Francisella tularensis, Dorofeev, 1947
|
publication ID |
https://doi.org/ 10.1016/j.ijppaw.2023.11.005 |
|
DOI |
https://doi.org/10.5281/zenodo.11043156 |
|
persistent identifier |
https://treatment.plazi.org/id/03B75452-1566-FF8C-3624-FE1CFA8181D6 |
|
treatment provided by |
Felipe |
|
scientific name |
Francisella tularensis |
| status |
|
3.2. Detection of Francisella tularensis View in CoL subspecies
The presence of F. tularensis was not detected through the pdpD-2 gene within both blood and tick samples. However, the RD1 gene did yield amplification, producing a 520 bp product size. In tick samples, the outcomes based on the RD1 gene indicated the presence of 15 samples belonging to the holarctica . Similarly, from blood samples, seven instances were isolated that also fell under the holarctica .
3.3. Phylogenetic analysis
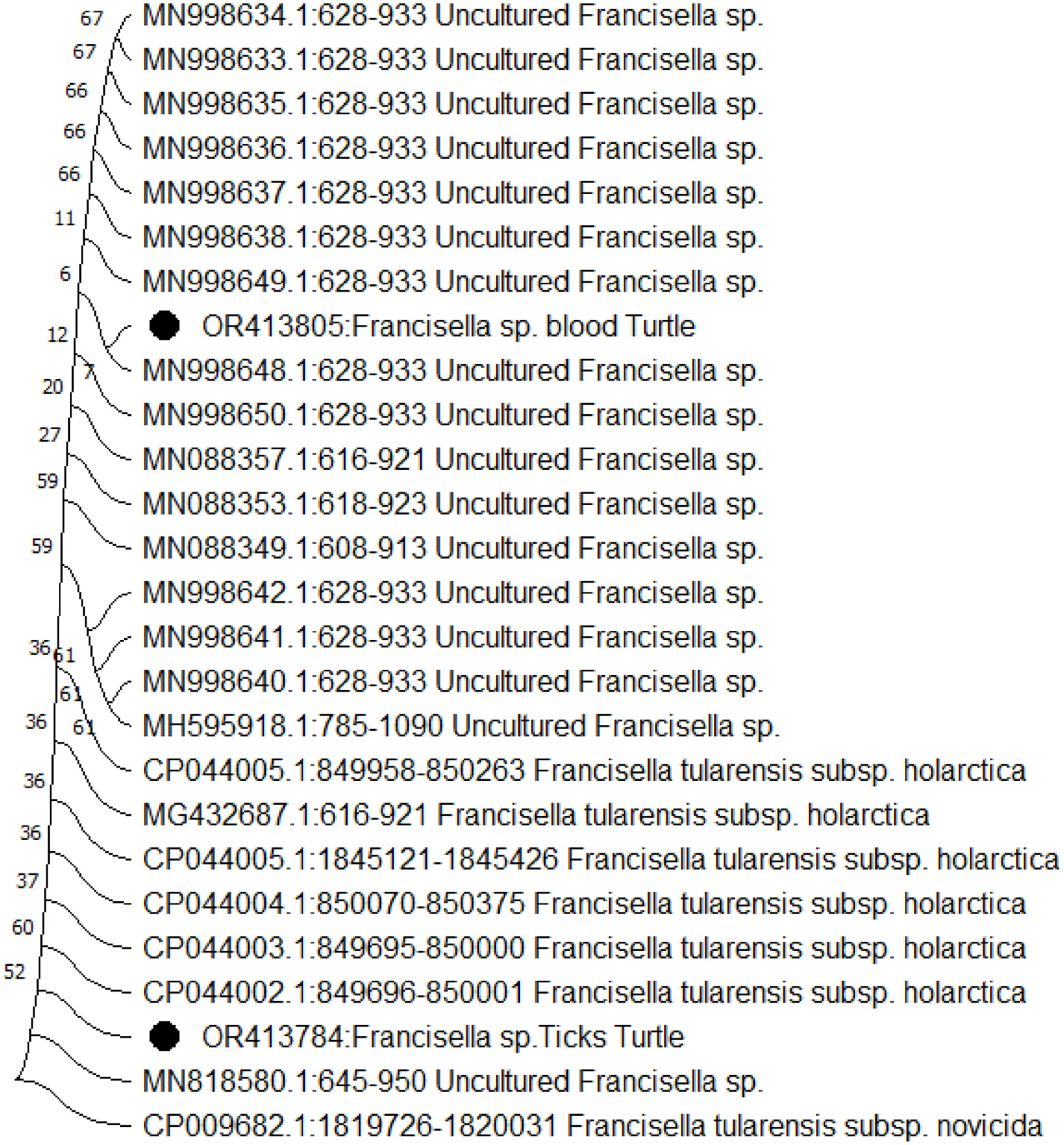
Through the construction of a phylogenetic tree utilizing neighborjoining analysis of both the 16S rRNA and RD1 partial genes, it was discerned that four isolates exhibited close clustering. These isolates showcased a high degree of similarity, ranging between 99.9% and 100%, signifying their similarity as practically identical ( Figs. 2 View Fig and 3 View Fig ).
Figs. 2 View Fig and 3 View Fig illustrate the outcomes of phylogenetic analyses carried out utilizing RD1 and 16S rRNA sequences. The nucleotide sequences recorded in this study have been assigned accession numbers (16S rRNA: OR413784, OR413805) and (RD1: OR396897, OR396898). These sequences have been added to the GenBank database, which can be accessed at https://www.ncbi.nlm.nih.gov/genbank/.
4. Discussion
The present study was the first study on Francisella in the blood samples and tick pool of West Azerbaijan province (Oshnavieh). Results of the present study revealed that 23% of the all examined tick samples were positive for Francisella . Also, the contamination of Francisella genus in blood samples was 7%.
Ticks play a significant role in transporting critical pathogens for both humans and animals, making them indicators of infection within natural ecosystems ( Rizzoli et al., 2011). In recent times, there has been an expansion in the geographical range and habitats of diverse and adaptable tick species. This growth trend is substantially influenced by factors such as alterations in land utilization, shifts in climate patterns, and the global interconnectedness ( Gubler et al., 2001; Harrus and Baneth, 2005). On the flip side, specific tick species, like H. aegyptium , demonstrate a pattern of shrinking geographical distribution, closely linked to the diminishing populations of their vulnerable hosts ( Mihalca et al., 2011).
Nevertheless, as a broader principle, the decline in the presence of natural host populations may instigate a phenomenon known as hostswitching behavior ( Keesing et al., 2010). Given that H. aegyptium is known to shift its feeding preference towards a variety of other hosts, particularly during its pre-adult stages, the assessment of its zoonotic pathogen load holds significant importance.
In terms of their contribution to the ecology of zoonotic infectious diseases, tortoises and their ticks have received considerably less focus in comparison to mammals and birds. Among small mammals, hedgehogs ( Erinaceus spp. ) hold particular importance in synanthropic environments. In such contexts, they act as reservoir hosts for important human pathogens, including A. phagocytophilum , Babesia spp. ( Silaghi et al., 2012), and B. burgdorferi s.l. ( Skuballa et al., 2012). Considering H. aegyptium ’s occasional feeding on hedgehogs and its potential interaction with humans ( Bursali et al., 2010), it becomes essential to evaluate the role of this species as a carrier host for zoonotic pathogens.
In this study, the Real Time-PCR technique was employed to examine the presence of F. tularensis in both small ruminants and the associated ticks in the western region of Iran. Through this method, F. tularensis DNA was identified in 0.82% of tick samples collected from the Kurdistan province. However, no presence of the bacteria was observed in the sheep and goat populations ( Rahravani et al., 2022).
Starting from 2011, the Pasteur Institute of Iran has shown growing interest in exploring tularemia within the country. Most of the research efforts in Iran have predominantly concentrated on rodents, the environment, and the human populations vulnerable to the disease. These studies have sounded cautionary notes about the potential emergence of tularemia outbreaks in the country.
Despite these previous warnings, there remains a substantial dearth of research conducted on F. tularensis in arthropod vectors within the Iranian context. Hence, a vital goal is to comprehend the origins of infection within the lifecycle of Francisella in Iran. As a part of this ongoing investigation, the DNAs of F. tularensis found in tick samples underwent molecular subtyping assays, leading to their categorization as F. tularensis subsp. This particular subspecies, holarctica , exhibits reduced virulence in both humans and animals and is widely distributed across Asia, Europe, and Eurasia (Sj¨ostedt, 2007; Rahravani et al., 2022). Research conducted in Turkey, a neighboring country to the northwest, has emphasized the existence of F. tularensis subsp. holarctica within its territory ( Yeşilyurt et al., 2011; Duzlu et al., 2016).
This particular subspecies of F. tularensis is frequently linked to water sources that have been contaminated, such as lakes, rivers, and ponds. A previous investigation also proposed waterborne transmission as a potential avenue for a tularemia outbreak in Iran ( Esmaeili et al., 2021).
Taking into account this collection of evidence along with the findings of the present study, there is a potential indication that subsp. holarctica is present within Iran. Given the infrequent occurrence of F. tularensis bacteremia ( Haristoy et al., 2003), the task of isolating F. tularensis from blood samples of livestock presents a considerable challenge. The intricacies of this lifecycle could plausibly contribute to the difficulties encountered in successfully identifying F. tularensis within livestock blood samples. Past seroepidemiological investigations carried out in this area revealed that 16% of butchers had displayed positive results for tularemia antibodies ( Esmaeili et al., 2014).
Moreover, instances of infection were documented among captured rodents (4.8%) and hunters (18%) within the Kurdistan province ( Mostafavi et al., 2017). Hence, it strongly advocates for the initiation of comprehensive research endeavors on F. tularensis involving diverse tick species and other arthropods. Such investigations are vital to ascertain their plausible contribution to the epidemiological cycle of F. tularensis in Iran, particularly within regions prone to endemicity. A molecular study conducted in Egypt unveiled the presence of Francisella spp. among 4.7% of ticks found on camels.
Nevertheless, aligning with our findings, Francisella spp. was not identified in the blood and fecal samples of camels. Intriguingly, their study unveiled a remarkable seroprevalence of F. tularensis among individuals working in slaughterhouses, indicating a greater prevalence of tick bites (20.7%) in comparison to sporadic exposure (2.2%) among workers who encountered ticks less frequently ( Ghoneim et al., 2017). Conversely, reinforcing the disparities observed, findings opposing these results were found in a study conducted in Malaysia.
The absence of Francisella spp. in ticks and animal samples collected from livestock farms suggests that its presence might be restricted to Dermacentor questing ticks, without spreading to different tick species and livestock animals. The study highlighted that 11.3% of questing ticks gathered from forest reserves exhibited positive results for the 16S rRNA of Francisella spp. In stark contrast, no ticks attached to livestock animals such as sheep, goats, and cattle, nor their corresponding blood samples, displayed any indication of Francisella spp. presence ( Koh et al., 2019).
Even though F. tularensis remains prevalent among human populations in Turkey ( Akalın et al., 2009), employing the same method has not revealed its presence in different tick and mosquito species ( Haristoy et al., 2003; Akalın et al., 2009; Duzlu et al., 2016; Demir et al., 2020). Consequently, it has been postulated that the pattern of tularemia outbreaks in Turkey might be driven by water-borne transmission rather than vector-borne transmission ( Duzlu et al., 2016). In a Japanese study, F. tularensis demonstrated prevalence within Ixodes monospinosus ticks (8.22%), yet its occurrence in Ixodes persulcatus ticks was significantly lower at 0.66% (Suzuki et al., 2016). In this study, out of the 244 ticks collected from sheep and goats, the majority were identified as D. marginatus (66.4%), with Rh. sanguineus accounting for the minority (10%). The presence of F. tularensis DNA was exclusively detected in D. marginatus ticks (1.22%). These findings indicate a potential involvement of D. marginatus in the lifecycle of F. tularensis in the Kurdistan region.
Notably, in the United States, Dermacentor variabilis ( D. variabilis ) assumes a pivotal role as a vector for F. tularensis , actively participating in the bacterium’ s natural life cycle ( Whitten et al., 2019; Zellner and Huntley, 2019). Whitten et al. have documented a tularemia prevalence of 34% in D. variabilis through a comprehensive RT-PCR study ( Whitten et al., 2019). Similar to our study, previous research conducted in the USA and Europe has typically reported F. tularensis presence in various Dermacentor species ranging from less than 1% to less than 10% ( Goethert et al., 2004; Bielawska-Drozd et al., 2018; Hubalek and Rudolf, 2017).
Employing a real-time TaqMan PCR assay targeting the tul4 and ISFtu2 genes, an examination was conducted on ticks acquired from both wild and domestic animals across the Iberian Peninsula. The research revealed the presence of F. tularensis in 0.6% of ticks. Notably, it’ s important to mention that positive instances were primarily linked to Rh. sanguineus (25.7%) and D. marginatus (2.4%) ( de Carvalho et al., 2016).
Instances of Francisella and Francisella -like endosymbionts (FLEs) have been documented in Rh. sanguineus ticks in diverse locations, including Bulgaria ( Ivanov et al., 2011), Romania ( Andersson et al., 2018), and Thailand ( Rakthong et al., 2016).
Given the insights from previous studies, our results suggest that the prevalence of F. tularensis in ticks could vary depending on the specific tick species. For a thorough understanding of the disease dynamics, encompassing reservoirs and the primary agents of infection transmission in areas where positive cases have been detected, it is recommended to conduct a study with a larger sample size.
5. Conclusions
During the current research, the blood of examined turtles and ticks collected from various regions in Iran were positive for F. tularensis bacteremia through the PCR and Nested-PCR techniques. Importantly, a proportion of 1.6% of the ticks found on these turtles ( H. aegyptium ticks) were determined to harbor F. tularensis subsp. holarctica . Hence, it is advisable to evaluate the contribution of H. aegyptium ticks to the epidemiological cycle and the persistence of F. tularensis in Iran. Conducting more extensive screenings of both livestock and their associated ticks, encompassing a substantial volume of samples from diverse geographical regions throughout Iran is of great importance.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |