Haematoloechus occidentalis, León-Règagnon & Topan, 2018
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4526.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:4DF63CE5-4838-46CA-BB0E-2F91841D5CB1 |
|
DOI |
https://doi.org/10.5281/zenodo.5970243 |
|
persistent identifier |
https://treatment.plazi.org/id/03B987D9-FFF9-8802-B6E0-0579FB7EF9BB |
|
treatment provided by |
Plazi |
|
scientific name |
Haematoloechus occidentalis |
| status |
sp. nov. |
Haematoloechus occidentalis n. sp.
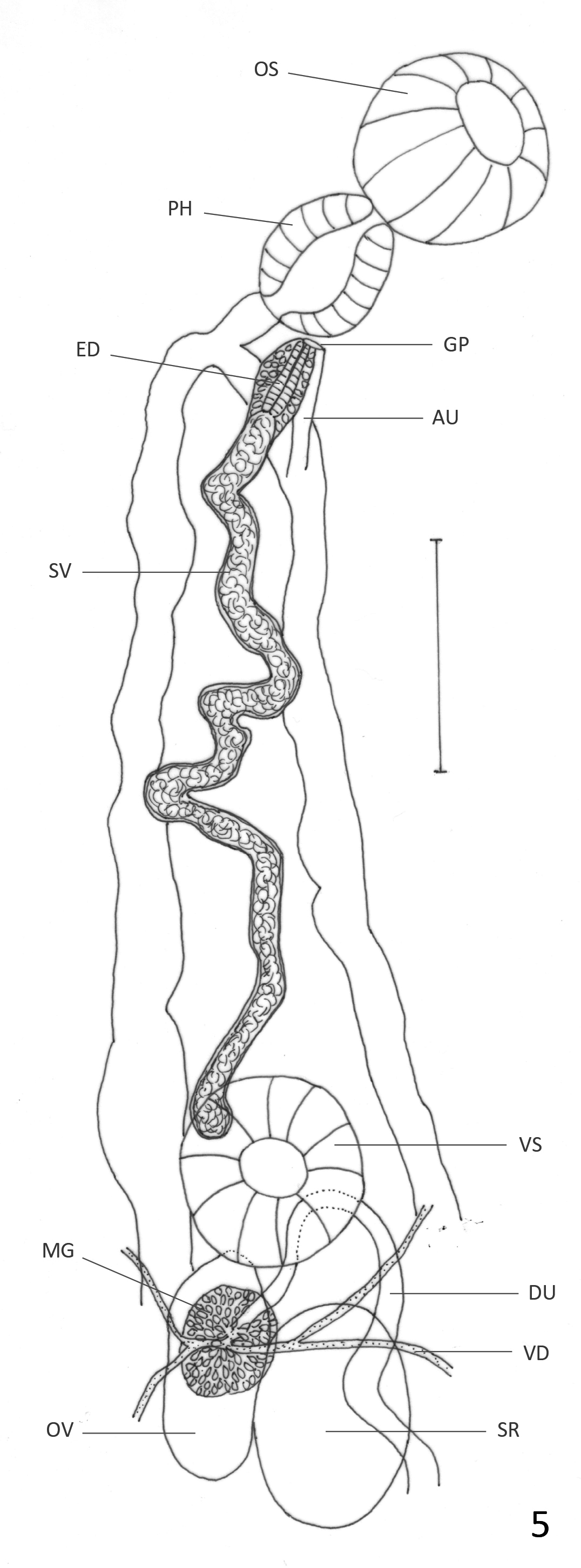
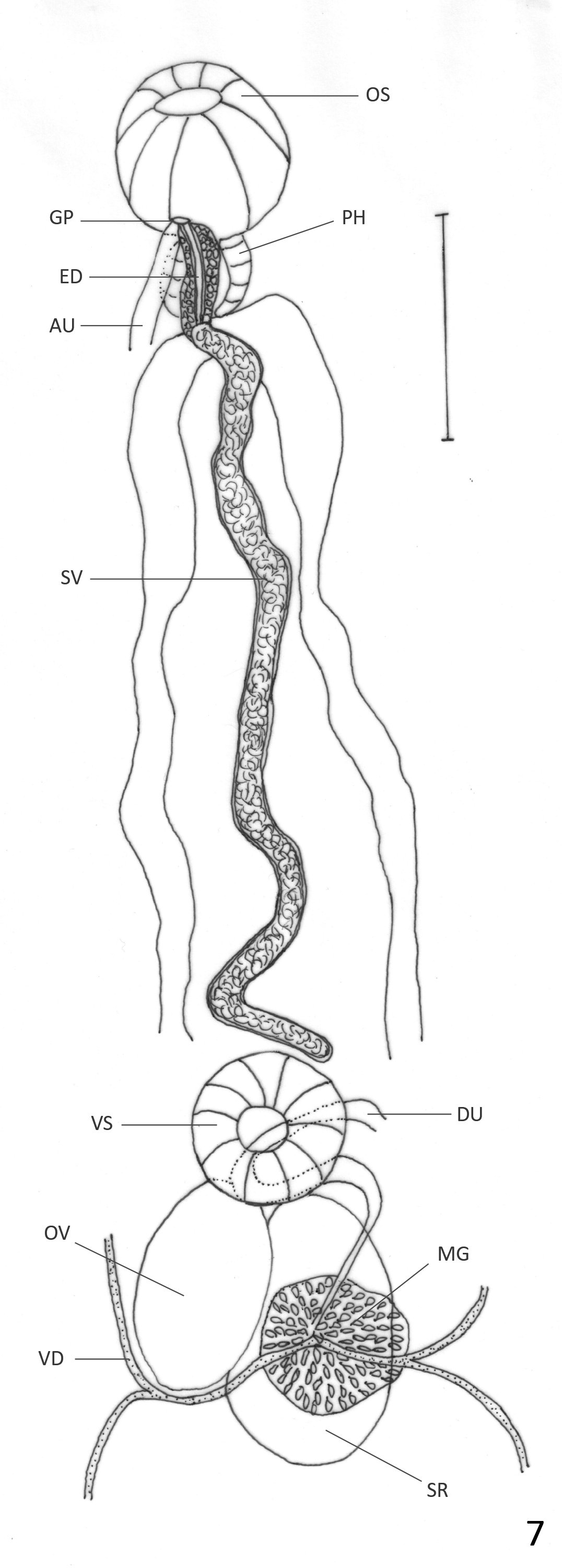
( Figs. 6 View FIGURE 6 & 7 View FIGURE 7 )
Type host: Rana sp. These host specimens belong to an undescribed species of leopard frog that is closely related to Forrer´s leopard frog Rana forreri Boulenger ( Zaldívar–Riverón et al. 2004) .
Type locality: Coquimatlán, Colima, México.
Site of infection: lungs.
Holotype: CNHE 10497
Paratypes: CNHE 10498–10504 , 10506 , 10507 , 4448
Other hosts and localities: Rio Grande leopard frog R. berlandieri , San Antonio Chimalapas, Oaxaca; Brown´s leopard frog R. brownorum, Rizo de Oro , Chiapas (Velazquez–Urrieta & León-Règagnon 2017); Forrer´s leopard frog R. cf. forreri , San Pedro las Playas, Guerrero ( Cabrera–Guzmán et al. 2007 as H. coloradensis ), Tehuantepec, Oaxaca (CNHE 10509), Santa María del Oro, Nayarit; Transverse Volcanic leopard frog R. neovolcanica, Ocotlán , Jalisco; showy leopard frog R. spectabilis, Mitla , Oaxaca (CNHE 10510); Rana sp., San Pablo Huitzo, Oaxaca (CNHE 10508), Nizanda, Oaxaca (CNHE 10511), San Fernando, Chiapas; cane toad Rhinella marina, Tres Palos, Guerrero.
Etymology: The species name refers to its distribution in western Mexico.
Description: Based on 21 mature specimens. Body elongate, with slender anterior region; 2.4–6.2 (4.5) mm long, 0.64–1.66 (1.24) mm of maximum width at testicular region. Tegument aspinose, even in live specimens. Oral sucker subterminal, round, 195–527 (392) long, 203–487 (385) wide. Pharynx oval, 114–252 (196) long, 122– 227 (187) wide; oral sucker: pharynx ratio 1: 0.43–0.65 (0.51). Pharynx and anterior region of esophagus surrounded by abundant glans cells. Esophagus 32–130 (84) long, sometimes obscured by uterus. Ceca bifurcated at 325–852 (621) from anterior extremity. Ceca terminate blindly near posterior extremity. Ventral sucker round, 162–365 (264) long, 162–365 (271) wide, at 0.96–2.5 (1.7) mm (26%–53% (38%) of BL) from anterior extremity. Sucker length ratio 1: 0.61–0.8 (0.70). Testes 2, oval, slightly lobed in some specimens, oblique, inmmediately posterior to ovary. Anterior testis opposite to ovary, 219–730 (477) long, 203–730 (466) wide. Posterior testis 259– 893 (553) long, 235–812 (482) wide. Cirrus sac reaches anterior border of ventral sucker, mostly obscured by ascending uterus; internal seminal vesicle, elongate, slightly coiled. Ejaculatory duct weakly muscular, 220–230 (225) long, surrounded by prostatic gland cells. Ovary oval, 252–536 (409) long, 187–446 (301) wide; at 1.1–2.8 (1.9) mm (33%–55% (42%) of BL) from anterior extremity. Seminal receptacle adjacent, partially overlaps with ovary; 244–730 (472) long, 162–511 (337) wide. Mehlis gland dorsal to seminal receptacle. Laurer’s canal not observed. Vitellaria in clusters overlapped with each other, distributed laterally, dorsally invade space between ceca in their anterior limit and in postesticular region. Anterior limit of distribution 487–1152 (778) (10%–25% (17%) of BL) from anterior end. Follicles extend asymmetrically, to level of anterior testis on ovarian side of body, and halfway between posterior testis and posterior end on side opposite to ovary. Uterine loops fill intra- and extracecal space, partially overlapped with testes and ovary. Descending and ascending parts of uterus form two lateral fields of transverse or diagonal loops that occasionally bend anteriorly or posteriorly and form very short longitudinal extracecal loops. One or two diagonal uterine loops oriented anteriorly often present in posterior end of body. Distal uterus fills entire preovarian region with diagonal loops. Genital pore median, ventral to pharynx. Eggs dark brown, 30–39 (34) long, 16–21 (19) wide. Excretory vesicle not observed. Excretory pore terminal.
Remarks: Haematoloechus occidentalis n. sp. belongs to the group of species that are similar to H. complexus , and as with H. caballeroi , it differs from many other species in the genus by its lack of longitudinal uterine loops at the posterior end of body reaching at least the posterior testis, by the large size of the ventral sucker which is more than half the size of the oral sucker, by having uterine loops invading the extracecal area and by having an oval ovary and testes (see H. caballeroi remarks).
Considering the characters mentioned above, Haematoloechus occidentalis n. sp. resembles H. caballeroi , H. complexus , H. elongatus , H. fuelleborni , H. humboldtensis , H. kernensis , H. longicollum , H. parcivitellarius , H. pukinensis and H. pulcher . It differs from H. humboldtensis , H. longicollum , H. parcivitellarius , and H. pulcher by its presence of diagonal uterine loops directed anteriorly at the posterior end of the body, which are either absent or directed posteriorly in those species ( Caballero 1942b; Bravo–Hollis 1943; Zamparo et al. 2011; León-Règagnon & Romero–Mayén 2017). Haematoloechus occidentalis n. sp. differs from H. kernensis , and H. pukinensis in having a smaller ventral sucker compared to the oral sucker (1:1 & 1:0.94 respectively, vs 1: 0.70 in H. occidentalis n. sp.) ( Ingles 1932; Ibáñez & Córdoba 1979). It also differs from those two species in the arrangement of the uterine loops; while in H. kernensis and H. pukinensis the ascending part of the uterus forms a few transverse loops in the post-testicular and in the pre-acetabular region ( Ibáñez & Córdoba 1979), in H. occidentalis n. sp. the ascending uterus fills both regions with transverse or diagonal loops. It differs from H. caballeroi in the uterine loops being transverse or diagonal in that species, never bending to form short longitudinal loops as happens in H. occidentalis n. sp.; also, the ejaculatory duct in H. caballeroi is strongly muscular while it is weakly muscular in H. occidentalis n. sp. ( Figs. 5 View FIGURE 5 & 7 View FIGURE 7 ). Haematoloechus occidentalis n. sp. differs from H. fuelleborni in the size of the ventral sucker compared to the oral sucker, which is larger in the new species (1: 0.5 in H. fuelleborni vs 1: 0.7 in H. occidentalis n. sp.). Also, the distribution of the vitellaria is different; in H. fuelleborni they are limited to two groups, one anterior to the ventral sucker and the other posterior to the testes, while they are distributed continuously extending asymmetrically, from the region anterior to the ventral sucker, to the level of anterior testis on the ovarian side of body, and halfway between the posterior testis and the posterior end on the side opposite to the ovary in H. occidentalis n. sp. Haematoloechus occidentalis n. sp. differs from H. elongatus in the distribution of the vitellaria, which are asymmetrical in H. occidentalis n. sp. while they reach the posterior region of testes on both sides of the body in H. elongatus . Also, that species is much larger in body size than H. occidentalis n. sp. (9.5 mm vs 4.5 mm) and in H. elongatus the uterine loops are transverse or diagonal, never bending to form short longitudinal loops as does in H. occidentalis n. sp. ( Caballero & Sokoloff 1934). Haematoloechus complexus and H. occidentalis n. sp. differ in the arrangement of the uterine loops. While in H. complexus the uterine loops are transverse in the post-acetabular region, and diagonal, oriented anteriorly in the posterior end ( Bolek & Janovy 2007a), in H. occidentalis n. sp. the uterine loops are transverse or diagonal often bending to form short longitudinal loops that can be oriented anteriorly or posteriorly.
Haematoloechus occidentalis n. sp. is one of the species that were differentiated using COI sequences by León-Règagnon (2010) (Ha63, Genbank HQ141705 View Materials ) and are included in the "complexus group".
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.