Eulioptera excavata, Hemp & Heller, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4671.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:FB9526DD-4A01-422A-ACC3-A50AB0A6AF40 |
|
persistent identifier |
https://treatment.plazi.org/id/03F74008-FFCF-FFA2-FF4C-83796A1B9B47 |
|
treatment provided by |
Plazi |
|
scientific name |
Eulioptera excavata |
| status |
sp. nov. |
Eulioptera excavata n. sp. Hemp C.
( Figs. 21 View FIGURE 21 , 22 View FIGURE 22 , 23 View FIGURE 23 , 31 View FIGURE 31 , 32 View FIGURE 32 , 33 View FIGURE 33 , 34 View FIGURE 34 )
Holotype. Male. Tanzania, Bahi District, Dodoma Region, East Chenene Forest Reserve, Miombo woodlands, on bushes and trees in forest, 1500 m, March 2018 . Paratypes 3 males, 6 females, same data as holotype and July 2017 . 3 males, 2 females, Tanzania, Mpwapwa District, Dodoma Region, near Gulwe , Miombo woodlands, 1000 m, March 2017 . 1 male, Tanzania, Mpwapwa District , Dodoma Region, Changalawe Hill, March 2015 . Depository : Collection C. Hemp .
Description. Male. General habitus and colour pattern. Predominantly green with numerous brown to reddish dots scattered over legs, pronotum and body as typical for most Eulioptera species. Stridulatory area reddish-brown marked ( Fig. 21 View FIGURE 21 A–C). Head and antennae. Antennae thin, greenish to dark, longer than body length. Fastigium verticis sulcate ridge, pointed at apex. Thorax. Surface of pronotum smooth. Stridulatory file on underside of left tegmen continuous, curved, with median wide and densely set teeth; teeth getting more widely spaced at both ends of the stridulatory file and stouter in shape; with about 170 teeth ( Fig. 22 D View FIGURE 22 ). Legs. Fore femora unarmed, mid femora with few (up to 5) very tiny and tightly to the ventral femora attached spinules; hind femora with ventral double row of few spinules, some individuals unarmed or with only 1–2 apically spinules ventrally. Hind tibiae with 4 rows of spines along length, at apex with three spurs at each side. Abdomen. Posterior margin of 10 th abdominal tergite median up-lifted, lateral with two lobes surrounding deep groove formed by tongue-like supra-anal plate ( Fig. 22 A, B View FIGURE 22 ); surface of 10 th abdominal tergite and supra-anal plate densely hairy ( Fig. 22 B View FIGURE 22 ). Cerci getting gradually more slender to apex, hairy; apices of cerci abruptly in-curved ( Fig. 22 A, C View FIGURE 22 ). Subgenital plate deeply lobed ( Fig. 22 C View FIGURE 22 ).
Female. In size and habitus similar to male, also with faint reddish-brown marking at base of tegmina ( Fig. 21 D View FIGURE 21 ). Ovipositor typical for genus, comparatively small and strongly up-curved ( Fig. 23 A View FIGURE 23 ). Subgenital plate tonguelike broad with two shallow lobes at posterior margin ( Fig. 23 B View FIGURE 23 ).
Diagnosis. From its habitus and outer genitalic morphology of the male very similar to E. incisa ( Ragge, 1980) from Togo, West Africa. E. incisa has a similar grove or excavation on the 10 th abdominal tergite, a similar subgenital plate divided into two lobes and similar male cerci that are slender and abruptly incurved at their tips. However, the ratio of the length of lobes to the basal part of the subgenital plate is different (1.3 in E. excavata n. sp. and 1.6 in E. incisa ) and the stridulatory file is different between the two species as well. In E. incisa the stridulatory file is only slighly curved with 143 densely set broad teeth over almost the whole length (figures in Ragge 1980)—while in E. excavata n. sp. the stridulatory file is more strongly curved at its ends with broad and densely set teeth in the middle part and more widely spaced thicker and shorter teeth at the ends of the file. Further the file consists of more than 170 teeth. Very likely these two species are closely related and in current speciation due to geographical separation. Unfortunately only the male holotype is known for E. incisa and thus the females of both species cannot be compared. E. montana Ragge, 1980 known only from the Chyulu Hills of southern Kenya also has a deeply lobed subgenital plate. However, the ends of the lobes are leaf-like expanded and thus E. montana can be easily distinguished from E. excavata n. sp. E. monticola Ragge, 1980 has a lobed subgenital plate as well, however not as broadly lobed and much shorter than in E. excavata n. sp. E. monticola is widely distributed in the Eastern Arc Mountains and along the coast thus occupying a different habitat than E. excavata n. sp. Females are not known for four Eulioptera species including the morphologically very similar E. incisa and are best identified with associate males.
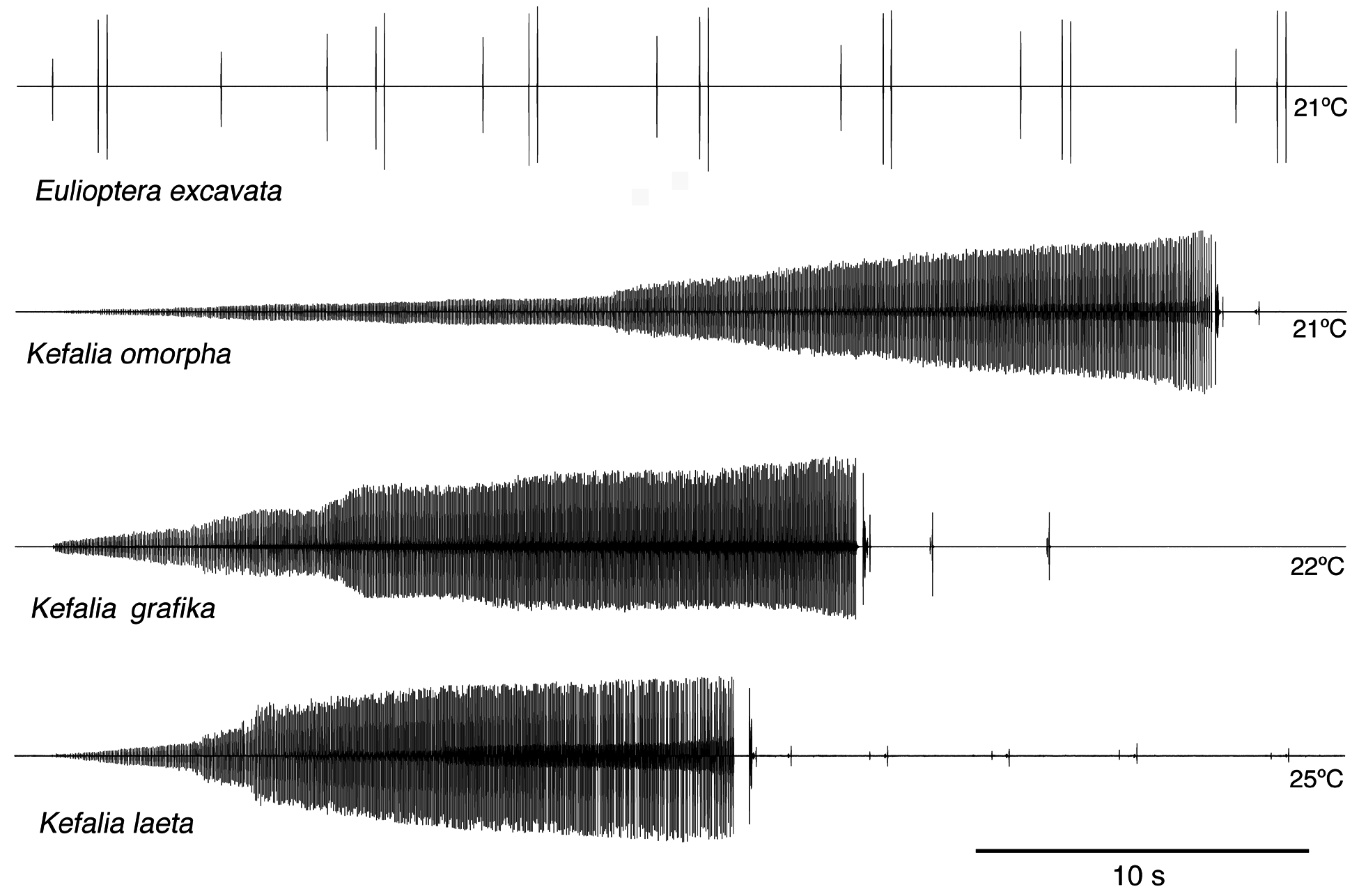
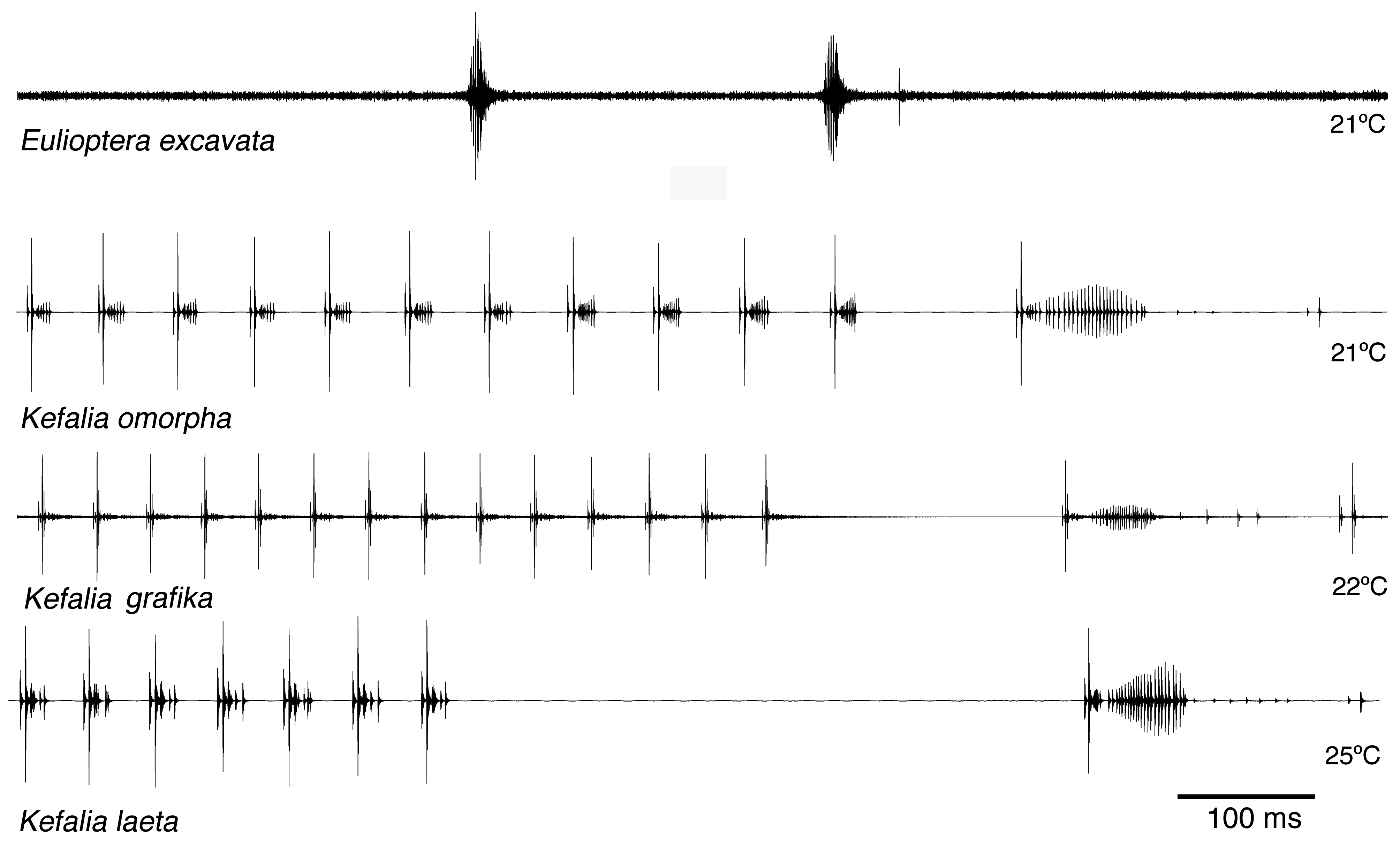
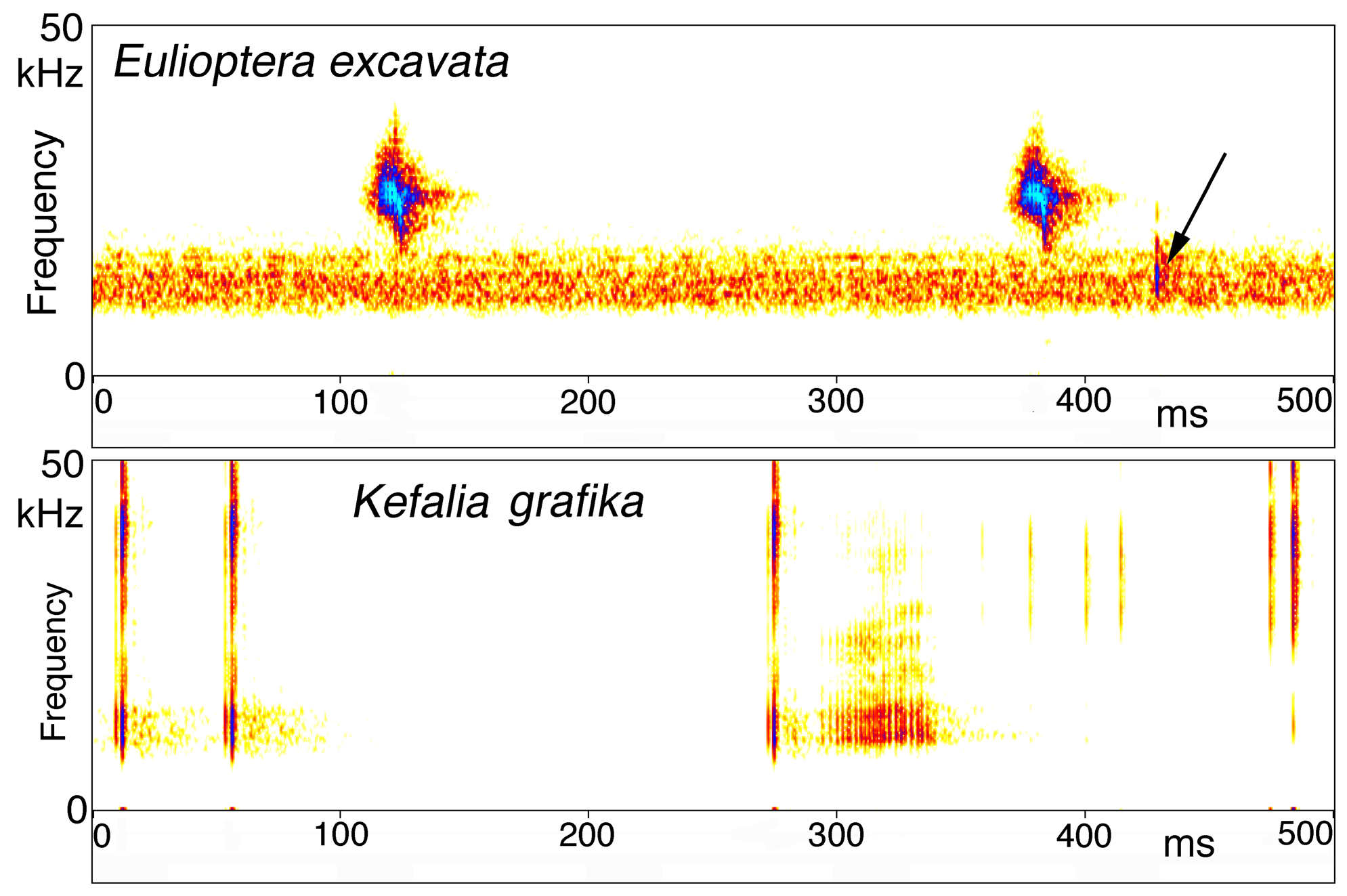
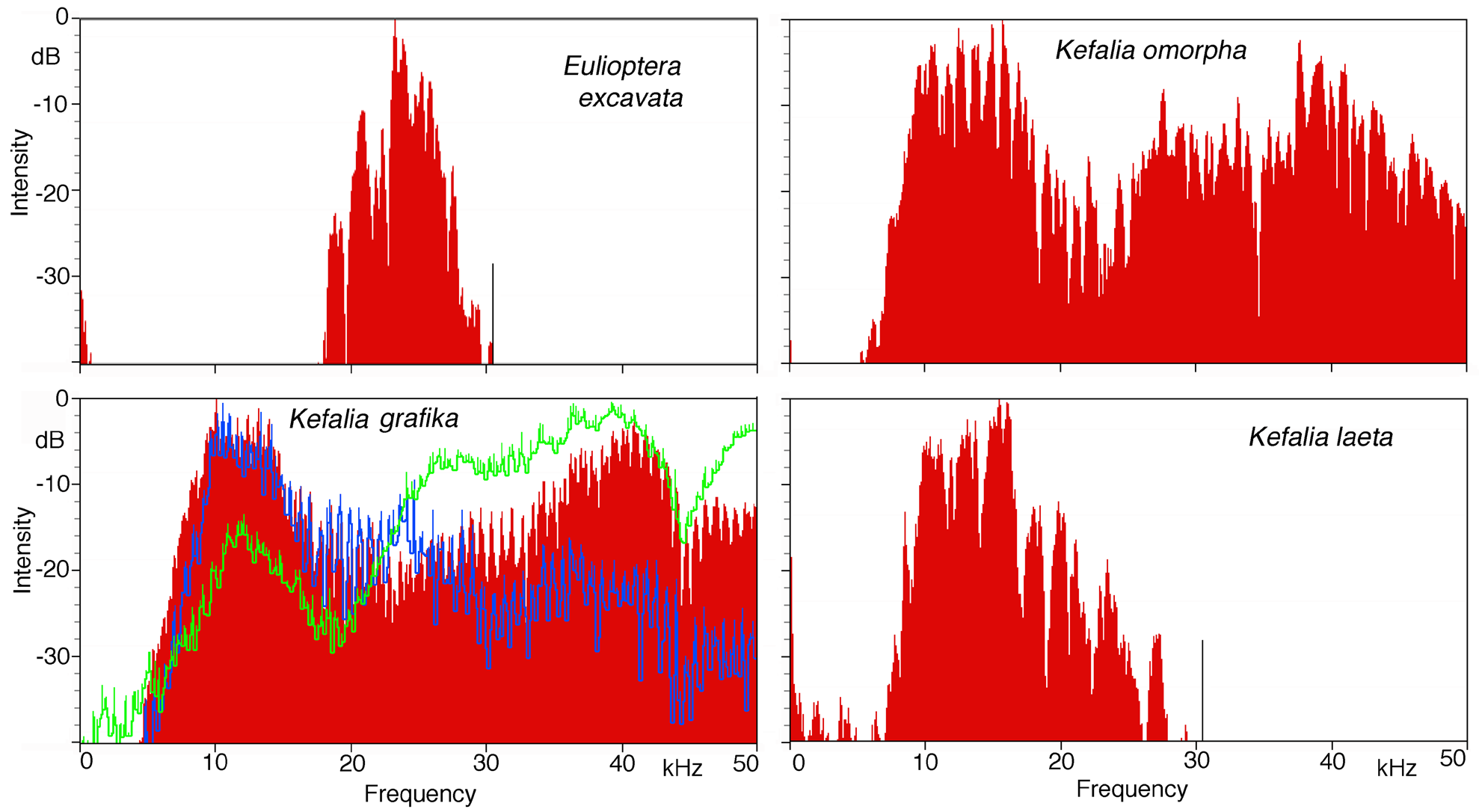
Song. The male calling song consisted of long sequences (50±20 s; n=10) of short (ca. 16.2±2.3 ms) isolated syllables. Pairs of syllables (intra-pair interval 259±9 ms) alternated with singe syllables (interval before pair 1269±48 ms, interval after pair 3365±686 ms; T=21ºC; Fig. 31 View FIGURE 31 ). In structure, the song is similar to that of other Eulioptera species (Hemp, in prep.), which also consist of sequences of short, isolated syllables. In this aspect the song resembles also that of species of the genus Tylopsis (Hemp, in prep). In one cage recording from the field, immediately after the second syllable of the male syllable pair an isolated impulse can be recognized which may be the response of a (captivated) female. It differs in spectral composition from the male song ( Fig. 33 View FIGURE 33 ). In some species males are able to mimic the frequency of the female ( Heller & Hemp 2017), but this seems to be an unusual case. The response delay of 56.9±0.8 ms (n=8; measured from beginning of male syllable) is quite similar to that of Tylopsis species, e.g. T. lilifolia with a latency of 70 ms for the female response ( Heller et al. 2015). The spectrum of the male song showed a relatively narrow peak at about 25 kHz ( Fig. 33 View FIGURE 33 , 34 View FIGURE 34 ), while the assumed female response had most energy around 15 kHz ( Fig. 33 View FIGURE 33 ).
Habitat. On bushes and trees in Miombo woodlands.
Distribution. Central Tanzania. At present only known from the Dodoma Region, Bahi and Mpwapwa Dis- tricts.
Etymology. Named after the strongly hollowed out posterior margin of the 10 th abdominal tergite, from Lat- in:— excavata .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.