Dromothelphusa longipes (A. Milne-Edwards, 1869 )
|
publication ID |
https://doi.org/ 10.26107/RBZ-2023-0048 |
|
publication LSID |
lsid:zoobank.org:pub:19D286F4-F712-4645-93FF-1AC19A55EDE9 |
|
persistent identifier |
https://treatment.plazi.org/id/882487B0-FFEA-FFFA-F69F-FC61EEFCFB85 |
|
treatment provided by |
Felipe |
|
scientific name |
Dromothelphusa longipes (A. Milne-Edwards, 1869 ) |
| status |
|
Dromothelphusa longipes (A. Milne-Edwards, 1869) View in CoL
( Figs. 8–14 View Fig View Fig View Fig View Fig View Fig View Fig View Fig )
Thelphusa longipes A. Milne-Edwards, 1869: 183 View in CoL , pl. 9 fig. 55. Potamon (Potamonautes) longipes – Ortmann, 1897: 304, 306;
De Man, 1904: 15. Potamon longipes – De Man, 1898: 437. Potamon (Potamon) longipes – Rathbun, 1904: 284, pl. 11 fig. 5. Ranguna (Ranguna) longipes – Bott, 1970: 168, pl. 43 fig. 7 (partim);
Chuensri, 1974: 25; Bott & Türkay, 1977: 93, figs. 1, 2. Demanietta (Dromothelphusa) longipes – Naiyanetr, 1992: 114. Dromothelphusa longipes – Yeo & Ng, 1999: 640; Ng et al., 2008:
162; Cumberlidge et al., 2009: supplementary table.
Material examined. Lectotype: male (56.5 × 43.6 mm) (MNHN-IU-2014-23055), Île de Poulo Condore , coll. R. Germain . Paralectotypes: 1 female (54.9 × 41.3 mm) (MNHN-IU-2014-23074, previously MNHN-B 4037 S) , 2 females ( MNHN-B 3827 S) , 1 male, 1 female ( MNHN-B 3828 S), same data as lectotype. Others : 2 males, 1 juvenile female (smaller male 33.9 × 27.4 mm) ( MNHN-B 17703 ) , 1 male, 2 females ( MNHN-B 17705 ), same locality as above, coll. Harmand, 1877 ; 1 female (54.1 × 40.8 mm) ( ZRC 1984.7034 View Materials ), coll. unknown, 2 November 1920 ; 1 female (53.5 × 41.5 mm) ( MBA 908 a), coll. unknown, November 1930 ; 1 female (47.2 × 36.5 mm) ( ZRC 2022.0057 View Materials ), stream at 100–200 m asl., coll. Bai, 25 June 1989 ; 3 males (30.3 × 25.4 mm, 30.1 × 25.2 mm, 34.6 × 28.1 mm), 1 female (36.6 × 29.7 mm), 1 ovigerous female (49.7 × 39.4 mm) ( ZRC 2021.0858 View Materials ) , 5 males (16.9 × 14.2 mm – 23.9 × 19.9 mm), 10 females (20.5 × 16.8 mm – 41.1 × 33.2 mm), 4 juveniles (9.3 × 7.7 mm – 17.2 × 14.3 mm) ( ZRC 2021.0861 View Materials ), station CDI-03, remnant freshwater pool about 100 m upstream, Dam Trau area , Nuoc Nong stream (= Đầm Tr ầu, suối Nư ớc Nóng), 8°43.697′N 106°37.152′E, coll. V. T. Nguyen et al., 13 April 2010; 1 ovigerous female (50.0 × 39.2 mm) GoogleMaps , 2 juvenile females, 1 broken carapace ( ZRC 2021.0863 View Materials ), station CDI-07, freshwater stream, westward of river mouth, 200 m upstream, Dam Tre (= Đầm Tre), coll. V. T. Nguyen et al., coll. 14 April 2010 ; 1 male (29.9 × 24.6 mm), 1 female (38.6 × 30.4 mm), 3 subadult males (16.2 × 13.7 mm – 21.2 × 17.5 mm), 3 subadult females (18.6 × 15.3 mm – 20.6 × 17.4 mm), 3 juveniles (10.4 × 9.0 mm – 12.4 × 10.5 mm) ( ZRC 2021.0862 View Materials ), station CDI-11, under rocks, dry stream bed, Bong Huong stream (= suối Bông Hư ờng), 08°42.210′N 106°37.012′E, coll. V. T. Nguyen et al., 15 April 2010; 2 dried carapaces (56.6 × 44.0 mm, 59.5 × 46.5 mm) ( ZRC 2021.0864 View Materials ), station CDI-11, dry stream bed, Bong Huong stream (= suối Bông Hường), 08°42.210′N 106°37.012′E, coll. V. T. Nguyen et al., 15 April 2010. All specimens from Condore, Vietnam GoogleMaps .
Diagnosis. As for genus.
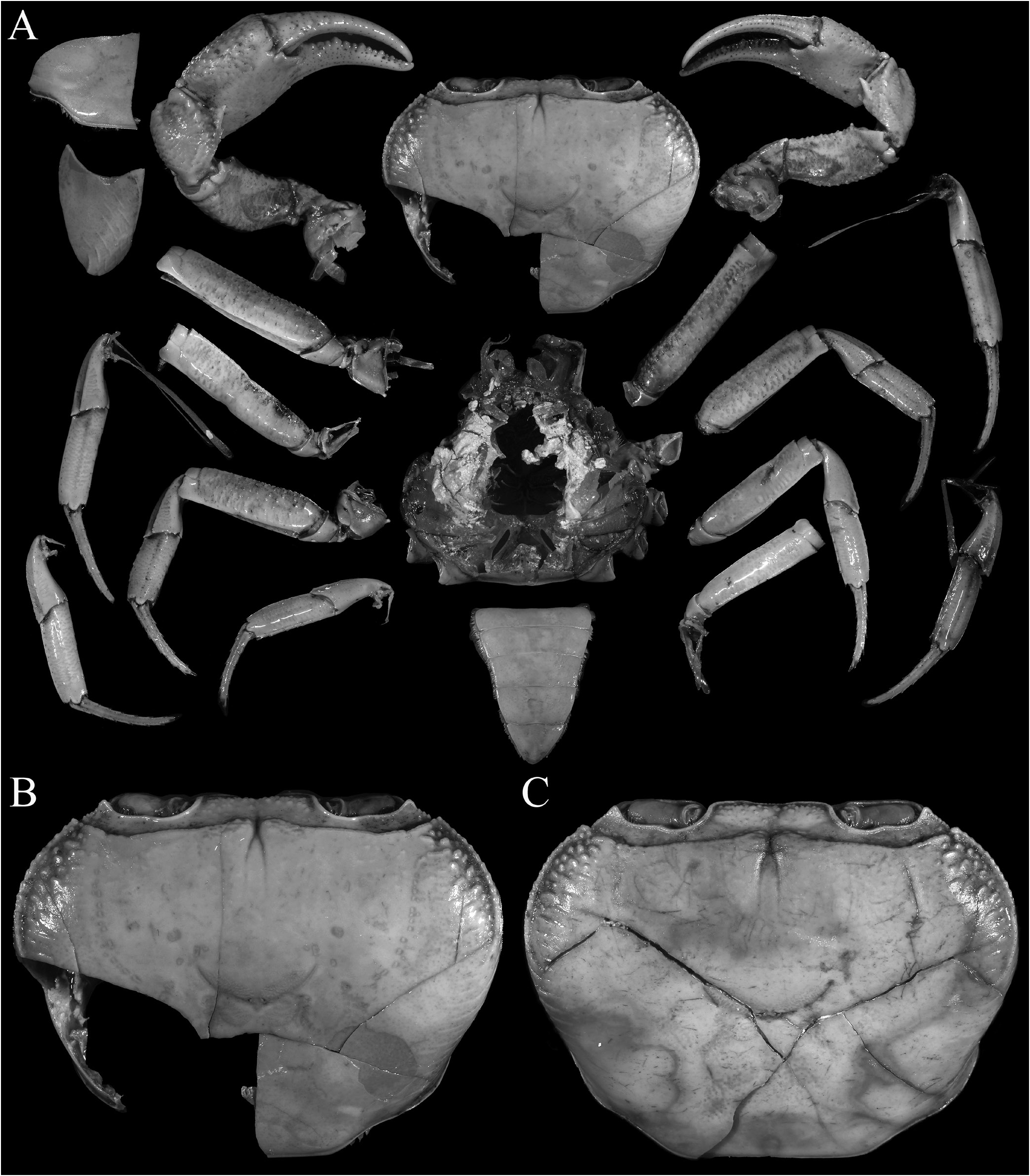
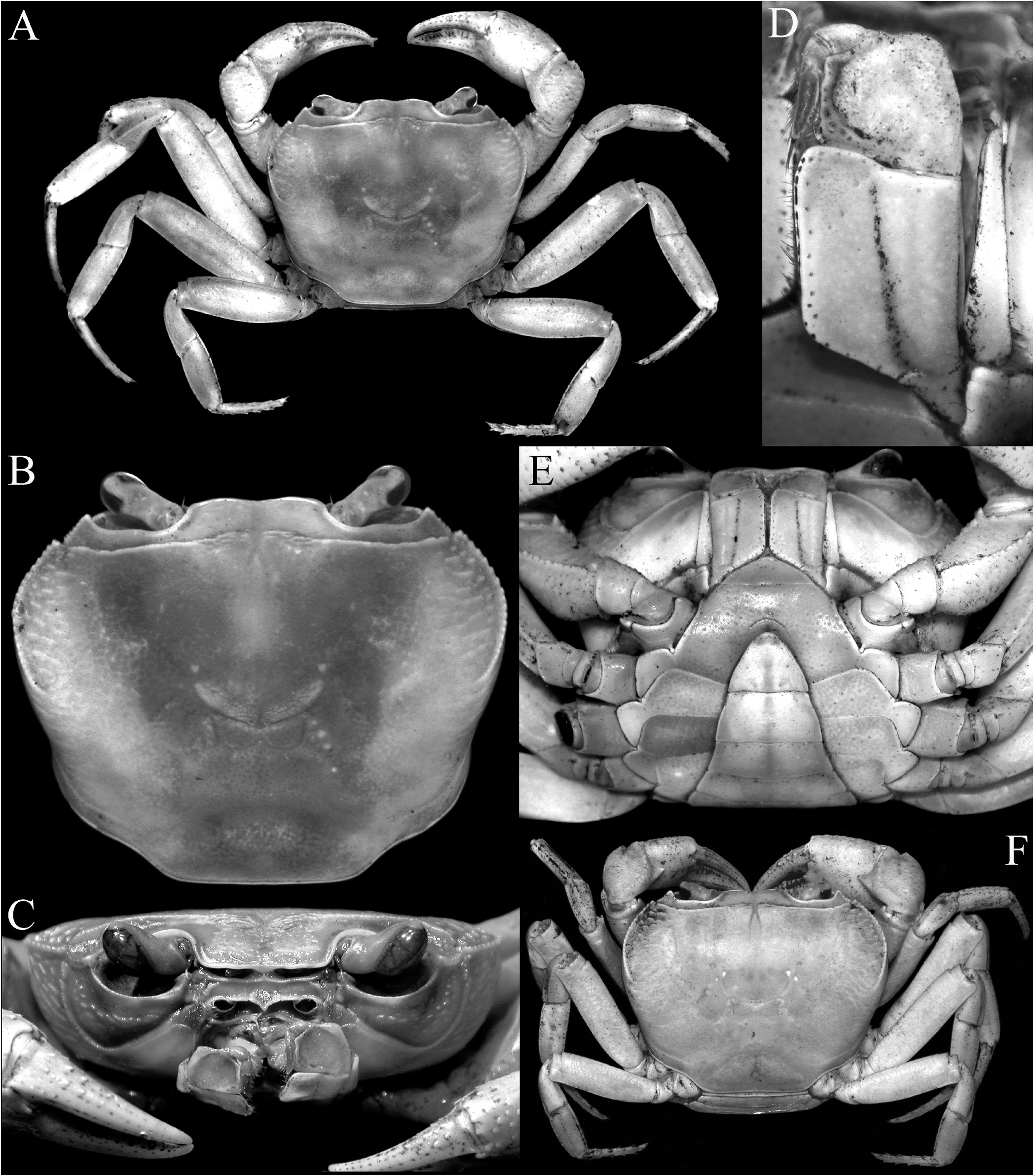
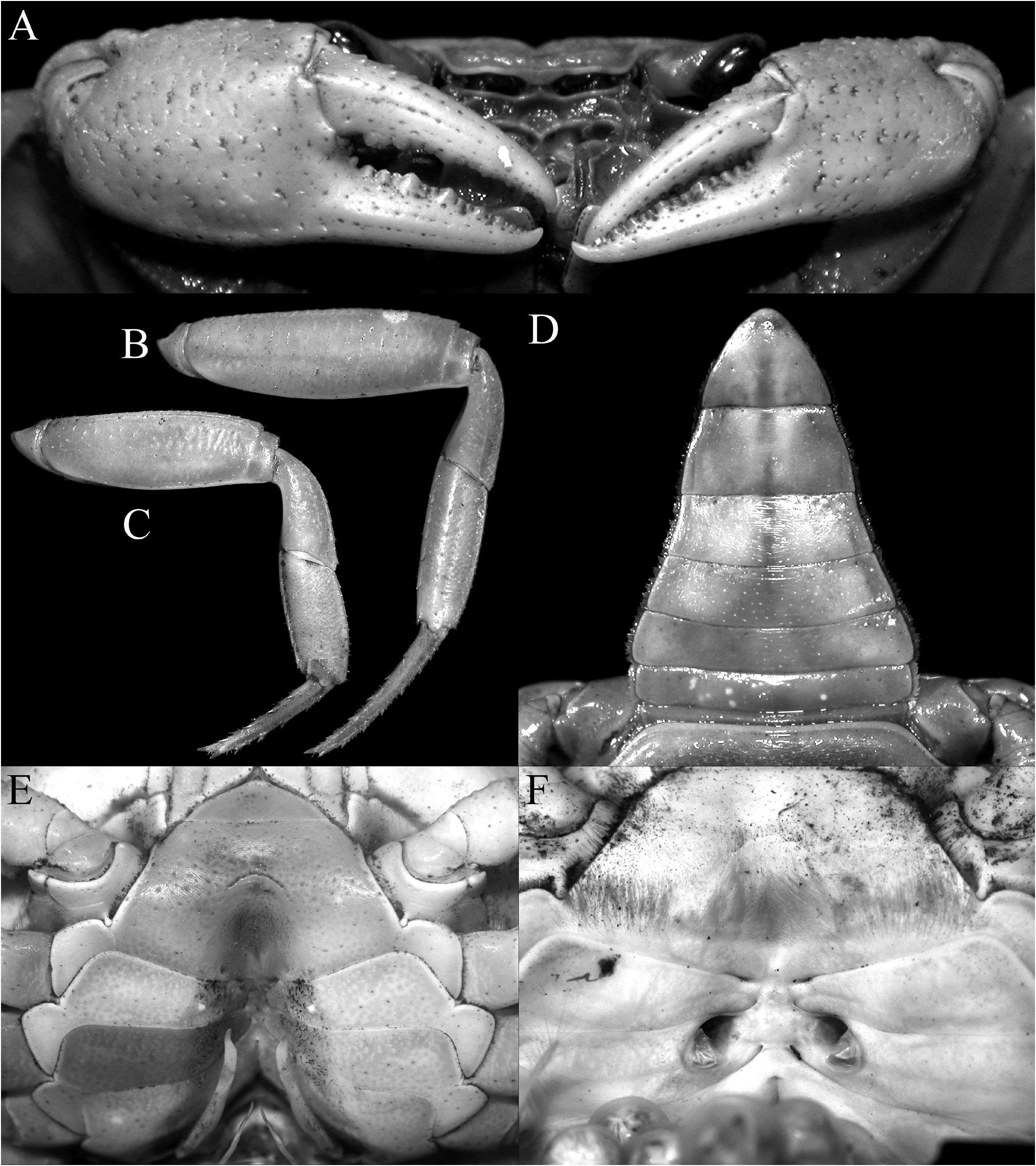
Description of male. Carapace wider than long, relatively high, dorsal surface convex transversely and longitudinally in frontal view; epigastric cristae well developed, separated by distinct groove which opens up into inverted V-shape posteriorly, slightly anterior to postorbital cristae, more or less confluent with postorbital cristae, not clearly separated; postorbital cristae sharp, almost confluent with epibranchial tooth ( Figs. 10A, B View Fig , 11A, B View Fig ); frontal and postorbital areas not compressed, with these regions relatively wide in dorsal view ( Figs. 10A, B View Fig , 11A, B View Fig ); regions behind epigastric and postorbital cristae almost smooth; pterygostomial, sub-hepatic, suborbital and sub-branchial regions gently rugose; cervical grooves shallow, just visible, posteriorly joining H-shaped gastric grooves ( Fig. 11A–C View Fig ). Front gently deflexed downwards frontal margin gently bilobed, separated by shallow median concavity ( Figs. 10A, B View Fig , 11B, C View Fig ). External orbital angle triangular, outer margin longer than inner margin; epibranchial tooth low but distinct, separated by clear cleft; anterolateral margins cristate, serrated, gently convex, not confluent with posterolateral margins; posterolateral margins gently converging posteriorly; branchial regions gently rugose ( Figs. 10A, B View Fig , 11A, B View Fig ). Orbits large, oblique in position; supraorbital margin sinuous, cristate; eyes large, almost occupying orbit, peduncle short, stout; cornea large ( Figs. 10B View Fig , 11B, C View Fig ). Antennular fossae longitudinally narrow, slit-like ( Fig. 11C View Fig ); antenna small, flagellum not extending beyond orbit ( Fig. 11C View Fig ). Epistome posterior margin with acutely triangular median tooth, outer part gently sinuous ( Fig. 11C View Fig ). Endostome smooth, no clear ridge visible.
Third maxilliped with surface glabrous, inner margin lined with short setae; merus subquadrate, ca. half ischium length, anteroexternal angle rounded; median part gently depressed; ischium relatively short, broadly rectangular, with distinct oblique median sulcus; exopod slender, long, distal part exceeding distal edge of ischium, reaching one-third length of merus, flagellum vestigial or absent in adults ( Figs. 11D View Fig , 13F–K View Fig ).
Chelipeds stout, nor elongate, heterochelous ( Figs. 10A View Fig , 11A View Fig , 12A View Fig ); outer surfaces with well-spaced low tubercles; margins of merus lined with round tubercles, without obvious subterminal spine, outer surface rugose ( Figs. 10A View Fig , 11A View Fig ); carpus margins uneven, inner distal angle with large, subdistal spine and basal tubercle ( Figs. 10A View Fig , 11A View Fig ); fingers longer than palm, dorsal and ventral margins uneven; cutting edges of fingers with strong cutting teeth ( Figs. 10A View Fig , 12A View Fig ).
Ambulatory legs not elongate, stout, glabrous; merus subdistal spine absent, upper margin weakly serrated; propodus relatively long; dactylus long, slender ( Figs. 10A View Fig , 11A View Fig , 12B, C View Fig ).
Anterior thoracic sternites relatively wider transversely; suture between anterior thoracic sternites 2 and 3 visible but not deep, complete, reaching edge of sternum; no suture between anterior thoracic sternites 3 and 4; sternopleonal cavity reaching imaginary line joining midpoint of cheliped bases; tubercle of male press-button locking mechanism small, on posterior third of thoracic sternite 5 ( Figs. 11E View Fig , 12E View Fig ).
Pleon triangular, all somites and telson free; somites 3–5 trapezoidal, lateral margin gently concave to convex; somite 6 trapezoidal, broader than long, lateral margins gently convex;
telson triangular with gently convex lateral margins, as long as somite 6 ( Figs. 10A View Fig , 11E View Fig , 12D View Fig ).
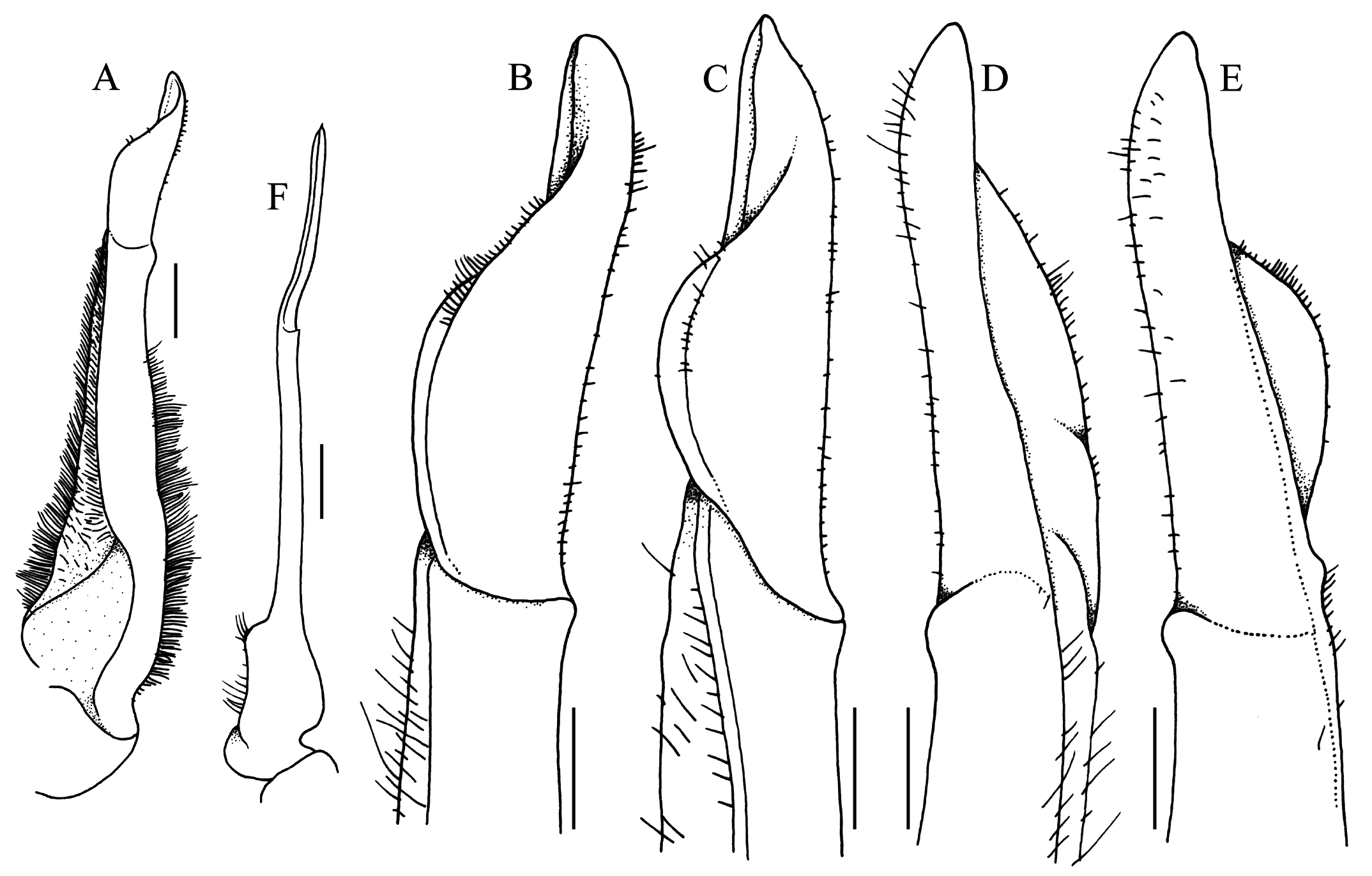
G1 almost straight, relatively slender; terminal and subterminal segments clearly demarcated; subterminal segment relatively slender, not neck-like distally, without shelf on upper part of outer margin; terminal segment almost straight, subconical, with prominent dorsal flap on proximal half, without longitudinal torque, without swelling on inner margin, groove for G2 marginal ( Fig. 14A–E View Fig ). G2 subequal in length to G1; distal segment about half length of basal segment; basal segment relatively narrow, with outer margin concave ( Fig. 14F View Fig ).
Females and variation. Female pleon ovate, covering most of thoracic sternum. Vulvae large, occupying ¾ anterior part of thoracic sternite 6, operculum present, sternal vulvar cover narrow, on posterior margin, opening obliquely inwards ( Fig. 12F View Fig ). For variation in the length and/or presence of a flagellum on the exopod of the third maxilliped, see remarks later.
Colour. The dorsal surfaces vary from pale red to orange in adults ( Figs. 8A, B, D–F View Fig , 9B–E View Fig ); with smaller specimens pale orangish-brown to beige ( Figs. 8C View Fig , 9A View Fig ); the ventral surfaces being pinkish-white to white ( Fig. 9F–H View Fig ). The eggs are bright orange, even when the eyes are visible ( Figs. 8G, H View Fig , 9H View Fig ).
Remarks. Alphonse Milne-Edwards (1869: 183, pl. 9 fig. 5) described Thelphusa longipes from the island of Condore from material collected by R. Germain but did not say how many specimens he had. He provided measurements for one specimen (sex not stated): 55.0 × 45.0 mm; and his figure depicts a female (A. Milne-Edwards, 1869: pl. 9 fig. 5). Rathbun (1904: 284, pl. 11 fig. 5) reported that the type series in MNHN consisted of two males and four females, provided measurements for two males and a female, and the larger male, 56.5 × 43.6 mm was designated as the “type” ( Rathbun, 1904: 285). Bott (1970: 168) did not examine the type material but reported on two female specimens, one from Condore in MBA and another supposedly from Trang, Thailand, in MNHN. The MNHN specimen ( Bott, 1970: pl. 51 fig. 55) was originally identified by Rathbun (1904) as Potamon (Potamon) abbotti Rathbun, 1898 , but Bott (1970) disagreed and assigned it to the present species. Ng (1986), however, showed that Bott (1970) had misidentified the specimen, and referred it back to Terrapotamon abbotti ( Rathbun, 1898) . Ng & Naiyanetr (1993: 21) also doubted the identity of Bott’s (1970: pl. 43 fig. 7) MBA specimen, commenting that, “…from his figures, these specimens do not appear to be that species at all”. This specimen (MBA 908a), however, has been re-examined and is clearly conspecific with the type material of D. longipes (present study; see also Bott & Türkay, 1977). The present specimen identified as the lectotype (56.5 × 43.6 mm, MNHN-IU-2014-23055) is the specimen selected by Rathbun (1904) as the “type” and we regard it as a valid lectotype selection (sensu ICZN, 1999: Article 74.5).
Bott & Türkay (1977) subsequently reported two specimens in MNHN, one of which was a male, but no measurements were given for that either. The figure of the male ( Bott & Türkay, 1977: fig. 2), however, has a scale bar with it, and it approximately agrees with the measurement provided by Rathbun (1904) of the largest male. Bott & Türkay (1977: fig. 1) also figured the distal part of the G1. The G1 figure in Bott & Türkay (1977) differs in that the dorsal fold appears to be lower and folded, and the distal part appears sharper ( Bott & Türkay, 1977: fig. 1) (cf. Fig. 14A–E View Fig ). These differences are not regarded as significant and are probably a consequence of the type specimen being dried for a long time.
The presence of a long flagellum on the exopod of the third maxilliped is an important character separating many genera. Although the value of this character has been questioned at times (e.g., see Brandis, 2000), it has proven to be useful to separate groups of species, and in many cases, the genera have also been supported by genetic data (e.g., see Shih et al., 2009). While it is known the flagellum may be broken in some individuals or slightly variable, no one yet demonstrated that this character may vary with size. We examined the good series of specimens of D. longipes on hand, which includes many small specimens.
Most juveniles (in which the pleopods are not visible) have a long flagellum on the exopods of both third maxillipeds (as long as the width of the merus) (e.g., 9.3 × 7.7 mm, 12.1 × 10.5 mm, 17.2 × 14.3 mm, ZRC 2021.0861; 10.4 × 9.0 mm, 10.7 × 9.2 mm, ZRC 2021.0862); in two specimens (12.4 × 10.7 mm, ZRC 2021.0861; 12.4 × 10.5 mm, ZRC 2021.0862), there is a long flagellum on one exopod while the other has only a vestigial one. Among the subadult individuals (in which the pleopods are visible but poorly developed), two subadult males (16.9 × 14.2 mm, ZRC 2021.0861; 18.2 × 14.7 mm, ZRC 2021.0862; Fig. 13D, E View Fig ) have a long flagellum on both exopods, while a subadult female (20.6 × 17.4 mm, ZRC 2021.0862; Fig. 13A, B View Fig ) has one long and one vestigial flagellum on each exopod. All the other juveniles, subadult males and females, as well as all specimens with carapace widths wider than 21 mm carapace width have only vestigial flagella on their exopods ( Fig. 13F–K View Fig ). The present study of this good series of specimens indicates that the flagellum on the exopod of the third maxilliped is a taxonomic character valid only for adult specimens. While this character is often important in delineating potamid genera, it should be relied upon for diagnosing adults only and is less reliable with respect to subadults and juveniles. This is usually not discussed in taxonomic treatments and should be considered in future studies of terrestrial and semiterrestrial potamids (and possibly gecarcinucids as well). The variation in the length of the flagellum here may be associated with the fact that juveniles and subadults are usually more aquatic in habit, spending more time in water (where the flagellum in more useful in respiration), with adults having more terrestrial habits and having less use for the structure.
Distribution. Known only from Condore island, southern Vietnam.
| R |
Departamento de Geologia, Universidad de Chile |
| V |
Royal British Columbia Museum - Herbarium |
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Dromothelphusa longipes (A. Milne-Edwards, 1869 )
| Ng, Peter K. L. & Yeo, Darren C. J. 2023 |
Thelphusa longipes A. Milne-Edwards, 1869: 183
| Ortmann A 1897: 304 |
| Milne-Edwards A 1869: 183 |