Goussia bayae, Matsche & Adams & Blazer, 2019
|
publication ID |
https://doi.org/ 10.1645/18-67 |
|
publication LSID |
lsid:zoobank.org:pub:91055D6F-6DF6-4093-A39E-C977D2AE2575 |
|
DOI |
https://doi.org/10.5281/zenodo.7752621 |
|
persistent identifier |
https://treatment.plazi.org/id/BEAD36F7-B4A7-48C1-A2B3-49A6A1B9069F |
|
taxon LSID |
lsid:zoobank.org:act:BEAD36F7-B4A7-48C1-A2B3-49A6A1B9069F |
|
treatment provided by |
Felipe |
|
scientific name |
Goussia bayae |
| status |
sp. nov. |
Goussia bayae n. sp.
( Figs. 1–4 View Figure 1 View Figure 2 View Figure 3 View Figure 4 )
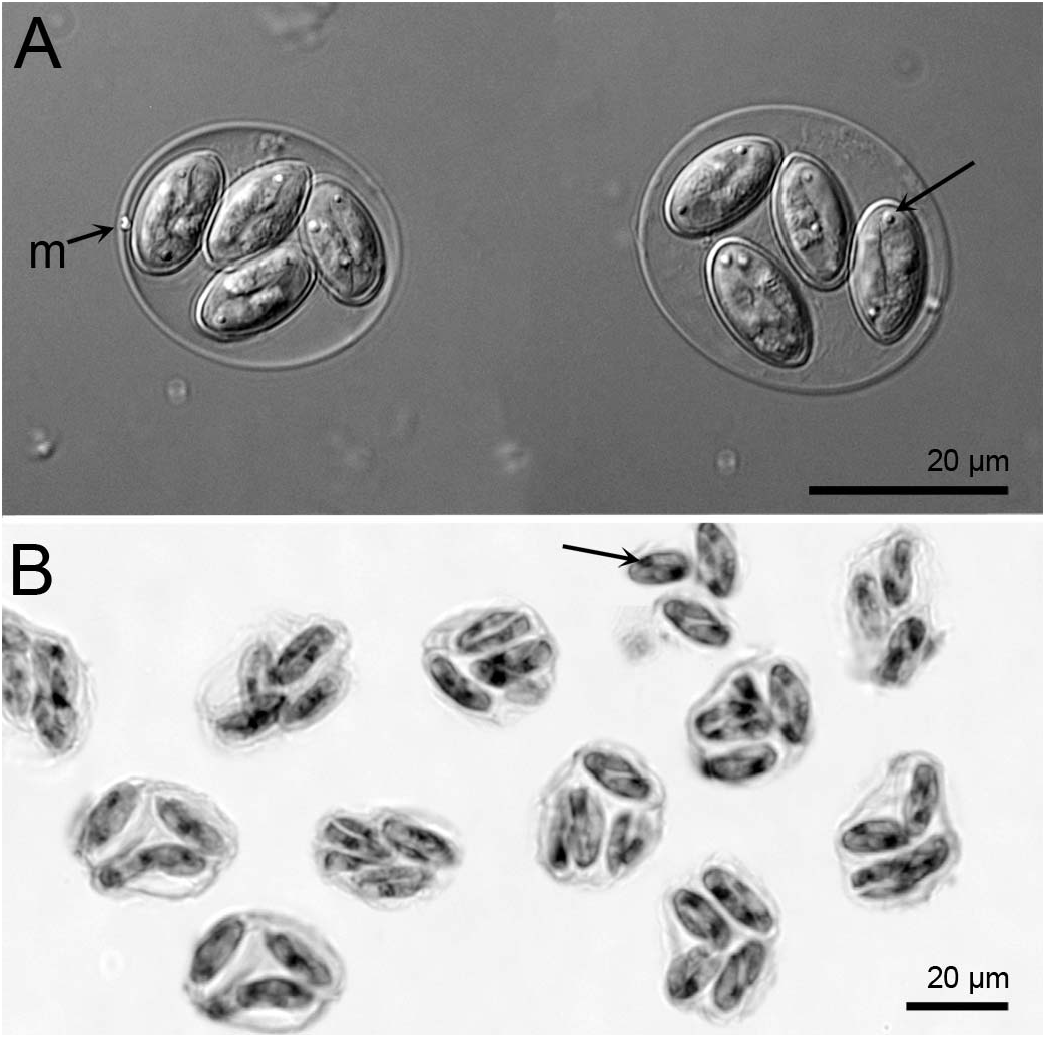
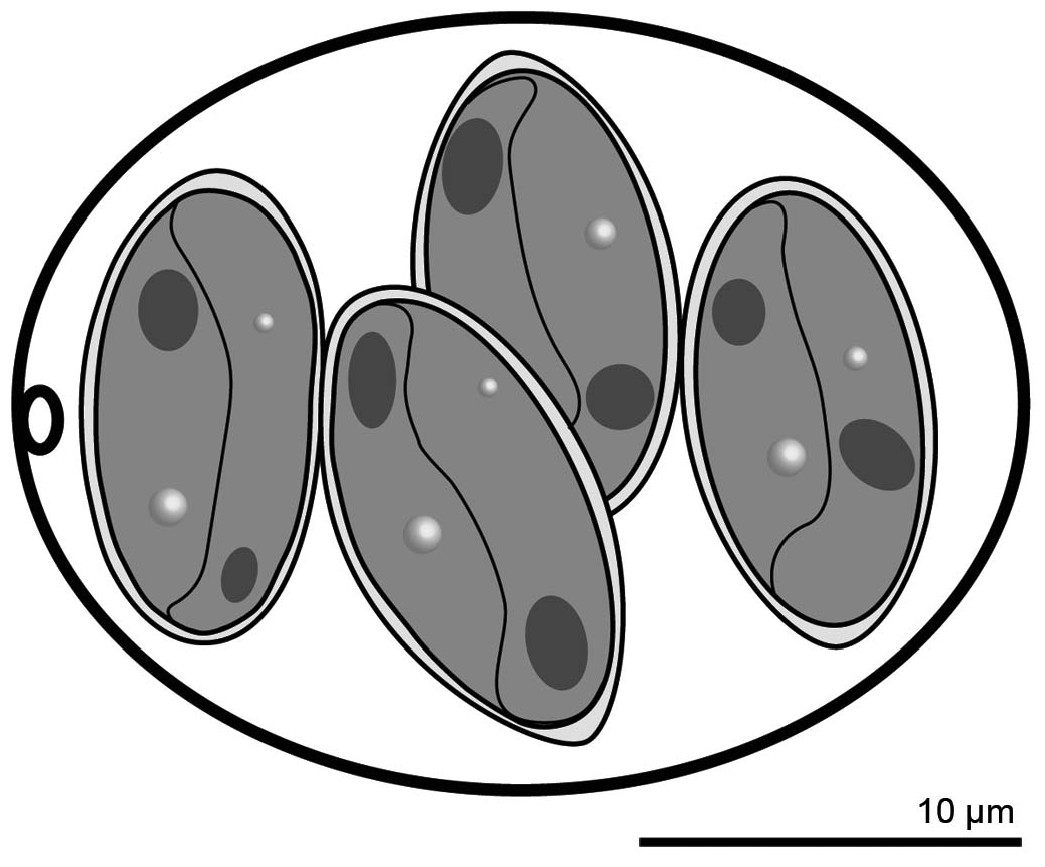
Diagnosis: Oocyst shape: subspheroidal, with a single-layered smooth wall, ~ 0.4–0.6 thick; oocyst length (L) X width (W): 26.2 ± 2.1 SD X 21.8 ± 2.1 SD (ranges: 22–30 X 18–25); oocyst L/ W 1.2 ± 0.05 SD (range: 1.1–1.3) ( Figs. 1 View Figure 1 , 2 View Figure 2 ); micropyle present; oocyst residuum and polar granules absent; sporocyst shape: ellipsoidal, slightly tapered on 1 end, ~ 12.6 ± 0.6 SD X 7.8 ± 0.8 SD (ranges: 10–14 X 6–9), sporocyst L/ W 1.6 ± 0.1 SD (range: 1.4–1.9), with a smooth wall composed of 2 valves joined by a longitudinal suture; Stieda body, sub-Stieda body, sporocyst residuum absent; excysted sporozoite shape: slightly arcuate, 1 end tapered to a point; nucleus and refractile bodies present.
Taxonomic summary
Type host: White perch, M. americana (Gmelin 1789) ( Perciformes : Moronidae ).
Other hosts: Unknown.
Site of infection: Hepatic bile ducts, common duct, and gallbladder.
Prevalence of infection: Of 150 fish examined, 100%.
Type locality: Choptank River, Preston , Maryland (38°46 ′ 36.0 ′′ N, 75°58 ′ 10.4 ′′ W) GoogleMaps .
Type specimens: Histological sections of infected tissues stained with H&E ( USNM 1507371 View Materials and 1507381) and photosyntypes ( USNM 1490787 View Materials , 1490788 View Materials , 1490789 View Materials , and 1490790) were deposited in the National Museum of Natural History, Smithsonian Institution , Washington , D.C.
Etymology: The specific name is in honor of colleague and mentor Ana Baya.
Remarks
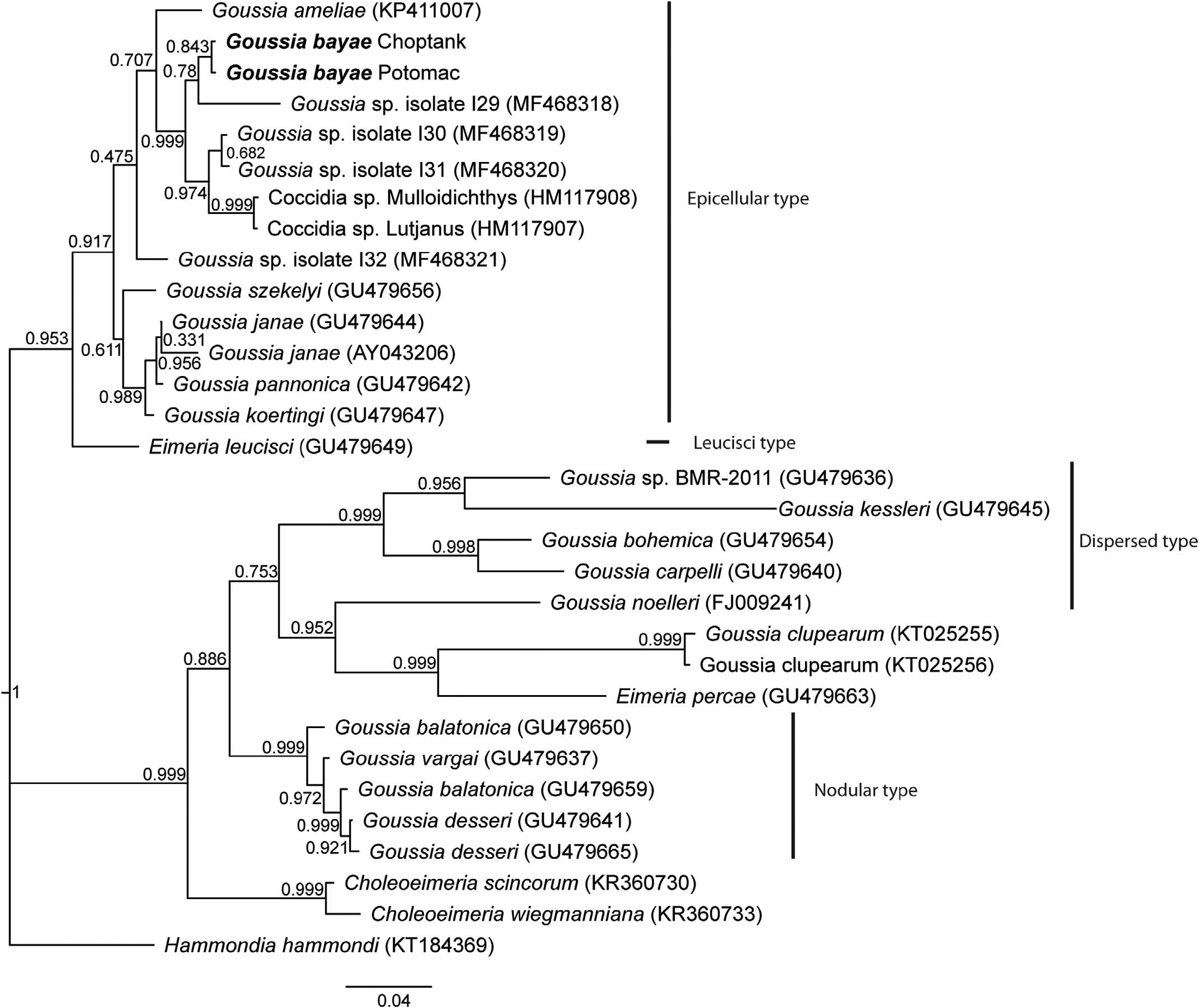
Phylogenetic analyses of other Goussia spp. using partial 18S rDNA placed G. bayae n. sp. in a clade of Goussia ( Fig. 5 View Figure 5 ), defined as epicellular (sensu Rosenthal et al., 2016). Goussia bayae samples isolated from both the Choptank and Potomac were identical. The closest relative to G. bayae by Bayesian analysis was Goussia sp. isolate I29 ( MF468318 View Materials ), despite sharing only 95.4% identity. The isolate is morphologically undescribed, but was found in the intestine of a farmed European bass ( Dicentrarchus labrax ) ( Xavier et al., 2018a). The next nearest neighbors were also not fully morphologically described, but isolated from the heart and kidney of wild-caught chub mackerel Scomber japonicas ( MF468319 View Materials , MF468320 View Materials ), the spleen and kidney of the bluestripe snapper Lutjanus kasmira (HM117907), and the liver and spleen of the orange goatfish Mulloidichthys pfluegeri (HM117908). Goussia bayae shared 98.3% identity with Goussia sp. from the chub mackerel and 98.4% identity with sequences from the bluestripe snapper and orange goatfish. Of the remaining Goussia in the epicellular clade, only Goussia ameliae from alewives Alosa pseudoharengus has been reported from North America (Lovy and Friend, 2015).
Goussia bayae possesses a micropyle ( Figs. 1 View Figure 1 , 2 View Figure 2 ), whereas the oocysts of all other epicellular species compared with here lack a micropyle and are distinctly smaller. The closest in size to G. bayae is G. ameliae (oocyst L X W: 18.6 X 14.1) from landlocked alewives and Goussia janae (oocyst L X W: 18.1 X 12.7). However, G. ameliae has abundant sporocyst residuum and G. janae sporocysts are longer (L: 13.5) and more slender (W: 5, L/W: 2.7) compared with the sporocysts of G. bayae .
Goussia bayae is morphologically distinct from 2 eimerians previously reported from white perch. Oocyst diameters of E. glenorensis (12.0–10.5) and E. moronei (8.0–7.2) were markedly smaller than G. bayae and a micropyle was absent in both eimerians (Molnar and Fernando, 1974). Also, a Stieda body was present and refractile bodies were absent in E. glenorensi and E. moronei sporocysts.
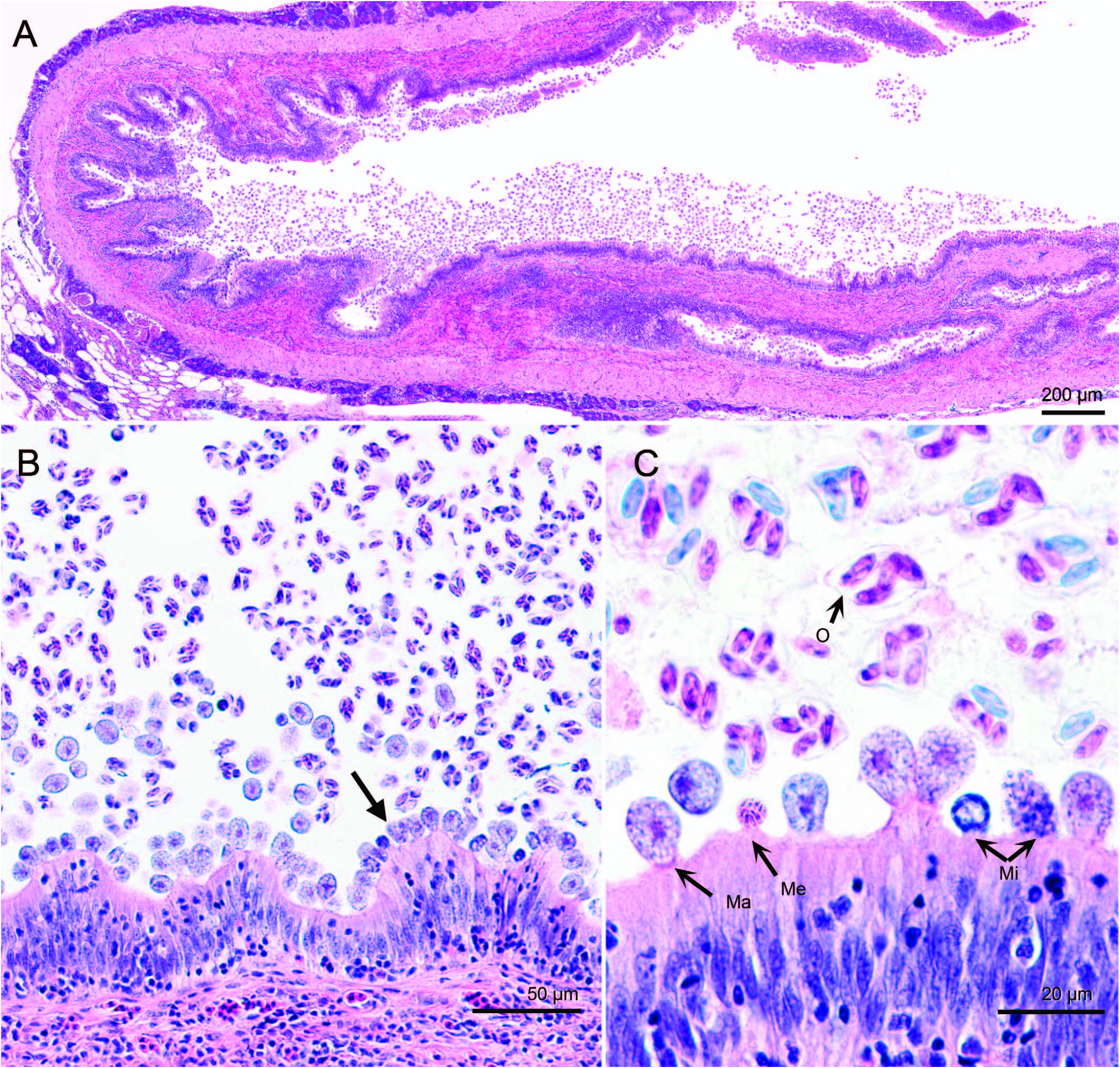
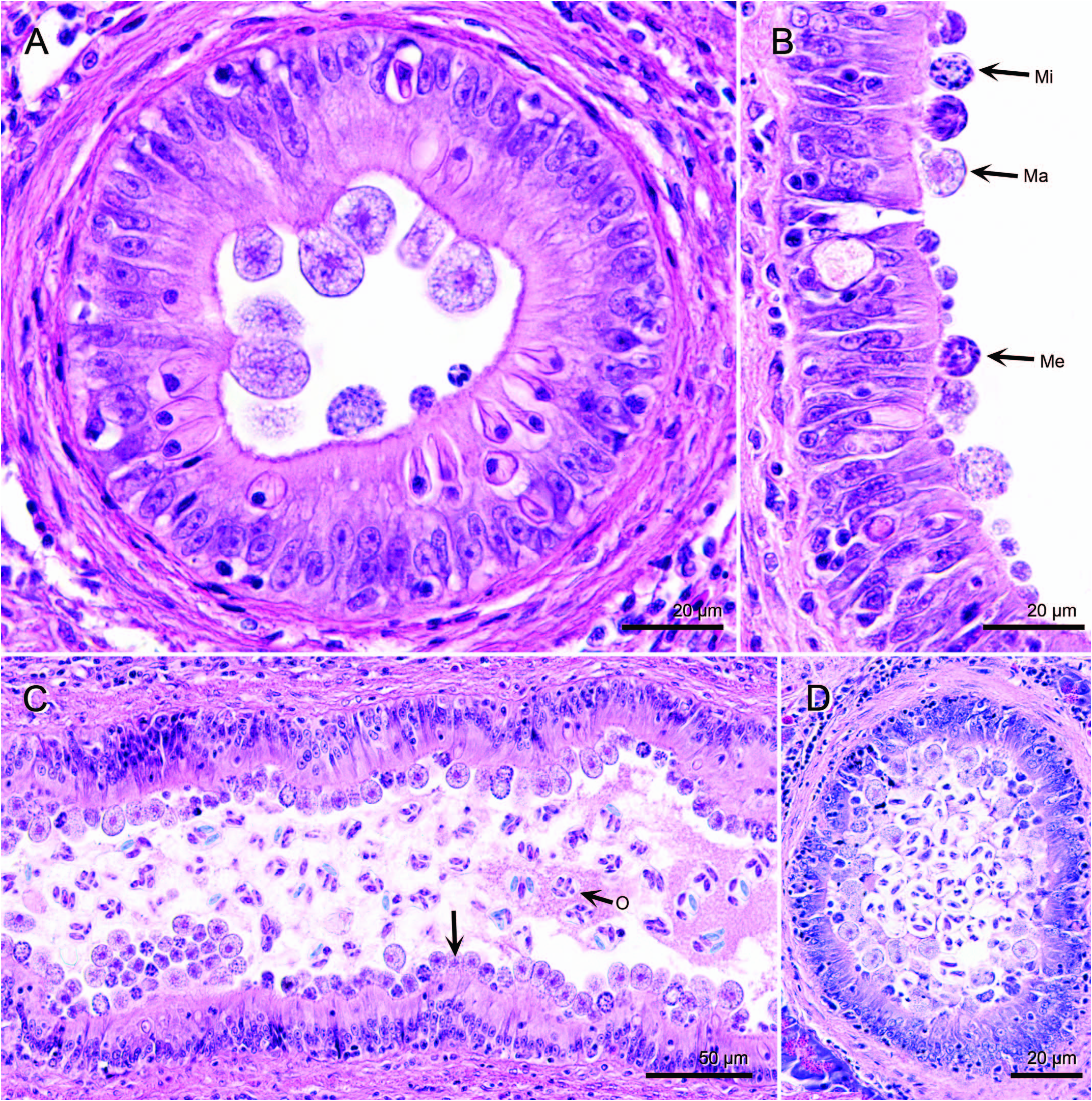
Histological examination of tissues indicated large numbers of coccidia in the gallbladder ( Fig. 3 View Figure 3 ). Coccidian development was asynchronous and sporulation was endogenous. Meronts and gamonts were epicellular to the biliary epithelium, whereas oocysts were in the lumen ( Fig. 3B, C View Figure 3 ). These stages of G. bayae were also observed in hepatic bile ducts and common duct ( Fig. 4 View Figure 4 ). Meronts contained elongate merozoites that stained basophilic ( Figs. 3C View Figure 3 , 4B View Figure 4 ). Microgamonts contained microgametocytes that were scattered throughout the cytoplasm or aligned near the wall ( Figs. 3C View Figure 3 , 4B View Figure 4 ). Macrogamonts were larger than meronts and microgamonts, and contained a central eosinophilic nucleus and a coarse cytoplasm ( Figs. 3C View Figure 3 , 4B View Figure 4 ). Although prevalence was 100%, the severity of infection was highly variable among fish, ranging from mild to severe. In fish with mild infections, not all intrahepatic bile duct branches contained coccidia, whereas duct branches in severely infected fish were generally enlarged with numerous coccidia. Developing stages of G. bayae were not detected in the stomach, pyloric cecae, or intestine of fish. However, bile that passed through the ductus choledochus into the intestine contained sporulated oocysts of G. bayae .
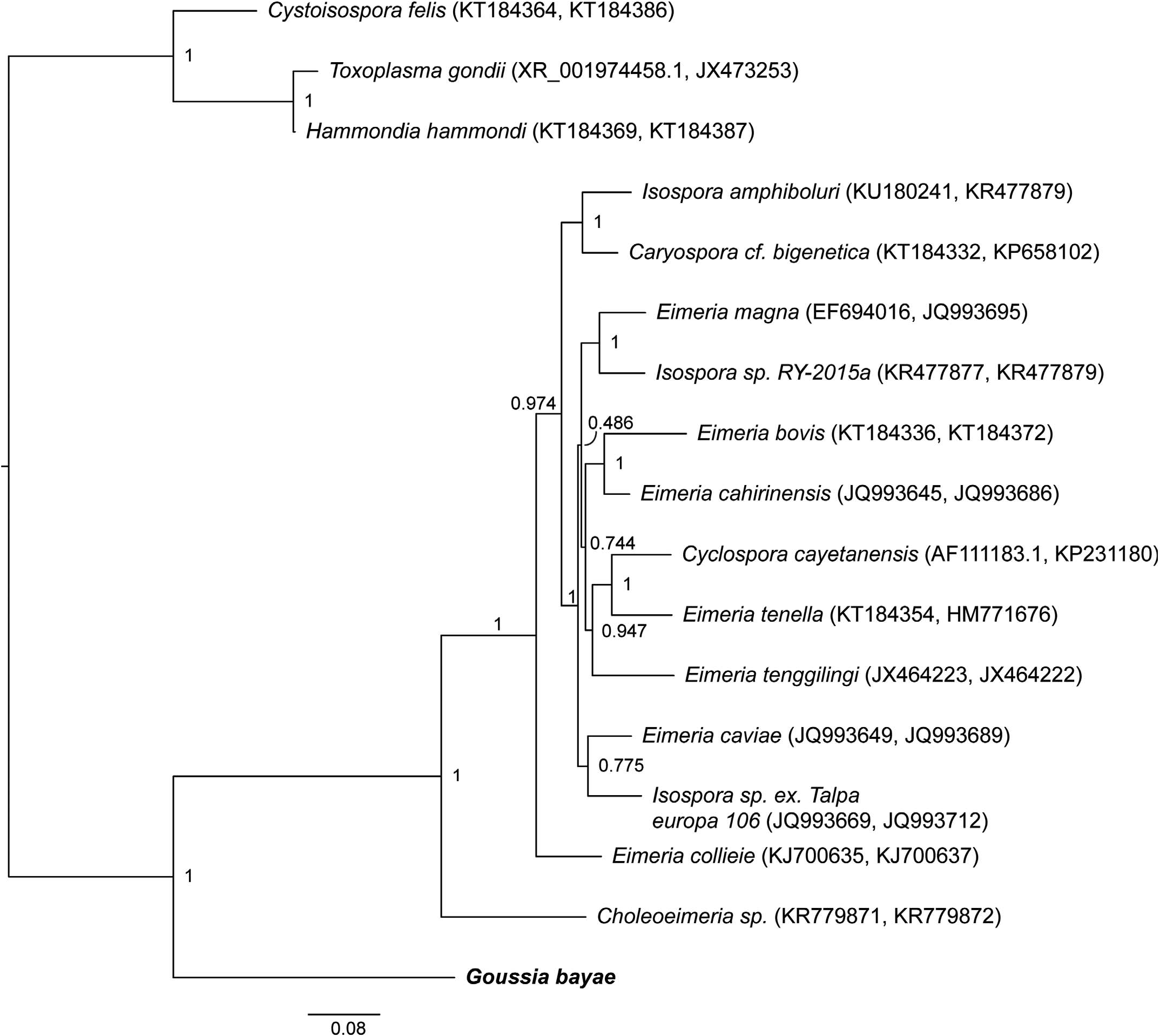
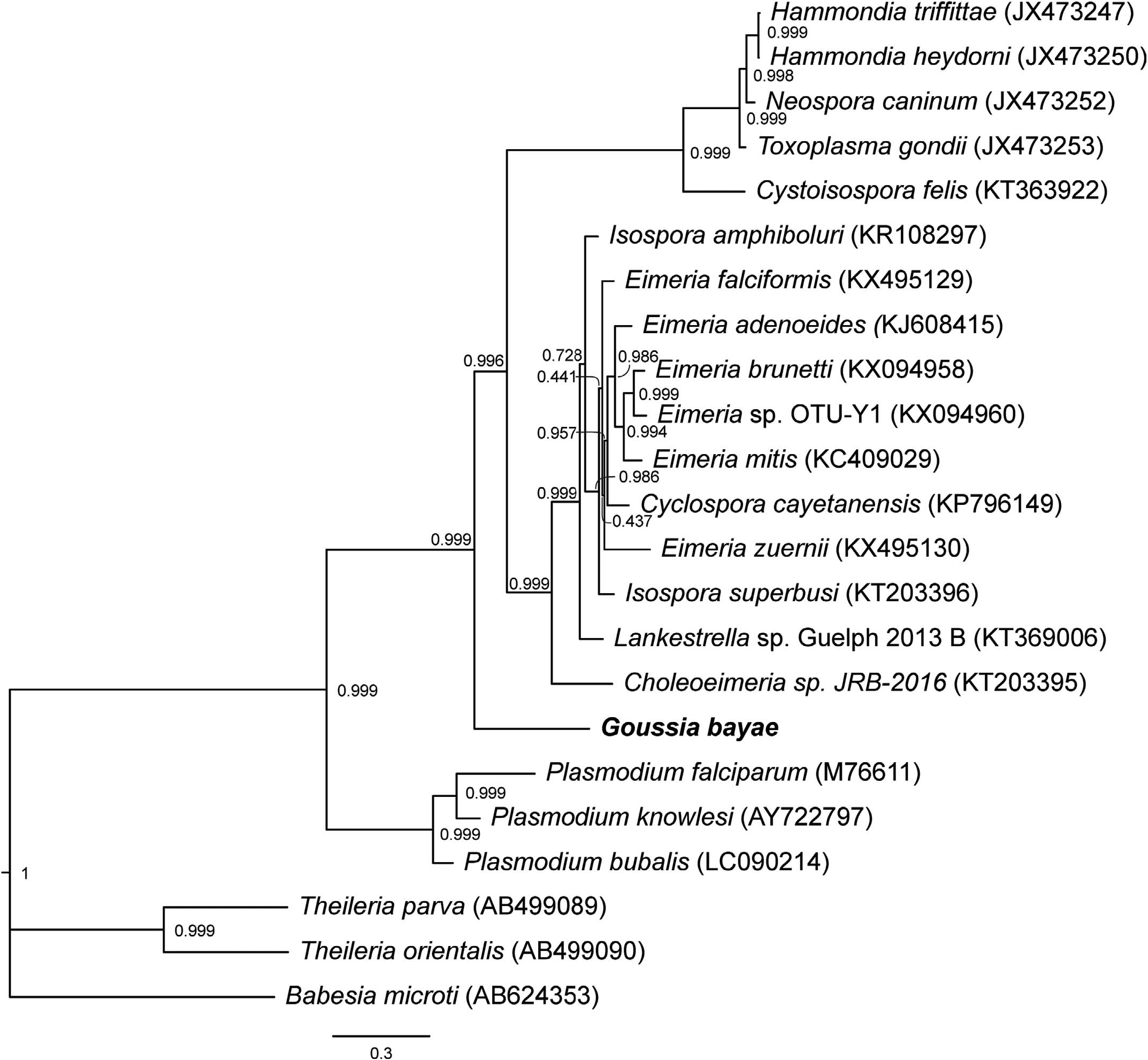
Molecular analyses also report the first mitochondrial DNA sequence from a Goussia species. The other evolutionarily distinct lineages of Goussia shown in Figure 5 View Figure 5 do not yet have mitochondrial DNA sequences available. Bayesian analyses of the combination of gene markers 18S rDNA and COI ( Fig. 6 View Figure 6 ), as well as of the partitioned mitochondrial genes (COI and Cytb) ( Fig. 7 View Figure 7 ), definitively placed G. bayae basal to Choleoeimeria sp. and other Eimeria species with 0.999 bootstrap support. The nearest-neighbor by distance was Choleoeimeria sp. , which is similar in size, sporocyst, sporozoite, and excystation structures, and in biliary tissue localization, but infects reptiles ( Szczepaniak et al., 2016). In comparing mitochondrial genes, haematozoeans Babesia , Theileria , and Plasmodium are ancestrally related to the coccidians ( Fig. 7 View Figure 7 ). Goussia bayae is sister group and basal to Eimeriidae , Choleoeimeria, and Sarcocystidae ( Fig. 7 View Figure 7 ). Basal placement of G. bayae to Eimeriidae was also supported by analysis of all 3 mitochondrial genes (data not shown).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.