Formica bruni Kutter, 1967
|
publication ID |
https://doi.org/ 10.5281/zenodo.5392741 |
|
persistent identifier |
https://treatment.plazi.org/id/03BB87B2-FF97-F175-4D22-F9E8FE78FA2B |
|
treatment provided by |
Marcus |
|
scientific name |
Formica bruni Kutter, 1967 |
| status |
|
Formica bruni Kutter, 1967 View in CoL
TYPE LOCALITY. — Zermatt, Switzerland.
TYPE MATERIAL. — Syntypes 1 male, 4 queens, 3 workers ( MZ) [investigated].
GEOGRAPHIC ORIGIN OF THE MATERIAL STUDIED. — The numerically evaluated 201 specimens (161 workers, 17 queens, 23 males) came from Sweden 25, Germany 8, France 23, Switzerland 105, Austria 28, Yugoslavia 9, Spain 3. Total number of specimens seen> 350.
DESCRIPTION
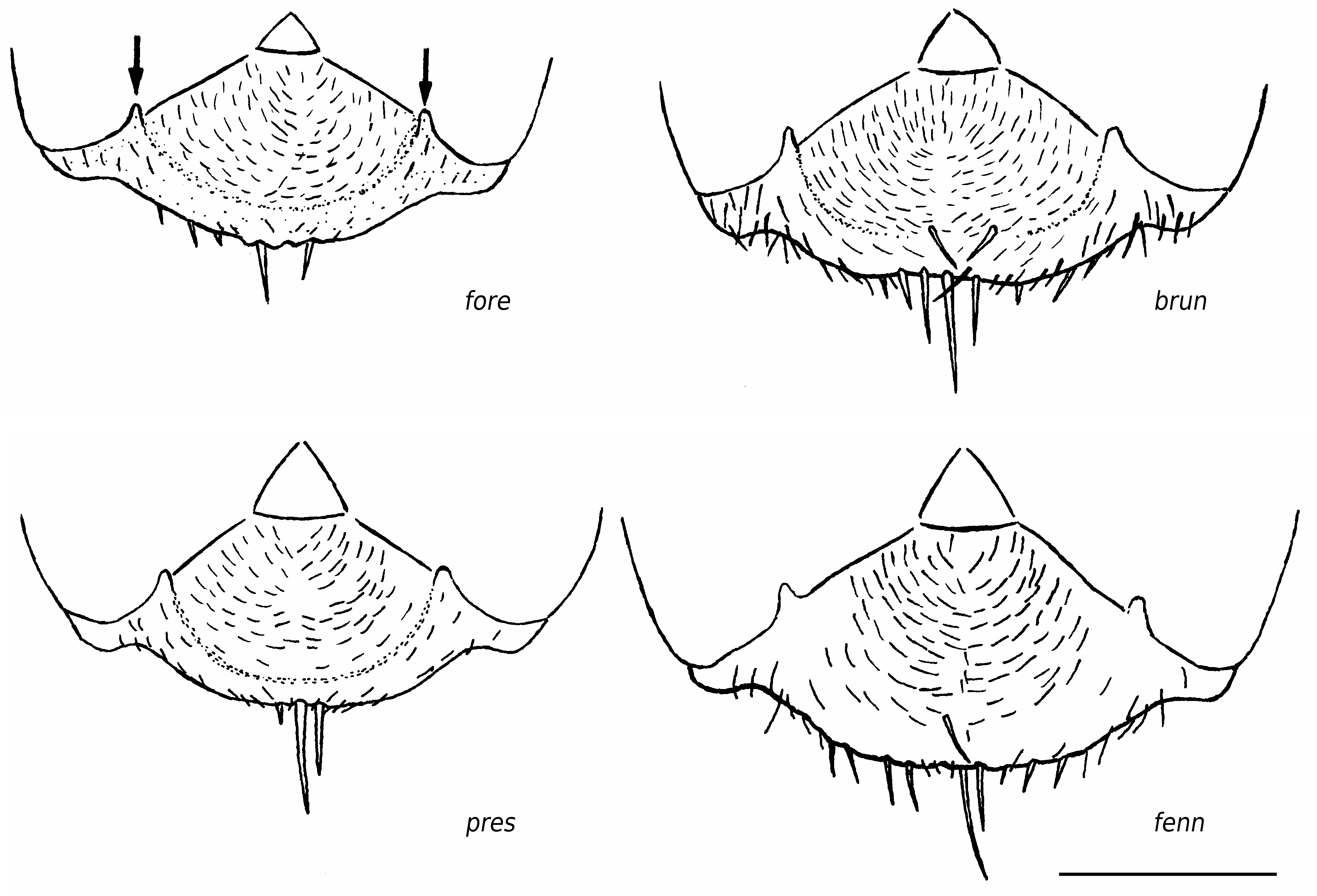
Worker ( Figs 2-4 View FIG View FIG View FIG ; 15 View FIG )
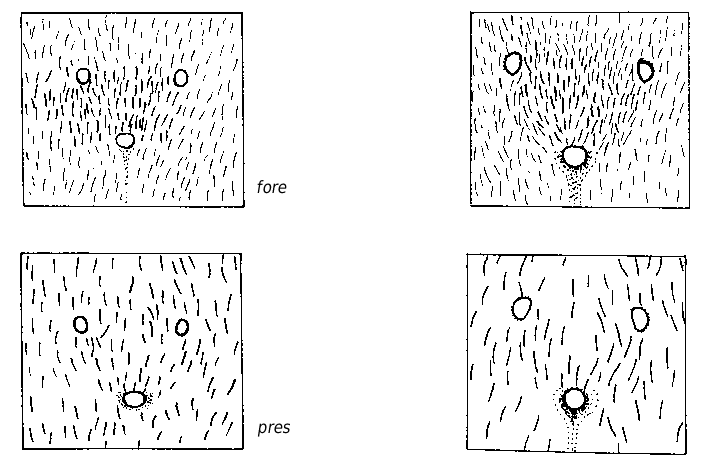
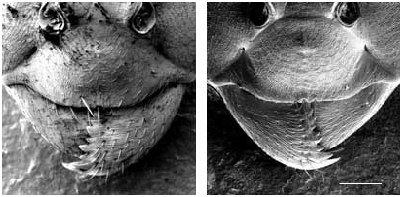
Medium-sized species (CL 1305 ± 72, 1090- 1484; CW 1244 ± 77, 1033-1449). Head of average Coptoformica shape (CL/CW 1.050 ± 0.016, 0.999-1.096). Scape rather long (SL/CL 1.013 ± 0.025, 0.941-1.082). Setae only present on anterior clypeus, long decumbent pubescence hairs on frontolateral clypeus always present (ClySet 1.77 ± 0.49,1-3; ClyPub 3.79 ± 1.17, 1.0-6.5; Figs 3 View FIG ; 15 View FIG ). Lateral semierect setae in the ocellar triangle rarely present (OceSet 22%). Eye hairs more or less developed, maximum eye hair length at least in few specimens of a nest sample> 10 µm (EyeHL 15.4 ± 5.2, 4-29). Pubescence hairs in the occellar triangle short and very dense ( Fig. 4 View FIG , sqrtPDF 4.40 ± 0.41, 3.31-5.87). Craniad profile of forecoxae without or very few semierect setae (nCOXA 0.22 ± 0.55, 0-3). Dorsal mesosoma, lateral metapleuron and ventrolateral propodeum without standing setae (nMET 0.00 ± 0.04, 0-0.5). Outer edge of the hind tibial flexor side on the distal half with few subdecumbent setae( Fig. 2 View FIG , nHTFL 4.11 ± 1.33, 1.0-8.0). Semierect setae on gaster tergites usually beginning at posterior border of third or fourth tergite (TERG 3.23 ± 0.70, 2-5). Pubescence density on first gaster tergite variable but usually rather low (sqrtPDG 6.36 ± 0.51, 5.10-7.75).
Queen
Medium-sized (CL 1429 ± 37, 1354-1488; CW 1430 ± 44, 1340-1518; ML 2359 ± 91, 2232- 2535). Head slightly elongated, but shorterheaded specimens occur (CL/CW 1.000 ± 0.025, 0.935-1.030), scape long (SL/CL 0.949 ± 0.016, 0.926-0.982). Setae restricted to anterior clypeus. Clypeus lateral of the tentorial pit level with pubescence hairs surpassing the anterior margin by more than 10 µm. Lateral semierect setae in the ocellar triangle usually absent but differentiation from pubescence difficult. Eye hairs relatively long (EyeHL 23.9 ± 4.8, 16-34). Pubescence in the occellar triangle short and extremely dense (sqrtPDF 3.46 ± 0.29, 3.05- 3.98). Occipital corners of head with subdecumbent to decumbent pubescence (OccHD 23.6 ± 5.4, 12-30). Dorsal head surface relatively matt or weakly shining (GLANZ 1.33 ± 0.28, 1.0-2.0). Craniad profile of forecoxae without or very few decumbent setae but differentiation from pubescence difficult (nCOXA 1.00 ± 1.02, 0-3). Dorsum of head, mesosoma, and gaster with profuse and dense subdecumbent to decumbent pubescence. Dorsal mesosoma usually without clearly-defined strong setae but always with dense and long subdecumbent pubescence hairs single hairs of which approach in strength to setae (MnHL 73.2 ± 10.6, 48-91). Outer edge of the hind tibial flexor side on the distal half with few subdecumbent setae and with short decumbent pubescence (nHTFL 3.41 ± 1.37, 2.0-7.0). Semierect setae on gaster tergites usually beginning on the second to fourth tergite (TERG 2.88 ± 1.05, 1-4). Pubescence on first gaster tergite very dense (sqrtPDG 4.54 ± 0.47, 3.76-5.28).
TAXONOMIC COMMENTS AND
DIFFERENTIAL DIAGNOSIS
Formica bruni shows rather constant characters throughout its geographic range and is usually well-separable from the other species. However, specimens with reduced eye hairs could be confused in particular with foreli and occasionally with pressilabris . In such cases, both species can be separated from bruni by the absence of projecting frontolateral clypeal pubescense and the absence of second level clypeal setae. F. pressilabris additionally differs by the shorter SL/CL and very sparse frontal pubescence. Details of the most useful characters to differentiate bruni , foreli , and pressilabris are given in Table 7. Queens of bruni are separable from each W Palaearctic species on the individual level; the data in Tables 7 and 8 need no further comment. The best difference to the long-headed, continental manchufennica group is the more dense frontal and tergite pubescence and the sigificantly shorter head. The most reliable difference to large foreli queens with above-average EyeHL is the presence of lateral, projecting clypeal pubescence and of long and strong subdecumbent pubescence on promesonotum (MnHL> 40, in foreli always 0).
BIOLOGY AND DISTRIBUTION
Geographic range
Formica bruni represents a submediterraneansuboceanic type with European origin. The frequent confusion with foreli and pressilabris led to an underestimation of its distribution. 112 samples identified by the author came from altogether 50 localities in Spain, France, Italy, Yugoslavia, Switzerland, Austria, Germany and S Sweden (two sites in Skåne). The French and Swiss Alps seem to be a distributional centre with 31 known sites. A record from the North Sea island of Terschelling/ Netherlands (leg. Preuss) given by Agosti (1989) seems credible but needs confirmation. The vertical distribution in the W Alps ranges from 370 to 2150 m and is, as a consequence of grassland distribution, bimodal: 13 sites are situated at 708 ± 289 [370-1240] m and 18 sites at 1638 ± 231 [1380-2150] m.
Habitat selection
F. bruni is a specialised species of thermophilous, oligotrophic grasslands. In the W Alps, the main habitats of the colline/submontane population are xerothermous grasslands (preferentially on limestone) and the montane/subalpine population is usually found on sunny, S-exposed pastures or hay meadows. The German, Austrian, and Swedish populations were found in xerothermous to semidry grasslands on limestone or sand.
Status as threatened species
In Germany one (threatened by extinction). In Switzerland probably two (severely threatened). The causes of decline are similar as in exsecta .
Colony foundation
The host species for socially parasitic foundation is unknown. Isolated, monogynous colonies are rare, i.e. the transition from monogyny to polygyny is apparently easier than in exsecta . As a rule, large polycalic colonies are found.
Nest construction
There is no difference to the normal Coptoformica type. The mounds do not reach the size known for exsecta and their diameter is normally <50 cm. According to Feller (1985), nest entrances are always situated at the mound base. The subterranean part of an excavated summer nest showed a central vertical duct from which horizontal galleries branched off to chambers of 1-2 cm diameter that were distributed from near the surface down to 40 cm depth. Each chamber contained 2-3 queens.
Development and microclimatic requirements
In a polycalic colony near Martigny/ Switzerland investigated by M. A. Schneider, oviposition usually begins in late March. Males and queens develop from the egg to the imago within 55- 60 days (i.e., the first emerge from the pupae in late May). The developmental time of workers is 50 days in spring (first callows appear in mid- May) and 40 days in summer. The bulk of worker offspring ecloses from mid-July to mid-August.
Demography of nests and colonies
Two polycalic colonies in Switzerland comprised 250 nests/ 5644 m 2 (near Martigny, M.A. Schneider) and 61 nests/ 4000 m 2 (near Genolier, C. Feller). All nests were mutually friendly and exchanged populations. Two nests of 30-40 cm diameter were censused by Schneider for their winter population in late March. One nest contained 47500 workers and 79 queens and the other 51279 workers and 326 queens. Nest splitting in spring leads to a higher number of less populous summer nests. The corrected estimate of Feller (1985) for an “average” summer nest of 20 cm diameter was a total of 3500 workers and 24 queens (site near Genolier). Population concentration in autumn is believed to reduce winter mortality and dispersing in spring improves economic recource utilization and territory defence. 25% of the nests in Martigny and 20% of those in Genolier produced alates. A bimodal but largely overlapping size distribution of the workers was observed by C. Feller; the larger workers preferentially performed Innendienst tasks and the smaller workers mainly Aussendienst tasks.
Swarming
In Central Europe, mature alates are found in the nests 19 July ± 12.0 d (15 June-13 Aug, n = 15). Observations on swarming and mating were made in Switzerland (Schneider pers. comm.): in polycalic colonies only about 5% of females fly, the others are inseminated at the nest mounds. Micraners fly higher and farther than macraners. Macraners stay near the nest and transmit more sperm per mating: 220000 sperm cells against 120000 in micraners. Sufficient air temperatures given, swarming takes place from 7.00 to 11.00 h a.m., as soon as the first direct sunlight hits the mound surface. Completely clouded sky prevents the flight and beginning sunshine in the second half of the day can not release it.
Food sources
F. bruni can use a wide range of food sources. Trophobiosis with Aphidina is most important. Coccidae are used less frequent and also Cicadina sucking at the roots and lowest sprout parts of herbs are tended; in the latter case the Cicadina colonies are protected by walls of plant material. Floral and extrafloral nectaries of diverse plant species in the field layer are intensively exploited. Zoophagous activity may be considerable: different developmental stages of insects, spiders, and earthworms are consumed. The foraging activity is mainly diurnal. Nocturnal activity is 25% of the diurnal activity in mid-summer; in spring and autumn it is lower (Schneider pers. comm.). Foraging completely stops at surface temperatures> 40 °C. Different polygynous societies that do not exchange workers or broods may share territories and even food sources.
| MZ |
Museum of the Earth, Polish Academy of Sciences |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |