp. considering that the differences between the two species were recognized by Rosso (i.e. Rosso 2009; Rosso & Di Martino 2016) and extant specimens of H. mediterranea n. sp. from Rosso’s collection have been examined here. The hypothesis that H. mediterranea n. sp. results from a speciation from boreal populations of H. lichenoides having entered the Mediterranean during phases of glaciation can be regarded as tenable. On the other hand, the record of H. lichenoides from the Middle Miocene in Hungary by Moissette et al. (2007), without morphological details, remains questionable.
HABITAT DISTRIBUTION
All examined specimens of H. mediterranea n. sp. were collected within the depth range of 55-200 m ( Table 4 View TABLE ), on soft bottoms ranging from the circalittoral zone (sensu Pérès & Picard 1964) to the upper limit of the bathyal zone. The shallowest sampling stations were close to deep coralligenous outcrops or lower discrete rocks, and presented a large part of coarse detrital elements indicating the frequent occurrence of bottom currents. The largest specimens ( Fig. 5A, C View FIG ) were collected in this category of stations. In deeper soft bottoms (100-200 m), more distant from the coast and comprising a greater proportion of fine particles, colonies were smaller. Unlike H. frondiculata , colonies of this species were never collected on rocky walls, but all were lying free at the surface of soft bottoms, leaning on their lateral branches, the base attached on small to tiny substrates. The record at a very shallow depth (20-25 m) in Egypt by Abdelsalam (2014) of a fragment of colony identified as Hornera sp., sampled together with H. frondiculata , is questionable and probably does not correspond to H. mediterranea n. sp. All examined colonies of H. mediterranea n. sp. were free of epibionts except for one from Marseille (JGH-Stn 72.8, Riou Is., 90 m), which was encrusted by two small colonies of Amphiblestrum lyrulatum (Calvet, 1907) . Interestingly, as pointed out by Lopez de la Cuadra & Garcia-Gomez (1994) in their redescription of this species, A. lyrulatum is endemic to the Mediterranean and often confused with ‘ Ramphonotus minax’ (Busk, 1860) from the northern Atlantic. Thus, as with H. mediterranea n. sp., A. lyrulatum may be a ‘residual northern guest’ ( Rosso & Di Geronimo 1998), genetically differentiated in the Mediterranean after transfer after transfer of an Atlantic species during glaciation phases.
PROTECTION STATUS
As already mentioned, H. lichenoides ( Linnaeus, 1758) is the only bryozoan species in the list of endangered or threatened species in the Mediterranean (Barcelona Convention, Annex II: UNEP 2011). It is not clear which species this name refers to and why it was designated as threatened. Since H. mediterranea n. sp. is uncommon and has no spectacular features which would be noticed by anyone other than an expert bryozoologist, it is likely that there was a confusion with H. frondiculata .
GEOGRAPHICAL DISTRIBUTION
In the present state of knowledge, H. mediterranea n. sp. is only known from the Mediterranean. Almost all records of H. mediterranea n. sp. are from the western Mediterranean ( Table 4 View TABLE ). The only available record from the eastern Mediterranean is a small specimen collected at Santorini, Aegean Sea ( Harmelin 1968, listed as H. frondiculata ).
GEOLOGICAL DISTRIBUTION
Hornera mediterranea n. sp. was recorded (as H. lichenoides ) in Pleistocene deposits in southern Italy ( Costa et al. 1991; Di Geronimo et al. 1997; Rosso & Di Geronimo 1998; Di Geronimo et al. 2003; Rosso 2005) and submerged late Würmian to Holocene deposits from the Sicily Strait ( Di Geronimo et al. 1993), often co-occurring with H. frondiculata .
| MNHN |
Museum National d'Histoire Naturelle |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Hornera mediterranea
| Harmelin, Jean-Georges 2020 |
Hornera lichenoides ’
| ROSSO A. & DI MARTINO E. 2016: 570 |
Hornera
| ABDELSALAM K. M. 2014: 272 |
| ROSSO A. 2009: 134 |
| HARMELIN J. - G. 1976: 223 |
Hornera frondiculata
| HARMELIN J. - G. 1968: 1187 |
Hornera serrata
| NEVIANI A. 1939: 70 |
Hornera lichenoides (Linnaeus)
| DI GERONIMO I. & ROSSO A. & SANFILIPPO R. 1993: 92 |
| COSTA B. & ROSSO A. & SANFILIPPO R. & ZANINI A. 1991: 418 |
| ROSSO A. 1989: 270 |
| ZABALA M. & MALUQUER P. 1988: 182 |
| SAGUAR J. & BORONAT J. 1987: 413 |
| ZABALA M. 1986: 820 |
| LAUBIER L. 1966: 223 |
| CALVET L. 1931: 43 |
| Harmelin & d’Hondt 1992: 609 |
Hornera mediterranea
| SMITH A. M. & TAYLOR P. D. & SPENCER H. G. 2008: 390 |
| WATERS A. W. 1904: 94 |
Hornerra violocea var. proboscina
| O’Donoghue & de Watteville 1939: 8 |