Emerita portoricensis Schmitt, 1935
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5227.3.3 |
|
publication LSID |
lsid:zoobank.org:pub:CF7401B6-F906-40C7-8B7E-84963272BD39 |
|
DOI |
https://doi.org/10.5281/zenodo.7525447 |
|
persistent identifier |
https://treatment.plazi.org/id/A624A87C-FFFE-FFB7-FF38-FBDF9E744AF1 |
|
treatment provided by |
Plazi |
|
scientific name |
Emerita portoricensis Schmitt, 1935 |
| status |
|
Emerita portoricensis Schmitt, 1935 View in CoL
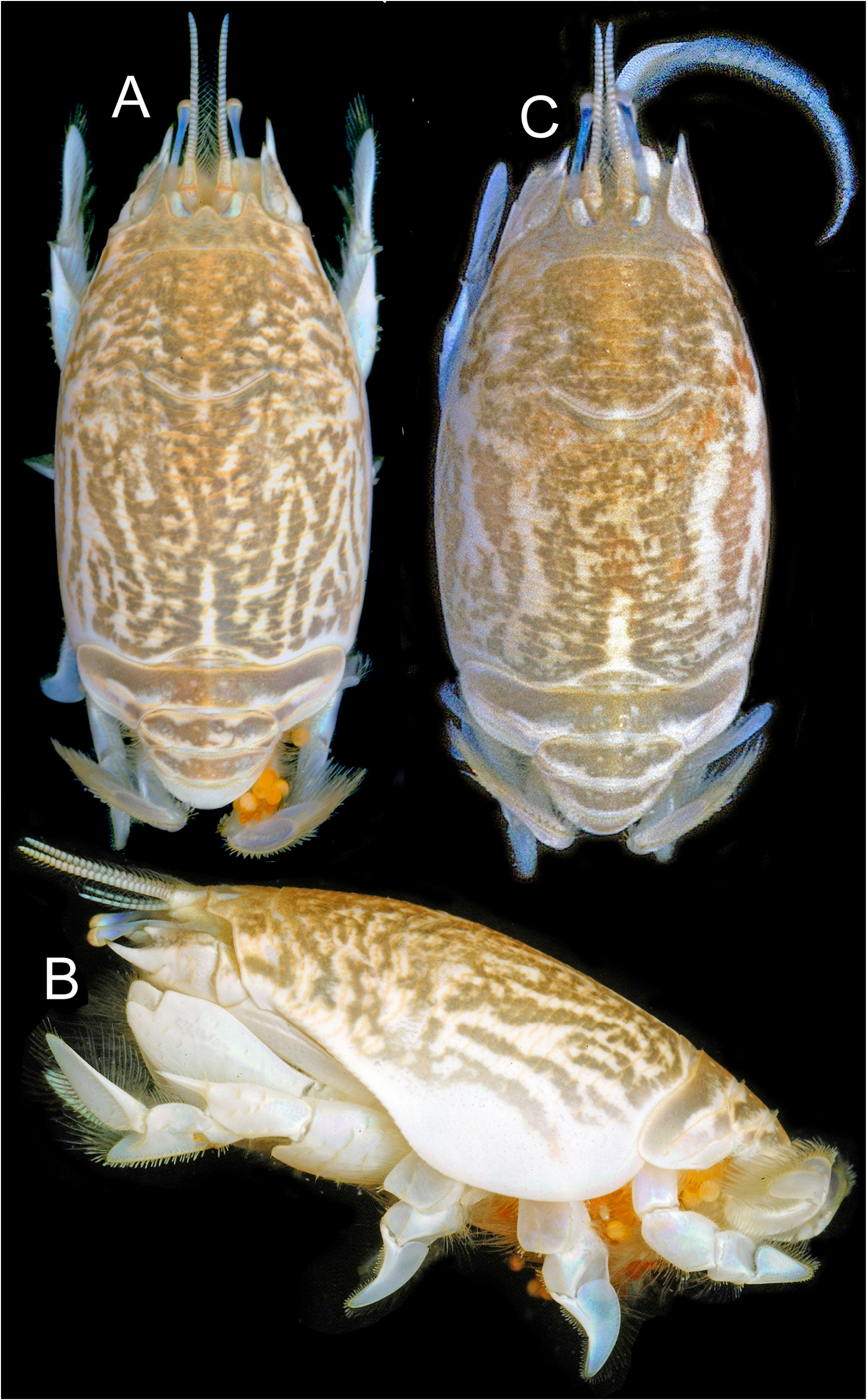
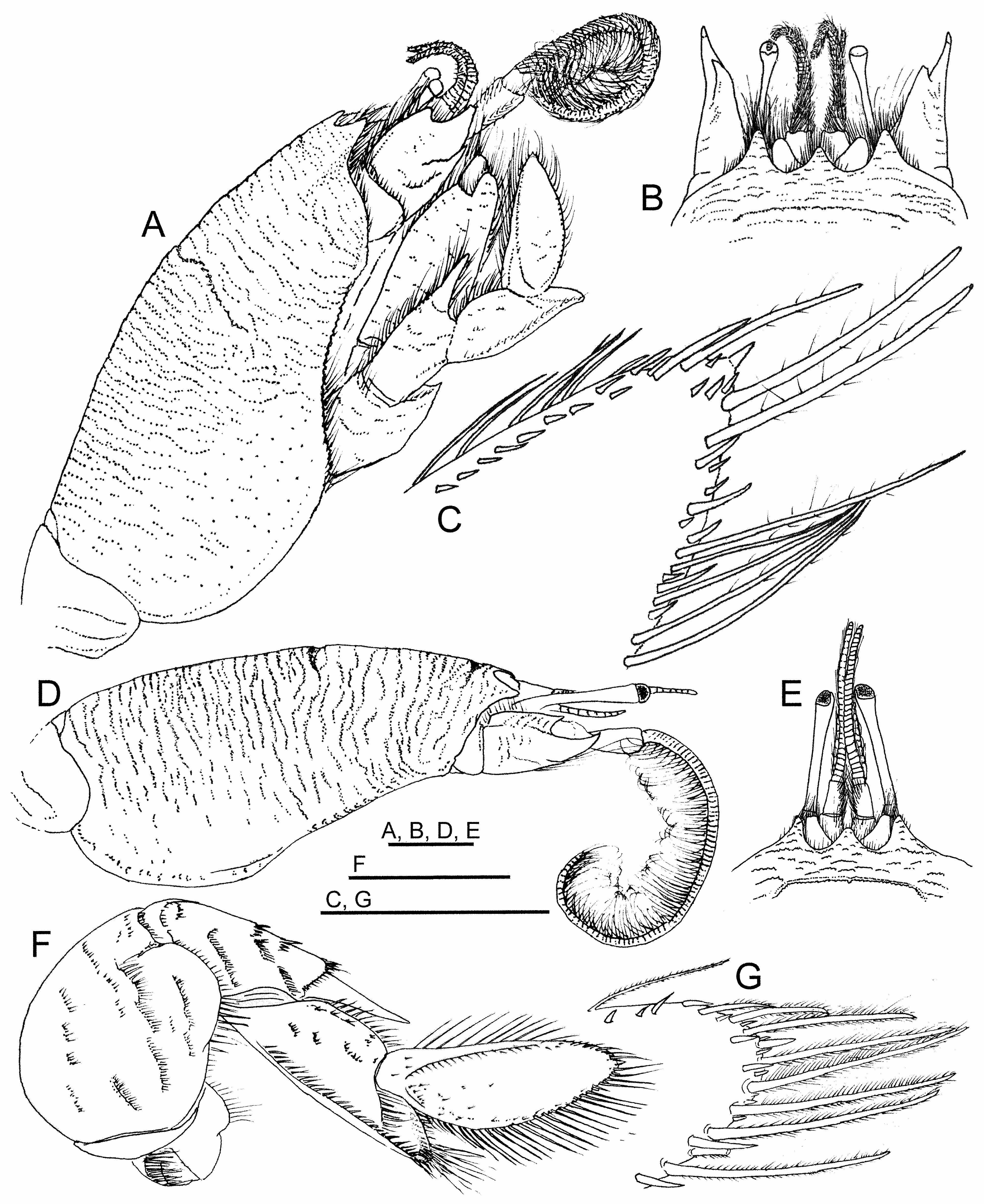
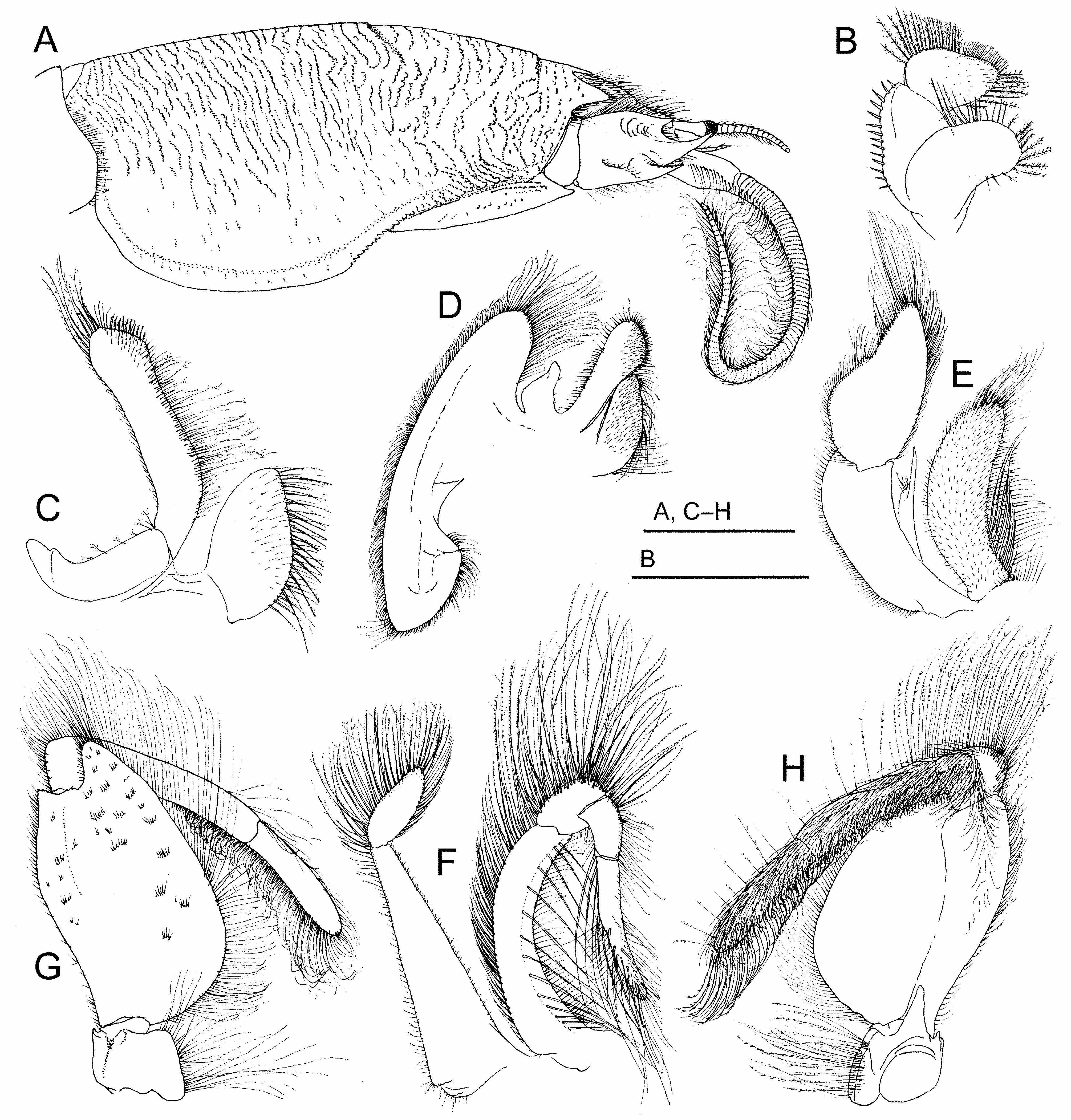
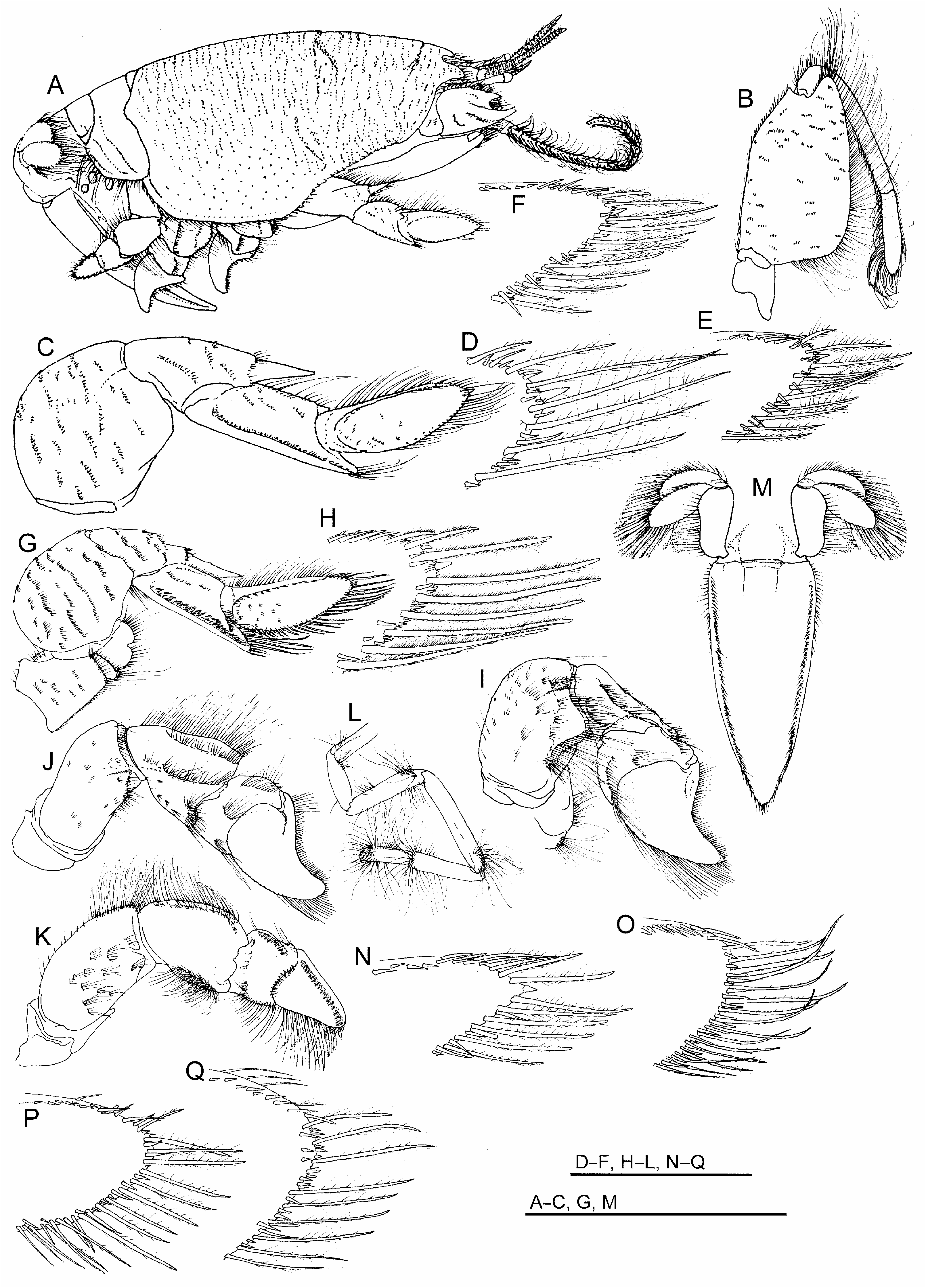
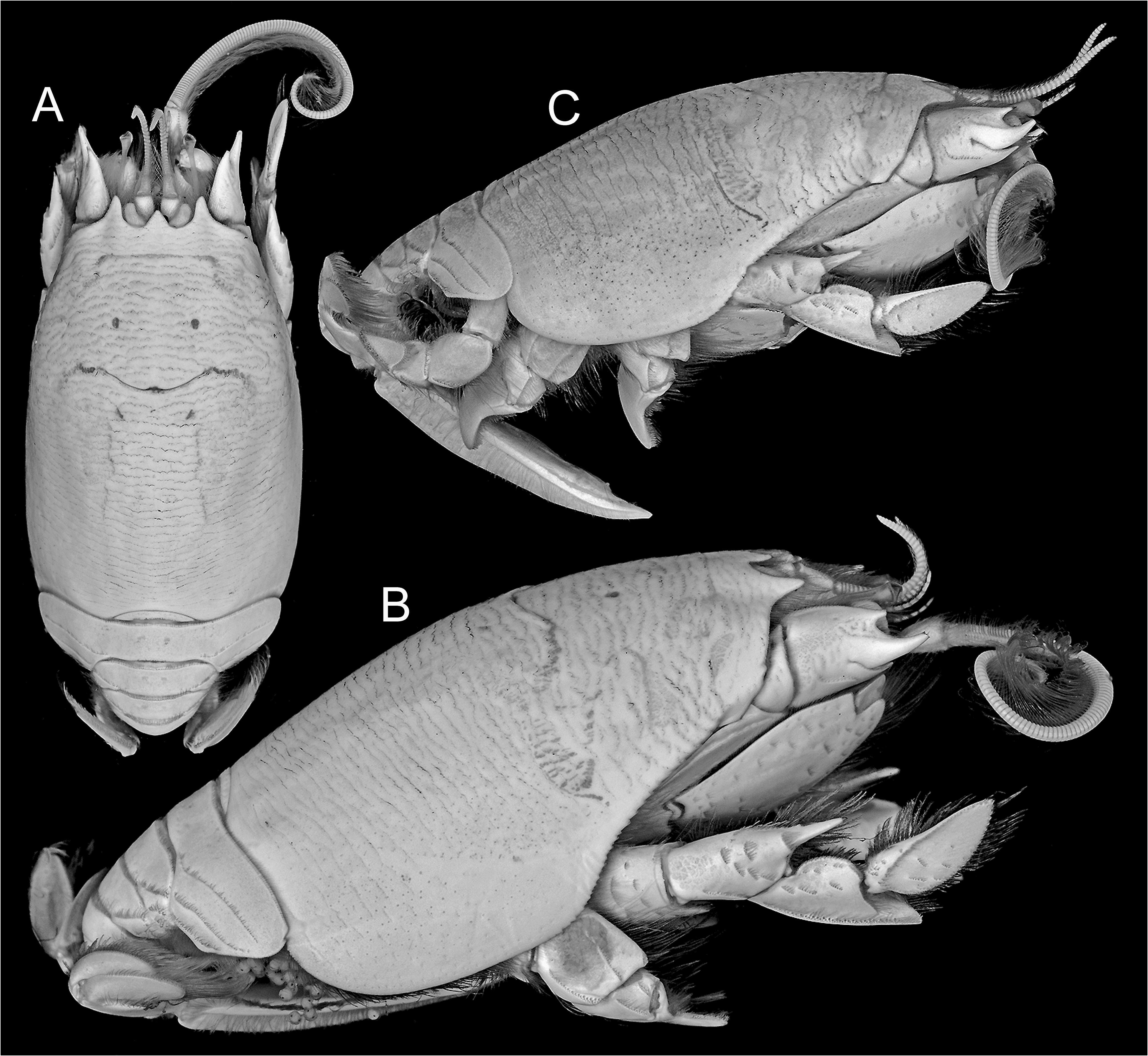
( Figs. 1A–C View FIGURE 1 ; 2A–G View FIGURE 2 ; 3A–H View FIGURE 3 ; 4A–Q View FIGURE 4 ; 5A–C View FIGURE 5 )
Emerita portoricensis Schmitt, 1935: 213 View in CoL (figs. 72a–b), 215, 217 (part, excluding Florida and Texas).
Emerita portoricensis View in CoL .— Goodbody, 1965: 195–197; Efford, 1967: 88–90; Abele & Efford, 1972: 506; Efford, 1976: 174 (part, excluding Florida), 175–178, 179 (fig. 7, part, excluding Florida and Brazil), 183; Rodriguez, 1980: 241 (part, excluding Florida and Texas); Abele & Kim, 1986: 38 (part, excluding Florida and Texas), 434 (unnumbered figure); Sastre, 1990: 526–533 (table 1; figs. 1–7); Sastre & Yoshioka, 1992: 456–463 (figs. 1–5); Tam et al., 1996: 489, 490 (fig. 1, part, excluding Brazil), 491 (table 1), 495 (fig. 4); Melo, 1999: 295, 298 (part, excluding Florida and Brazil, excluding 299, fig. 204); Pérez, 1999: 320–326; García et al., 2000: 215–223; Haye et al., 2002: 903, 904 (fig. 1, part, excluding Brazil), 905 (fig. 2), 906, 907 (table 1), 908 (fig. 3), 910 (table 2); Felder et al., 2009: 1068, (1095 footnote 177) (part, excluding Pensacola, NW Florida); Boyko & McLaughlin, 2010: 142; Hsueh, 2015: 254, 255 (table I), 256 (key); Poupin, 2018: 152, 254.
Emerita sp. — Venera-Ponton et al., 2020: 5 (table 1, BINs cluster BOLD:ACU0009).
Type material. Holotype: ovigerous female holotype, USNM 65731 View Materials , Mayaguez, Puerto Rico, 19–20 January 1899 . Paratype: ovigerous female, USNM 65729 View Materials , Isla de Vieques, Puerto Rico, 8 February 1899 .
Other material examined. Puerto Rico: 18 females, 11 ovigerous, USNM 42209 View Materials , San Juan Harbor , 13 January 1899 . Belize: 9 small females, USNM 22601 View Materials , Carrie Bow Cay , no date ; one lot, USNM 1672672 View Materials , sta. CB-38, Carrie Bow Cay , 15 May 1977 ; ovigerous female, USNM 1545049 View Materials (= ULLZ 10465 View Materials ), lower intertidal muddy, quartzite, riverine sand off wave-washed beach, 0.5 m, Pelican Beach Hotel, Dangriga , Stann Creek District, 18 April 1983 ; one lot, USNM 1672671 View Materials , collection data same as preceding . Costa Rica (Caribbean): ovigerous female, CCBD 4690 , Parque Nacional de Cahuita , 13 July 2013 ; ovigerous female, CCDB 4690 View Materials , Parque Nacional de Cahuita , Limón, 13 July 2013 ; ovigerous female, CCDB 4938 View Materials , low energy beach, Playa de Puerto Viejo , Limón, 19 July 2013 . Panama (Caribbean): ovigerous female, USNM 1672670 View Materials , Shimmey Beach, Fort Sherman , 10 August 1969 ; ovigerous female, USNM 1546871 View Materials (= ULLZ 13325 View Materials ), swash zone quartzite sand, low energy beach inshore of grassbed, Playa Boca del Drago , Isla Colon, 6 August 2011 ; ovigerous female, USNM 1546925 View Materials (= ULLZ 13456 View Materials ), collection data same as preceding ; juvenile female, USNM 1546926 View Materials (= ULLZ 13457 View Materials ), collection data same as preceding ; juvenile, USNM 1547046 View Materials ( ULLZ 13690 View Materials ), swash zone muddy quartzite sand, Bocas del Toro beach across road from STRI lab , Isla Colon, 9 August 2011 . Trinidad: ovigerous female, USNM 65727 View Materials , Cedros , January 1905 .
Diagnosis. Carapace dorsally convex, surface densely covered by transverse microcrenulate rugae not extensively disposed as short lines or short crescentic rows, many elongate and continuous across median longitudinal line of carapace, more than 17 crossing median line posterior to cervical furrow, rugae laterally becoming obsolete over most of broad, punctate, epimeral lobe of carapace; front terminating in three strong subacute lobes, median forming broad angular rostrum less advanced than narrower lateral lobe to each side.Antennular flagellum dorsal ramus comprised of approximately 30 articles.Antennal peduncle with second article terminating in large, distally upturned, median spine, shorter dorsal and ventral spines reaching no more than half length of median spine. Third maxilliped lacking exopod; endopod with merus mesial margin distal end terminating in strongly produced subtriangular lobe, lateral margin sinuous, distal third offset by weakly angular bend, distal end forming acute corner. First pereopod merus massive, distal half of flexor margin produced to form broad, truncate lobe; carpus terminating in narrow spine reaching to base of dactylus; propodus approximately three times longer than broad, subequal in length to dactylus; dactylus elongate, length more than two times greatest width, lateral surface narrowly ellipsoid in outline, terminus rounded, margins of article lined by long, plumose setae interspersed with short spiniform setae or spinules of varied sizes, rounded terminus of article bearing slightly enlarged single, short, spine. Pleon with second pleonite larger than all others, its dorsal tergite reaching to full width of carapace, lateral extremes forming broadly rounded flanges to each side of narrower median commissure, flange dorsal surfaces each bearing pair of long largely unbroken transverse rugae extending almost to ventrolateral margin of tergite. Diagnostic DNA sequence data for COI and 16S mitochondrial genes included in the Barcode of Life Database (dataset dx.doi.org/10.5883/DS-CRUSTACE) under BINs cluster BOLD:ACU0009 and in GenBank (www.ncbi.nlm.nih.gov/genbank) under accession numbers MN183852 View Materials , MN183956 View Materials , MN184098 View Materials , and MN189420 View Materials for COI, and L43111 View Materials , MK971283 View Materials , MK971390 View Materials , MK971536 View Materials , and MK971646 View Materials for 16S.
Description. Carapace ( Figs. 1A–C View FIGURE 1 ; 2A, D View FIGURE 2 ; 3A View FIGURE 3 ; 4A View FIGURE 4 ; 5A–C View FIGURE 5 ) elongate, subcylindrical, dorsally arched, strongly convex transversely, longitudinal arch strongest in anterior half; carapace surface densely textured by low transverse microcrenulate to microdenticulate rugae, many rugae continuously elongate, not extensively disposed in short lines or as short crescentic rows, many continuous across middorsal regions of anterior and posterior carapace, number extending across postcervical middorsal line typically exceeding 17; ventrolateral rugae of pterygostomial region separating weak anteriorly curved ridges near ventral margin, ridges abbreviated to produce minutely serrate appearance along anterior margin of broad epimeral lobe; front terminating in three strong subacute dentiform lobes ( Fig. 2B, E View FIGURE 2 ), median forming broad angular rostrum, rostrum less advanced to anterior than narrower, longer, lateral lobe to each side, separated from each lateral lobe by broad U-shaped sulcus overlying ocular plate; transverse frontal groove parallel to frontal margin, incised by weak median notch and short posteriorly directed bend at each lateral extreme, microdenticulate ridge marking posterior lip of frontal groove positioned about 1/5 distance from tip of rostrum to cervical groove, cervical groove just anterior to carapace midlength, posterior lip of cervical groove marked by microdenticulate ridge, overall broadly crescentic with lateral arms extending anteroventrally, obsolete across carapace midline; rugae laterally becoming broken to obsolescent over most of broad lower epimeral lobe otherwise marked by well-separated punctae, rugae weakly evident as short lines on ventralmost margins of lobe.
Eyes at swollen ends of very narrowly elongated peduncles, corneal end reaching anteriorly to near midlength of terminal article of antennal peduncle when extended, exceeding anterior reach of longest spine on penultimate article of antennal peduncle. Antennular flagellum ( Figs. 2A, B, D, E View FIGURE 2 ; 3A View FIGURE 3 ; 4A View FIGURE 4 ) dorsal ramus comprised of approximately 30 articles, length near twice that of ventral ramus. Antennal peduncle ( Figs. 2A, B, D View FIGURE 2 ; 3A View FIGURE 3 ; 4B View FIGURE 4 ; 5C View FIGURE 5 ) with second article terminating distally in large, upturned, median spine, spine originating from lateral crest on article, shorter dorsal and ventral spines reaching no more than half length of median spine, terminal article of peduncle with dense lateral row of elongate setae continuous with row on antennal flagellum; flagellum long, robust, typically comprised of more than 85 densely setose articles.
Mandible ( Fig. 3B View FIGURE 3 ) membranous, extensively fused with posterior margin of epistome, basally produced to form mesially directed gnathal lobe with long plumose setae along distal and mesial margins; palpus of two articles larger than gnathal lobe, proximal article of palp with line of stiff spiniform setules along lateral margin, terminal article with margins lined by rows of long plumose setae distally and mesially, separated by distomesial row of shorter setae, external surface with scattered short simple setae. First maxilla ( Fig. 3C View FIGURE 3 ) proximal endite developed as flattened lobe, loosely attached to remainder of appendage, mesial margin lined by row of long plumose setae adjacent to field of long simple setae, field of simple setae extended onto external surface; distal endite elongate with broadened terminal end, lateral slope of terminus bearing very elongate plumose setae, mesial slope of terminus bearing narrow field of short, stiff, serrate, spiniform setae, shaft of article setose along lateral and mesial margins, those of lateral margin short, those of mesial margin dense, long, plumose; endopodal palp elongate, sparsely setose, weakly hooked, narrowed to subacute rounded tip. Second maxilla ( Fig. 3D View FIGURE 3 ) exopod broadly developed as scaphognathite attached to base; proximal endite subdivided, larger lobe broad, flattened with mesial margin bearing overlapped rows of long plumose setae, short simple setae covering most of external surface; distal endite club-shaped, marginal setae longer, plumose laterally, those of distal end forming dense field of short stiff setae, continued as field of short simple setae on external surface; endopod short, stubby, narrowed, subacute tip deflected distally.
First maxilliped exopod ( Fig. 3E View FIGURE 3 ) arched, crescentic, comprised of two marginally setose articles, proximal article strap-like, subrectangular, distal article subovoid, margin concave laterally, narrowing toward terminus, distal margins lined by long plumose setae; endopod minute and strap-like, membranous, subterminal shoulder with small cluster of plumose setae, tip constricted to narrow shaft; distal endite prominent, elongate, exceeding length of exopod proximal article, strap-like, heavy setation including cover of short setae over external surface, long plumose setae in dense terminal field and along entire mesial margin, proximal half of mesial margin with additional row of longer, coarser, plumose setae overlying others.
Second maxilliped exopod ( Fig. 3F View FIGURE 3 ) comprised of two articles, proximal article elongate, length subequal to endopod ischiomerus, broadest proximally, tapered over full length to narrow articulation with distal article, sparsely setose, distal article subovoid, spatulate, slightly tapered in distal half to blunt tip, margins densely lined by long plumose setae; endopod comprised of four articles, ischiomerus (proximal article) narrow, distinctly arcuate, rib-shaped, lateral margin densely lined by long plumose setae, mesial margin lined by row of long plumose setae overlain by row of longer, heavier simple setae originating from weakly defined crest along external surface, carpus short, inflated, approximately 1/4 length of ischiomerus, distal and lateral margins covered by dense filed of heavy, elongate, plumose setae, propodus less robust, narrower than but subequal in length to carpus, margins setose but less coarse than on carpus, dactylus narrowly elongate, weakly tapered to rounded tip, length approximately 2/3 that of ischiomerus, margins heavily setose, longest setae coarse, simple, extending as row along entire flexor margin. expanded more broadly onto surface in distal half, comparable to those of ischiomeral surface, which are overlapped when article flexed.
Third maxilliped ( Fig. 3G, H View FIGURE 3 ) lacking exopod; endopod with merus broad, margin lined by short setae laterally, long plumose setae mesially and distally, length approximately 1.9 times greatest width, mesial margin strongly rounded in proximal third, broadly rounded distally, distal end terminating in strongly produced subtriangular lobe overlying external surface of carpus and proximal propodus, lateral margin sinuous, distal third offset by weak but distinct angular bend, distal end forming acute corner, carpus short, subquadrate, robust, long plumose setae on distal and lateral surfaces, dense field of plumose setae originating on distal half of internal surface, contiguous with field over entire internal and flexor surfaces of propodus and dactylus, propodus length approximately five times greatest width, dactylus subcylindrical, approximately 2/3 propodus length, terminus rounded.
First pereopod ( Figs. 2A, C, F, G View FIGURE 2 ; 4A, C–H View FIGURE 4 ) merus massive, broadly subovate in outline, lateral surface convex, crossed by broken, oblique lines setose rugae, extensor margin most strongly arched proximally, distal half of flexor margin produced to form broad, truncate lobe; carpus elongate, distally terminating in strong narrow spine reaching to base of dactylus, spine offset laterally from proximal portion of article by setose ridge, portion proximal to spine approximately two times longer than broad, lateral surface crossed by few broken oblique setose rugae; propodus approximately three times longer than broad, subequal in length to dactylus, lateral surface with longitudinal ridge along most of inferior margin and strong ridge extending obliquely from articulation with dactylus to near inferior margin, latter ridge marking origin of blade-like distal process, distal process narrowing to rounded terminus, its superolateral surface excavate to cup base of flexed dactylus, stiff bristle-like setation along ridges of article, terminal setation of distal process elongate, plumose; dactylus elongate, length distinctly exceeding two times greatest width, lateral surface narrowly ellipsoid in outline, terminus rounded, weakly arched ridge originating just below superior (extensor) margin proximally, merging with superior margin distally, surface between ridge and inferior (flexor) margin weakly concave, ridge lined by short bristle-like setae, margins of article lined by long, plumose setae interspersed with short spiniform setae or spinules of varied sizes, rounded terminus of article bearing slightly enlarged single, short, spine.
Second through fourth pereopods all heavy, similarly configured ( Fig. 4A, I–K View FIGURE 4 ); second with merus massive, sub-rectangular, slightly longer than broad, lateral surface convex with scattered short setose rugae, extensor margin most strongly arched distally, distal half of flexor margin produced to form subtriangular lobe, carpus robust, subtriangular in outline, approximately as long as broad, lateral surface with superior and arched inferior longitudinal ridges, both coarsely setose, propodus heavy, subquadrate, distal extreme produced, tapered to densely setose bluntly tipped process fitted against internal surface of flexed dactylus, dactylus distinctly flattened, articulated to distal end of superior propodal margin, broad proximally, distinctly hooked in lateral outline, concave superior margin lined by coarse short setae, strongly convex inferior (flexor) margin lined by short setae proximally, longer heavy setae distally, narrowing to upturned subacute distal tip; third pereopod overall with similar ridging and setation to second, merus more elongate, lacking subtriangular lobe on flexor margin, carpus more distinctly triangular in outline, dactylus less strongly hooked with less acute tip; fourth pereopod merus similar to that of third, carpus lateral surface subrectangular, propodus lacking tapered distal process fitted against internal surface of dactylus, dactylus heavy, less flattened than in second and third pereopods, indistinctly hooked in lateral outline, with dense longitudinal tract of short heavy setae on lateral surface near superior (extensor) margin; fifth pereopod concealed beneath posterior thorax, typically intruding into branchial chambers, comprised of seven articles, all narrowly elongate except for the very short hooked dactylus opposing a distal process on the propodus to form a minute chela, merus exceeding length of all other articles, long setae disposed primarily in short tracts or patches, especially near distal ends of distal articles.
Pleon ( Figs. 2A, D View FIGURE 2 ; 4A, M View FIGURE 4 ) with first pleonite exposed dorsally as short, transversely narrow sclerite (tergite) fitting into posterior concavity of carapace, second pleonite larger than all others, its dorsal tergite reaching to full width of carapace, lateral extremes forming broadly rounded flanges to each side of narrower median commissure, flange surfaces each bearing pair of long relatively unbroken transverse rugae extending almost to ventrolateral margin of tergite, in addition to raised submarginal lips, flanges framing posterior concavity where smaller third pleonite articulates, tergites of third through fifth pleonites decreasing in size, lateral flanges directed ventrolaterally, lateral flange of third rounded, largely concealed below flange of second, that of fourth triangular, that of fifth narrowly produced, sixth pleonite with tergite elongate to form enlarged plate with subtriangular lateral extremes, width approximating that of telson to which it articulates; pleopods not developed on first and fifth pleonites, each developed as three narrow setose articles on second through fourth pleonites in females; large uropods ( Fig. 3G View FIGURE 3 ) articulated laterally to ventrolaterally on sixth pleonites, each comprised of elongate basal article attached to flattened endopod and exopod, both elongate ovoid in shape with dense plumose marginal setation, setation longest distally; telson lanceolate, lateral margin setose, weakly convex in outline, longitudinal tract of short setae filling furrow just medial to distinct submarginal ridge, terminus subacute.
Habitat. Shallow infaunal, moving with tidal rise and fall, concealing by shallow burrowing; siliceous, calcareous, and mixed sandy substrates, some with molluscan shell or coral fragments; beach wave swash zones to shallow subtidal flats, some near estuarine tidal outflows; 0– 2 m.
Distribution. Tropical sandy island and Central American mainland shorelines of Caribbean Sea, confirmed to include, but not limited to, Puerto Rico, Dominican Republic, Virgin Islands, Jamaica, Belize, Costa Rica, Panama, Colombia St. Lucia, St. Thomas, Venezuela, and Trinidad; western Atlantic Ocean.
Remarks. The variably rounded terminus of the first pereopod dactylus in all but the largest specimens of E. portoricensis could perhaps lead to their misidentification as E. brasiliensis or E. talpoida , two western Atlantic species that to some degree share this character, even though their dactyli are usually broader and overall more ovoid in appearance ( Fig. 4O–Q View FIGURE 4 ; see also Williams 1965: fig. 115B). The narrowed, elongate shape of the first pereopod dactylus ( Figs. 2C, F, G View FIGURE 2 ; 4C–G View FIGURE 4 ), which is also slightly more tapered distally toward its rounded tip than in E. brasiliensis and E. talpoida , appears to account for its being labelled as a subacute tip ( Schmitt, 1935) in E. portoricensis . In only the largest of specimens does it approach the distinctly acute tip of E. benedicti ( Fig. 4N View FIGURE 4 ; see also Williams 1965: fig. 115A), the only other presently described western Atlantic species that shares the generally narrowed, more elongate shape of the first pereopod dactylus overall. While the shape of this article is somewhat alike in E. portoricensis and E. benedicti , specimens of these two species can be separated at most sizes by examination of the dactylus tip under magnification. In all mature adult specimens, the tip tapers triangularly to become a sharp corneous spine in E. benedicti , while the tip is to varying degrees rounded to a terminus at which it bears a short, slightly enlarged corneous spine in E. portoricensis . However, in the largest examples of E. portoricensis , including the holotype, the terminus tends to be less rounded than in all examined smaller specimens of these species from sampled populations, though it is nonetheless separable from large specimens of E. benedicti when they are compared under magnification.
Morphological separation of E. portoricensis from its western Atlantic congeners can also be based on patterning of the fine dorsal rugae of the carapace and second pleonite. Posterior to the cervical groove, fine broken transverse lines of rugae in E. portoricensis are at most little diminished across the mid-dorsal longitudinal line, while they are somewhat more broken into short cusps in adult E. brasiliensis and distinctly diminished to all but absent at the midline in adult E. talpoida . The pattern and density of transverse rugae are relatively similar dorsally in E. benedicti and E. portoricensis , but the relative absence of transverse rugae laterally on the epimeral lobe of the carapace in E. portoricensis was used as a key character by Schmitt (1935) to separate E. portoricensis from E. benedicti . This character, based upon Schmitt’s (1935) comparisons to the only three specimens of E. benedicti known at that time, was adopted in regional taxonomic references ( Felder 1973; Abele & Kim 1986) but appears to be of questionable utility, being somewhat subjective and dependent upon lighting and staining of the examined specimens. In almost all cases, some evidence of transverse rugae can be seen on the epimeral lobe in specimens of E. portoricensis , and these often resemble the only slightly denser patterns in specimens of E. benedicti of varied sizes. Lengths of rugae on the pleon are also useful in separating western Atlantic species, especially those on the dorsal surface of the lateral flange of the second pleonite, which in E. portoricensis ( Figs. 2A, D View FIGURE 2 ; 4A View FIGURE 4 ), much as in E. benedicti , is marked by a pair of relatively unbroken transverse rugae that extend almost to the ventrolateral margin of the tergite. In both species, these reach further laterally than in E. brasiliensis and E. talpoida .
Except for some depictions of third maxillipeds, published illustrations are lacking for other anterior oral appendages of most congeners, limiting potential comparisons to illustrations herein provided ( Fig. 3B–F View FIGURE 3 ). For the present, it can only be noted that those of E. portoricensis in general conform to descriptions and illustrations as provided for E. talpoida by Smith (1877) and Snodgrass (1952), adding to a baseline for future interspecific comparisons. From our own examinations it appears that the third maxilliped merus in E. portoricensis may bear a slightly more angular bend or shoulder on its lateral margin than in E. brasiliensis and E. talpoida , though this can be expected to vary somewhat with size and maturation.
It should also be noted that Schmitt (1935), as a footnote to his description of E. benedicti , noted that this species was nearest E. portoricensis in habitus, though “it tapers appreciably more anteriorly” than does that species. In the course of our examining a full range of sizes for both species, we could not confirm this to be a reliable character supported by either measurements or comparisons of dorsal outlines.
At present, applying diagnostic characters as herein defined, we have seen no vouchers to confirm that E. portoricensis ranges beyond Caribbean waters to the northern coast of Yucatán or onto shorelines of the Gulf of Mexico and southern Florida. Even so, there would seem little obstacle to its perhaps periodic occurrence in the more tropical of these waters, especially with warming of coastal waters, and one might expect it to range widely along shorelines of Cuba. In his descriptive paper, Schmitt (1935) included “South Florida and several Texas localities in the range he reported for E. portoricensis . Efford (1976) concluded these were all misidentifications of E. benedicti , but he confirmed a record of E. portoricensis from Pensacola, Florida (USNM 17919), this being the only one from within the Gulf of Mexico. Applying redefined characters in our own examination of this specimen, we find that this is instead a small representative of E. benedicti , with the terminus of its first pereopod dactylus being as in other specimens of E. benedicti ( Fig. 4N View FIGURE 4 ) more acute than it would in a specimen of E. portoricensis at a comparable size. Thus, we conclude that regional checklists and identification keys for waters of the eastern US and its coastal states ( Behre 1950; Felder 1973; Abele & Kim 1986; Camp et al. 1998; McLaughlin et al. 2005; Felder et al. 2009), being based on literature compilations, are in whole or part referring to records for E. benedicti within those regions, not to E. portoricensis . Regarding specifically the Gulf of Mexico, there is no basis for concluding that both species occur “throughout the region”, though the history of confusion among regional workers might explain why the two are believed by some to be the same species ( Britton & Morton 1989: 120).
Coloration in life is documented for only two specimens of E. portoricensis , but these conform to one another very closely and are for specimens taken from opposite ends of the known range, suggesting color could represent a relatively stable diagnostic character, at least for specimens of similar size and maturity ( Fig. 1A–C View FIGURE 1 ). As documented, the pattern includes longitudinal and diagonal dark bars of olive brown on the branchial regions and a light longitudinal bar marking the posterior of the median line. Clearly, this differs from much less striking color patterns in live specimens of E. benedicti , E. brasiliensis , and E. talpoida that we have documented to date (DLF photographic files). However, it remains to be shown whether this pattern is consistently evident in all fresh specimens of E. portoricensis . Should comparisons be made, it might also here be noted that the color image of a specimen reported to be Emerita talpoida from Guadeloupe, depicted in Poupin (2018: fig. 151), does not represent that species but instead Hippa testudinaria (Herbst, 1791) . Four related images reported as E. talpoida , accessible at http://crustiesfroverseas.free.fr/, also represent H. testudinaria . At present, there is no confirmed evidence that the distribution of E. talpoida extends into Caribbean waters.
The presently known world membership of Emerita Scopoli, 1777 stands at eleven, with a single new species being added since the listing of ten by Boyko & McLaughlin (2010). The addition of E. taiwanensis Hsueh, 2015 , was accompanied by a key to members of Emerita , albeit it apparently compiled from text and illustrations in literature rather than first-hand examinations of specimens representing species in the key ( Hsueh 2015: table I). Applying characters used therein, E. portoricensis might correctly key to that species depending upon whether the first pereopod dactylus was in fact interpreted to be “distally subacute in the first couplet. However, as we herein establish, this article in E. portoricensis is upon close study clearly rounded at the very terminus in all but the largest of specimens, and thus much less acute than for any of the other four species to which that character applies in the key. Even so, presence of a slightly enlarged terminal spine on the otherwise rounded margin in E. portoricensis suggests some degree of acuity if not examined under magnification. It should also be noted that the first key couplet separates members of the genus in large part on the basis of a length to width ratio in the first pereopod dactylus, measurement of which is not therein precisely defined. Among western Atlantic members of the genus, the length of the full article is not necessarily or conspicuously less than two times width in all specimens of E. talpoida and E. brasiliensis , depending on maturity of specimens and whether the length measurement fully encompasses the distance from articulation with the propodus to the distal terminus. Even so, a relatively narrow, elongate first pereopod dactylus is found in E. portoricensis , consistently longer than twice the greatest width.
We also here note that Hsueh (2015) includes an apparent error in both tabular data (table I) and the key (Appendix, couplet 8) regarding relative width of the second pleonite in E. benedicti , which is stated to be less than 2/3 the carapace width and thus is used as a distinguishing characters for its identification. The second pleonite actually equals or exceeds 2/3 the carapace width in E. benedicti and all other western Atlantic species examined to date, even when allowing for some variation in its width due to different sizes and degrees of maturation. If judged only from the poorly lighted type specimen photographs of Schmitt (1935: fig. 71a, b) this would not be readily apparent, but it is clear from our direct studies of both the type material and a large series of specimens from throughout the range of E. benedicti .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Emerita portoricensis Schmitt, 1935
| Felder, Darryl L., Lemaitre, Rafael & Mantelatto, Fernando L. 2023 |
Emerita sp.
| Venera-Ponton, D. E. & Driskell, A. C. & De Grave, S. & Felder, D. L. & Scioli, J. A. & Collin, R. 2020: 5 |
Emerita portoricensis
| Poupin, J. 2018: 152 |
| Hsueh, P. - W. 2015: 254 |
| Boyko, C. B. & McLaughlin, P. A. 2010: 142 |
| Felder, D. & Alvarez, F. & Goy, J. W. & Lemaitre, R. 2009: 1068 |
| Haye, P. A. & Tam, Y. K. & Kornfield, I. 2002: 903 |
| Garcia E. & Lemus, M. & Chung, K. S. 2000: 215 |
| Melo, G. A. S. de 1999: 295 |
| Perez, D. 1999: 320 |
| Tam, Y. K. & Kornfield, I. & Ojeda, F. P. 1996: 489 |
| Sastre, M. P. & Yoshioka, P. M. 1992: 456 |
| Sastre, M. P. 1990: 526 |
| Abele, L. G. & Kim, W. 1986: 38 |
| Rodriguez, G. 1980: 241 |
| Efford, I. E. 1976: 174 |
| Efford, I. E. 1967: 88 |
| Goodbody, I. 1965: 195 |
Emerita portoricensis
| Schmitt, W. L. 1935: 213 |