DORIPPINAE H. Milne Edwards, 1837
|
publication ID |
https://doi.org/ 10.5252/zoosystema2023v45a9 |
|
publication LSID |
urn:lsid:zoobank.org:pub:69C34731-8C25-4A1E-B336-B222CD3CBAC3 |
|
DOI |
https://doi.org/10.5281/zenodo.8071398 |
|
persistent identifier |
https://treatment.plazi.org/id/03CDBE74-9318-B507-CE72-FD15FEEAF8F4 |
|
treatment provided by |
Felipe |
|
scientific name |
DORIPPINAE H. Milne Edwards, 1837 |
| status |
stat. nov. |
Subfamily DORIPPINAE H. Milne Edwards, 1837 n. status
TYPE GENUS. — Dorippe Weber, 1795 View in CoL (type species by subsequent designation by Holthuis [1962]: Cancer quadridens Fabricius, 1793 ; the type species of Dorippe Fabricius, 1798 View in CoL is Cancer quadridens Fabricius, 1793 by subsequent selection by Latreille [1810], see Holthuis [1962]: 54; Holthuis & Manning [1990]: 7). Other included species: Cancer frascone Herbst, 1785 ; Dorippe glabra Manning, 1993 View in CoL ; Dorippe irrorata Manning & Holthuis, 1986 View in CoL ; Dorippe sinica Chen, 1980 View in CoL ; Dorippe tenuipes Chen, 1980 View in CoL ; Dorippe trilobata Manning, 1993 View in CoL .
DESCRIPTION
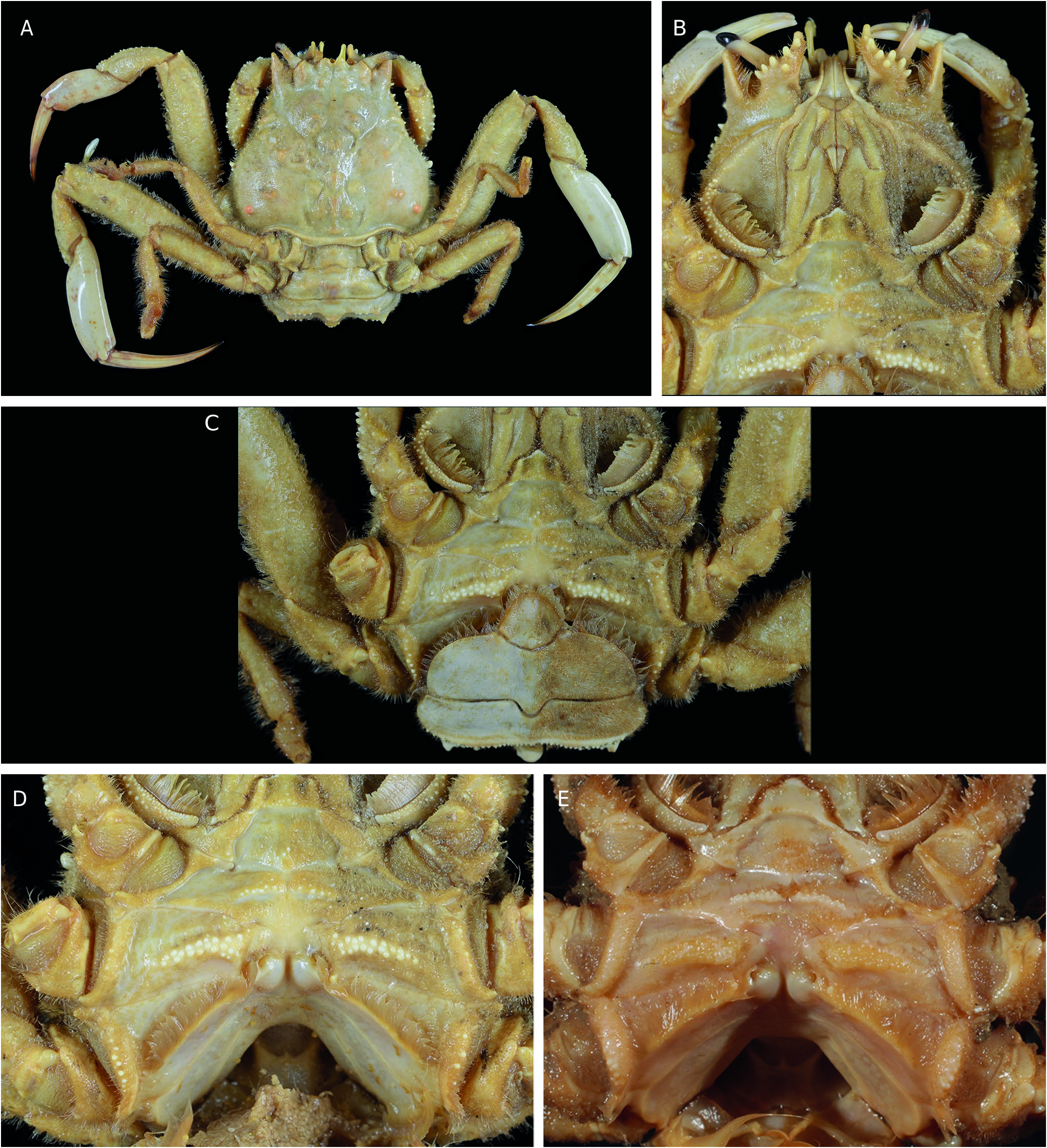
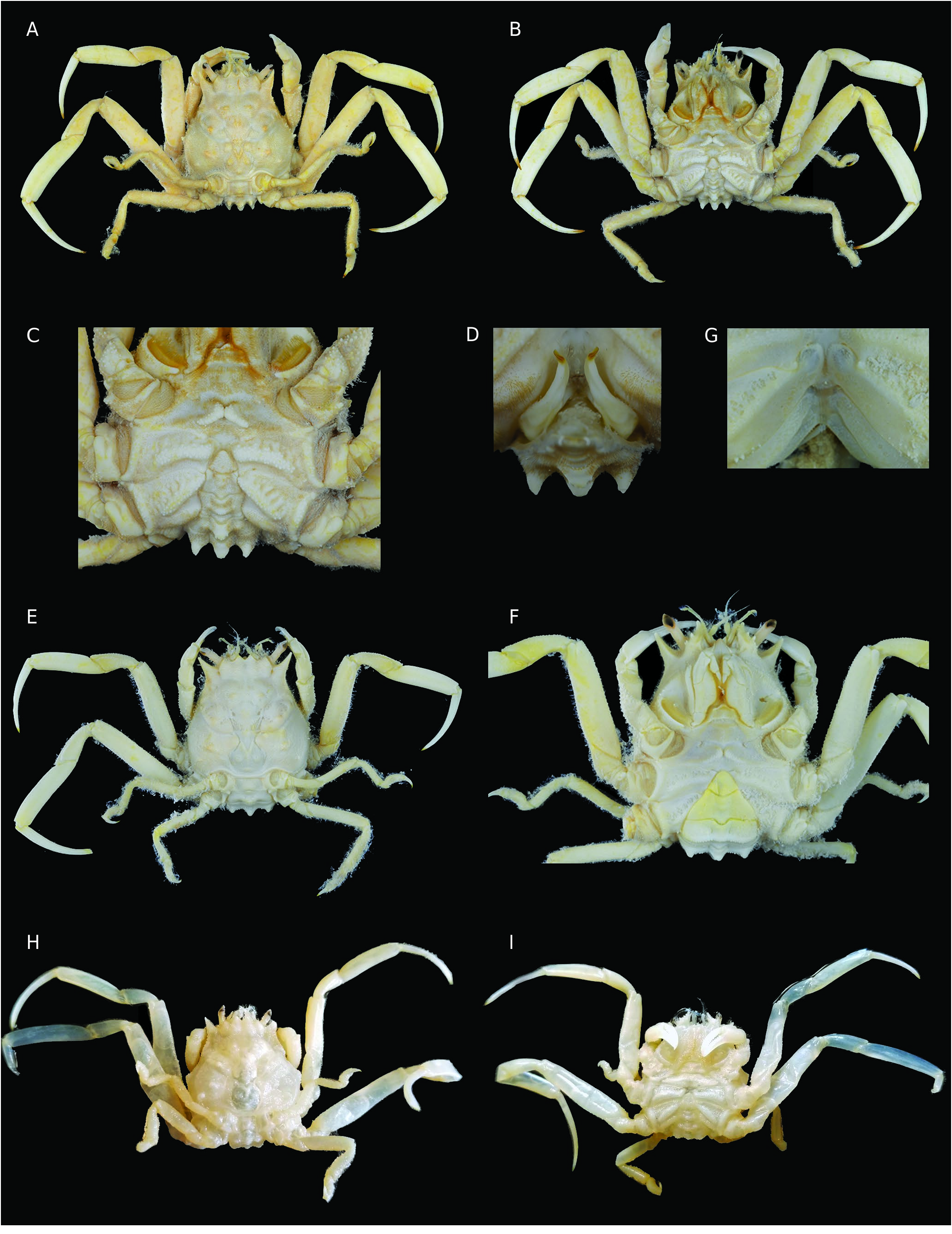
Carapace ( Figs 10 View FIG ; 12A View FIG ; 14A, E, H View FIG ; 15A, C, E, F View FIG )
Carapace wider than long, being much wider than long in larger individuals, but always narrowing distinctly forward. Dorsal surface strongly sculptured, subdivided into several regions, rough, uneven, with distinct tubercles, often with setae obscuring surface ornamentation; meso-, meta- and urogastric regions well recognisable. Precervical groove irregular, indistinct. Cervical groove distinct, wide. Branchiocardiac groove deep, clearly defined. A pair of small branchial lobes. At base of meso-metagastric region, two small, oblique, hardly distinct submedian gastric pits. Between base of outer orbital tooth and cervical groove, anterolateral margin of carapace long, with a few or many tubercles or denticles, otherwise smooth. Posterior margin of orbit with fissure. Outer orbital tooth triangular, slender and pointed, reaching beyond frontal teeth. Epibranchial angle marked, may be with spine. Front consisting of two closely spaced triangular submedian teeth, separated by more or less deep V-shaped emargination. Inner orbital teeth about as large as frontal teeth, reaching less far forward. Lower orbital margin with spines on outer margin of inner suborbital tooth. Carapace rim extending along posterolateral margin, lined posteriorly by narrow, straight strip, similar in both sexes.
Illustrations: Dorippe glabra: Manning 1993 : fig. 1a, b. D. frascone: Herbst 1785 : pl. 11, fig. 70 (reproduced by Holthuis & Manning 1990: fig. 3); Serène & Romimohtarto 1969: figs 1, 5, 10, 15A, B, pl. 1, figs A, B, pl. 3, figs A-C, as Dorippe (Dorippe) frascone ); Chen 1980: fig. 3.2, pl. 2, figs 1, 6, as D. (D.) frascone ; Holthuis & Manning 1990: fig. 2a. D. irrorata: Holthuis & Manning 1990 : fig. 4a-c. D. quadridens: Latreille 1818 : pl. 306, fig. 1; Guérin?1831-1833: pl. 13, fig. 2, as D. nodulosa (reproduced by Holthuis & Manning 1990: fig. 10A); Griffith & Pidgeon 1833: pl. 13, fig. 2, as D. nodulosa (reproduced by Holthuis & Manning 1990: fig. 10B); De Haan 1839: pl. 31, fig. 3, as D. quadridens (reproduced by Holthuis & Manning 1990: fig. 15); Borradaile 1903: pl. 22, fig. 1, as D. dorsipes (reproduced by Holthuis & Manning 1990: fig. 11b); Shen 1931: fig. 5 (reproduced by Holthuis & Manning 1990: fig. 11a; by Sin et al. 2009: fig 3A); Chen 1980: fig. 2a, b (reproduced by Holthuis & Manning 1990: fig. 12a); Holthuis & Manning 1990: figs 5a, 7a, 8, 9; Chen & Sun 2002: fig. 89.3, pl. 2.3; Naruse et al. 2014: fig. 2c; Takeda et al. 2019: 13, pl. 3E, F; Wong et al. 2021: fig. 8a, pl. 2B. D. sinica: Chen 1980 : fig. 1.2 (reproduced by Holthuis & Manning 1990: fig. 16a), pl. 1, figs 1, 3, 5, as D. (D.) sinica ; Miyake 1983: pl. 6, fig. 2; Takeda 1983: 231, fig. p. 121, as D. frascone ; Quintana 1987: fig. 20A, as D. frascone ; Holthuis & Manning 1990: figs 13a, 15; Yamaguchi & Baba 1993: fig. 89A, B; Chen & Sun 2002: fig. 90.1; Wong et al. 2021: fig. 9a, pl. 2C. D. tenuipes: Serène 1982 : pl. 1, fig. 1, pl. 2, fig. 1, as Dorippe miersi ; Chen 1980: fig. 2.1, as D. (D.) tenuipes (reproduced by Holthuis & Manning 1990: fig. 18a); Chen 1986a: figs 1, 2a; Holthuis & Manning 1990: fig.17a; Chen & Sun 2002: fig. 91.1; Takeda & Manuel-Santos 2006: fig. 6B. D. trilobata: Manning 1993 : fig. 2a, b.
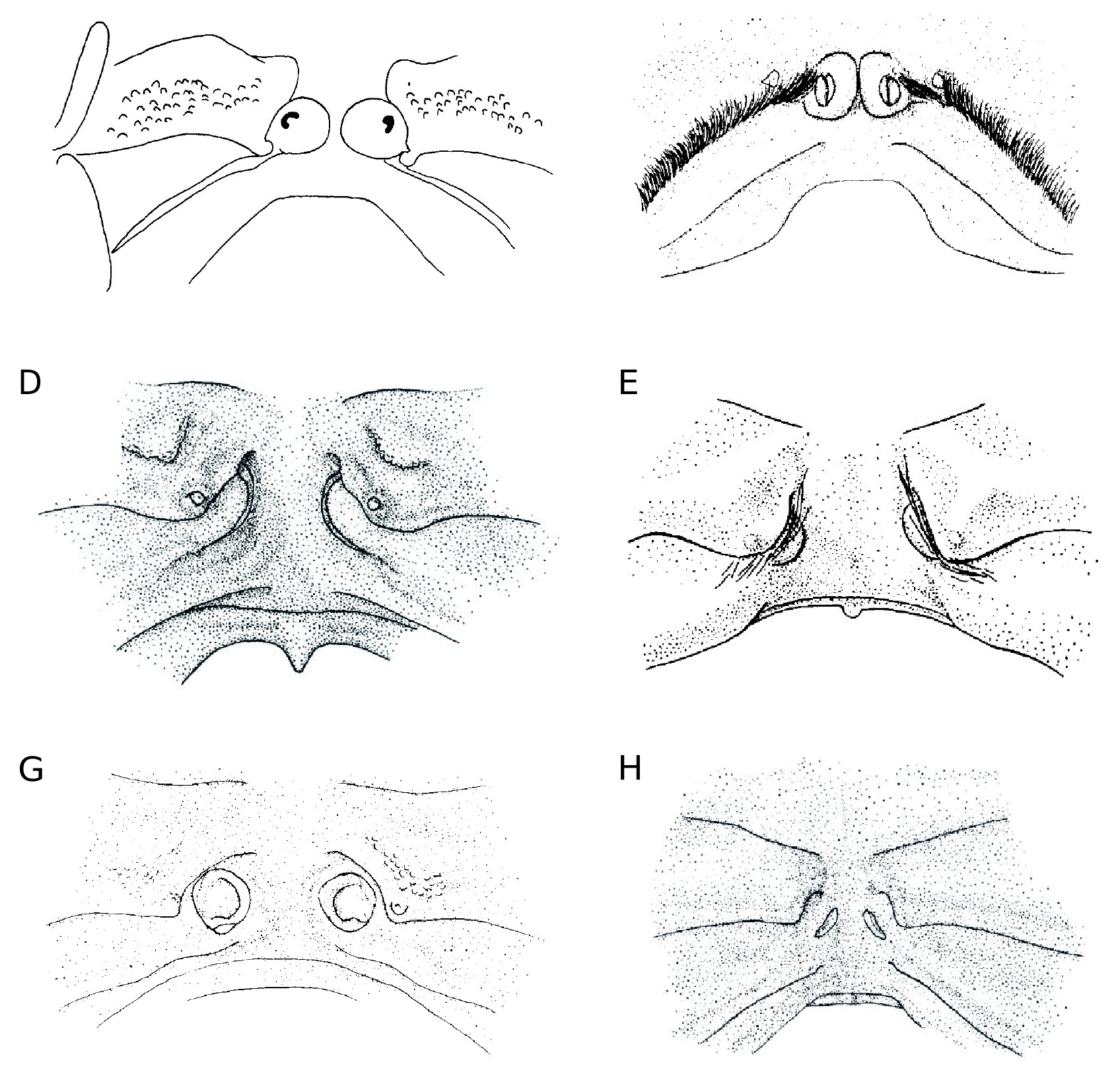
Cephalic structures ( Figs 12B View FIG ; 14B, F View FIG ; 15G View FIG )
Eyestalk elongated, slender, pointed; cornea ventrolateral. Antennule folded or incompletely folded into fossa. Antenna: article 2 + 3 long and immobile, wedged in narrow fossa, partially visible; articles 4 and 5 widened, very setose, directed forward; flagellum slightly bent outwards. Lower orbital margin with cluster of several spines.
Illustrations: Dorippe frascone: Chen 1980 : fig. 3.1, as D. (D.) frascone ; Holthuis & Manning 1990: fig. 3. Dorippe glabra: Manning 1993 : fig. 1b. D. quadridens: Ihle 1916 : figs 41, 45, as D. dorsipes ; Holthuis & Manning 1990: figs 6b, c, 12a; Chen & Sun 2002: fig. 89.1; Wong et al. 2021: fig. 8b. D. sinica: Chen 1980 : fig. 1b (reproduced by Holthuis & Manning 1990: fig. 16b); Quintana 1987: fig. 20A-a, as D. frascone ; Holthuis & Manning 1990: fig. 13b, c; Chen & Sun 2002: fig. 90.1; Wong et al. 2021: fig. 9b. D. tenuipes: Chen 1980 : fig. 2.2, as D. (D.) tenuipes (reproduced by Holthuis & Manning 1990: fig. 18b); Holthuis & Manning 1990: fig. 17b, c. D. trilobata: Manning 1993 : fig. 2b.
Oxystomatous disposition ( Figs 12B View FIG ; 14B View FIG ; 15G View FIG )
Openings of exhalant channels not visible in dorsal view.
Illustrations: Dorippe frascone: Holthuis & Manning 1990 : fig. 3. D. quadridens: Holthuis & Manning 1990 : figs 8, 10, 11; Wong et al. 2021: fig. 8b. D. sinica: Quintana 1987 : fig. 20a, as D. frascone ; Holthuis & Manning 1990: fig. 15; Wong et al. 2021: fig. 9b.
Pereiopods ( Figs 10 View FIG ; 12A View FIG ; 14A, B, E, F View FIG ; 15A, B, E, H, I View FIG ) Chelipeds equal in females and small males, but heterochely usually in large males. Carpus either smooth and naked or with distinct granules or tubercles and short hairs. Major cheliped with palm smooth or variously granulated, swollen, higher than long dorsally; lower margin convex, lacking teeth or tubercles. Minor cheliped with fingers 2-3 times longer than palm; both fingers with two grooves separated by ridge; cutting edges with 12-16 subequal triangular teeth, regularly distributed over edge.
Illustrations: Dorippe glabra: Manning 1993 : fig. 1c. D. irrorata Holthuis & Manning 1990 : fig. 4d. D. frascone: Chen 1980 : fig. 3.3, pl. 2, figs 1, 2, 4, 6, as D. (D.) frascone ; Chen 1986a: fig. 1c, as D. tenuipes ; Holthuis & Manning 1990: fig. 2b. D. quadridens: Holthuis & Manning 1990 : figs 5d, 6d; Wong et al. 2021: fig. 8c, d. D. sinica: Chen 1980 : fig. 1.3, as D. (D.) sinica (reproduced by Holthuis & Manning 1990: fig. 16c), pl. 1; Holthuis & Manning 1990: fig. 13d; Chen & Sun 2002: fig. 90.2. D. tenuipes: Chen 1980 : fig. 2.3 (reproduced by Holthuis & Manning 1990: fig. 18c), pl. 2, figs 3, 5, 7, 8, as D. (D.) tenuipes ; Holthuis & Manning 1990: fig. 17d; Chen & Sun 2002: fig. 91.2. D. trilobata: Manning 1993 : fig. 2c.
P2, P3 long or very long, P3 longest. Meri of P2 and P3 either nearly cylindrical or compressed, setose or glabrous, without dorsal spines or spinules, with numerous more or less acute granules in D. tenuipes . Sexual dimorphism of P2, P3 meri setation slight in D. frascone , with naked meri in males, hairy in females ( Fig. 14A, B and E, F View FIG , respectively), much more pronounced in D. sinica with merus of adult males ( Fig. 10C View FIG ) covered by dense pubescence but almost completely naked in females ( Fig.10D View FIG ). Propodi and dactyli entirely naked; dactyli of P2, P3 without hair fringes, naked or nearly so. P4, P5 reduced, with subcheliform apparatus.
Illustrations: Dorippe glabra: Manning 1993 : fig. 1d. D. irrorata Holthuis & Manning 1990 : fig. 4 e, f. D. frascone: Holthuis & Manning 1990 : fig. 2c, d. D. quadridens: Holthuis & Manning 1990 : figs 5e, f (reproduced by Sin et al. 2009: fig. 4A), 6e, f, 7b; Wong et al. 2021: fig. 8e, f. D. sinica: Holthuis & Manning 1990 : fig. 13e, f; Wong et al. 2021: fig.9c. D. tenuipes: Holthuis & Manning 1990 : fig. 17e, f. D. trilobata: Manning 1993 : fig. 2d.
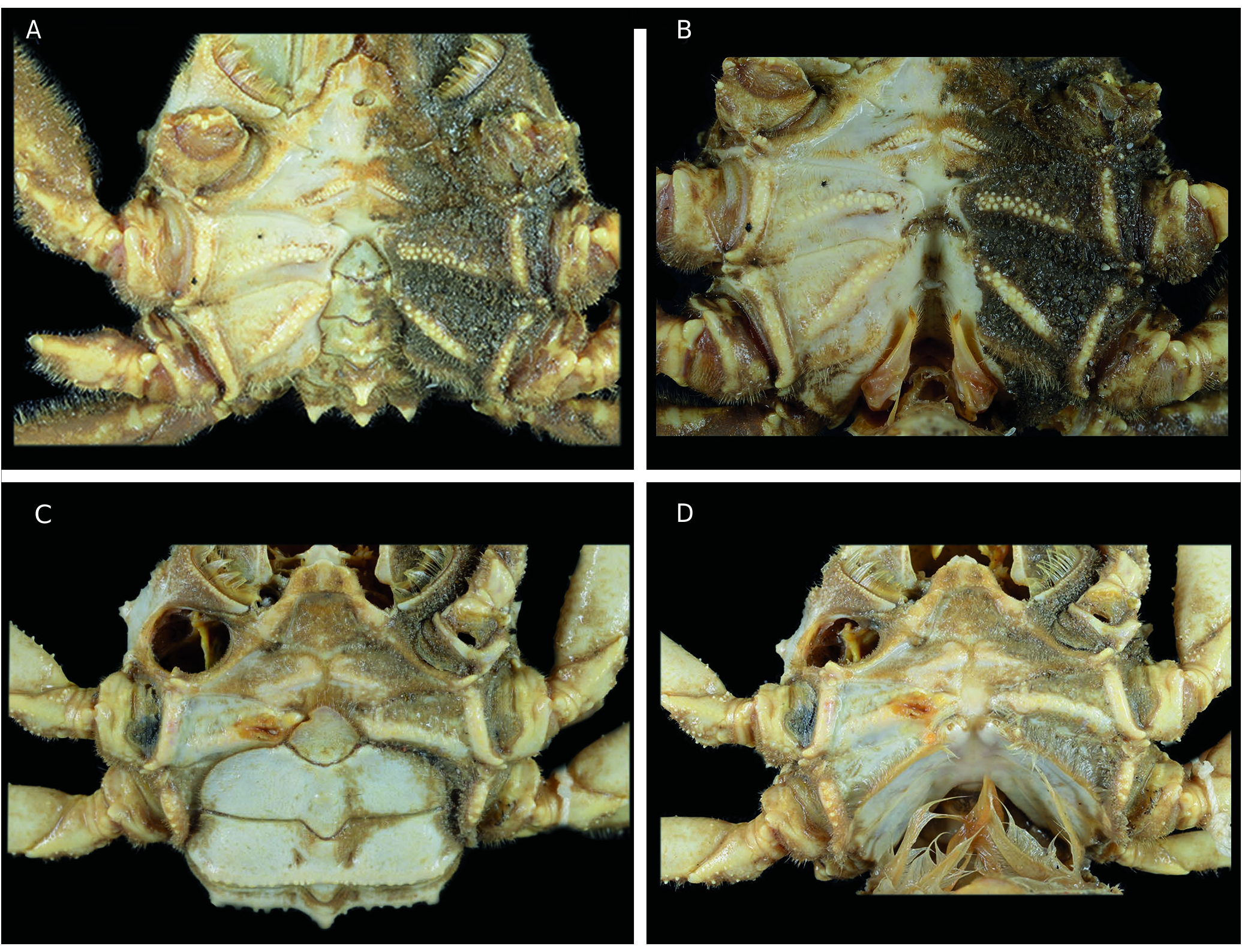
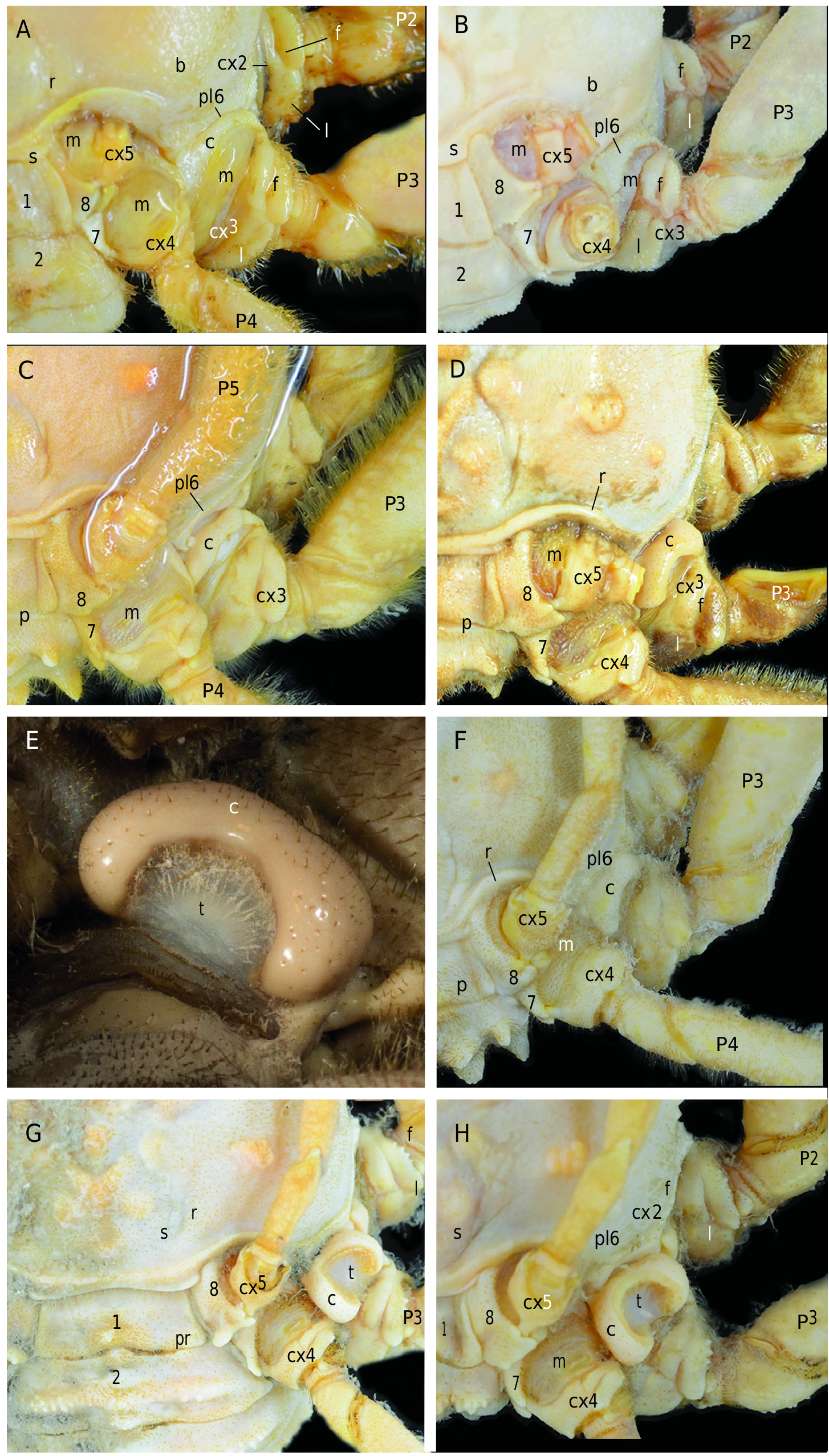
Thoracic sternum ( Figs 11 View FIG ; 12 View FIG B-E; 13; 14B, C, F; 15B, D, G) Thoracic sternum strongly sculptured in both sexes; thickened laterally at distal part of sternites 4 and especially 5, 6. Sternites 1 and 2 as pentagonal, raised shield: sternite 1 with small visible pointed portion; sternite 2 narrow, demarcated from sternite 3 by thick ridge; sternite 3 lowered, developed, separated from sternite 4 by short lateral suture (suture 3/4) that may or may not end in small boutonniere; sternite 4 with two strong curved submedian elevations or complete ridge; sternite 5 crossed by strong ridge; sternite 6 crossed by two elevations. Sutures 4/5-7/8 interrupted; 5/6 to 7/8 obliquely oriented; suture 5/6 strongly curved backwards, with press-button in curve. Female thoracic sternum extremely tilted backwards at level of ridge crossing entire sternite 6.
Pleon and telson ( Figs 8C View FIG ; 9A, C, D View FIG ; 10 View FIG ; 11B View FIG ; 12A, C View FIG ; 13A, C View FIG ; 14B, C, F, I View FIG ; 15 View FIG A-H)
Male pleon with all somites free; somite 1 trapezoidal, widening slightly posteriorly; somites 2, 3 each with transverse row of three strong teeth ( Dorippe frascone , D. quadridens , D. sima , D. trilobata ) or blunt granular elevations ( D. irrorata , D. tenuipes ); somite 4 narrower, narrowing posteriorly, with single median tooth ( D. frascone , D. quadridens , D. sima , D. trilobata ) or with granular elevations ( D. irrorata , D. tenuipes ); somite 5 laterally constricted; somite 6 posteriorly narrowing, with more or less produced posterolateral angles enclosing base of telson; telson triangular, with rounded apex, tip exceeding level of suture 5/6.
Illustrations: D. irrorata: Holthuis & Manning 1990 : fig. 4g, h. D. frascone: Chen 1980 : fig. 3.4, pl. 2, figs 1, 2, as D. (D.) frascone ; Holthuis & Manning 1990: fig. 2e, f. D. quadridens: Chen 1980 : fig. 2b, as D. (D.) frascone (reproduced by Holthuis & Manning 1990: fig. 12b); Holthuis & Manning 1990: figs 5g-i, 7c, d (reproduced by Davie et al. 2015a: fig. 71-2.22J); Chen & Sun 2002: fig. 89.2. D. sinica: Chen 1980 : fig. 1.4, as D. (D.) sinica (reproduced by Holthuis & Manning 1990: fig. 16d), pl. 1, figs 2, 6; Miyake 1983: pl. 6, fig. 2; Holthuis & Manning 1990: fig. 14c, d, e, f; Chen & Sun 2002: fig. 90.3. D. tenuipes: Chen 1980 : fig. 2.4 (reproduced by Holthuis & Manning 1990: fig. 18d), pl. 2, figs 3, 5, as D. (D.) tenuipes ; Holthuis & Manning 1990: fig. 17g, h; Chen & Sun 2002: fig. 90.3. D trilobata: Manning 1993 : fig. 2e, f.
Female pleon narrow in immature individuals, widening greatly in adults. Somites 3-5 with conspicuous transverse ridges; ridges on somites 3, 4, each with a median and two lateral teeth or low elevations, occasionally with small denticles; telson a little longer than wide, apex rounded (see below, Female pleonal-retention mechanism) (the triangular female pleon of D. frascone figured Fig. 14F View FIG belongs to a prepubertal female).
Illustrations. Dorippe glabra: Manning 1993 : fig. 1e. D. frascone: Chen 1980 : pl. 2, figs 4, 6, as D. (D.) frascone . D. quadridens: Holthuis & Manning 1990 : fig. 6h. D. sinica: Chen 1980 : pl. 1, figs 3, 4, as D. (D.) sinica ; Holthuis & Manning 1990: fig. 14a, b.
Pleonal-locking mechanism by press-button ( Figs 11C, D View FIG ; 12D, E View FIG ; 13B, D View FIG ; 14B, C, F, I View FIG ; 15B, D, G, H View FIG )
Press-button as small spine in curve of sternal suture 5/ 6 in both sexes.
Additional female pleonal-retention mechanism ( Figs 8C View FIG ; 9C View FIG , 10B, D View FIG ; 12A View FIG ; 15E, F View FIG )
In females, strong retention by wide process of dorsally exposed portion of sternite 8 overhanging pleonal somite 2. Small telson engaged between raised edges of sterno-pleonal cavity at level of sternite 5 ( Figs 12 View FIG ; 13C View FIG ; 15G, H View FIG ).
Male gonopore and penis
Male gonopore coxal. Coxo-sternal condition. Penis markedly angled, with membrane between inclined and vertical portions; penial bulb thick, sclerotised; exposed proximal penial portion sclerotised; next portion covered by pleon. Sternites 7 and 8 expanded over penis, very close to each other for short distance, then not completely joined in some cases; sternite 8 with bifid process over P5 coxo-sternal condyle, partially covering penial bulb, overhanging inclined portion of penis.
Illustrations: Dorippe quadridens: Guinot et al. 2013: 102 , fig. 16A-C. D. tenuipes: Guinot et al. 2013 : fig. 17A, B.
Gonopods ( Figs 11C, D View FIG ; 13B View FIG ; 14D View FIG ; 31A View FIG )
G1 relatively simple, rather straight, short, gradually tapering to single apex; subdistal setae; with narrow tongue-shaped corneous distal process; tip bluntly rounded; basal lobe present, covered with small denticles and with cluster of pappose setae at tip.
Illustrations: Dorippe frascone: Chen 1980 , fig. 3.5, as Dorippe (Dorippe) frascone ; Holthuis & Manning 1990: fig. 2g; Dai & Yang 1991: fig. 25.1, as D. (D.) frascone . D. irrorata: Holthuis & Manning 1990 : fig. 4i, j. D. quadridens: Stephensen 1946 : fig. 4A, as D. dorsipes ; Holthuis & Manning 1990: fig. 7e, f (reproduced by Sin et al. 2009: fig. 4A); Guinot et al. 2013: 102, fig. 16C, D; Chen & Sun 2002: fig. 89.4; Davie et al. 2015a: fig. 71-2.22J. D. sinica: Chen 1980 : fig. 1.5, as D. (D.) sinica (reproduced by Holthuis & Manning 1990: fig. 16e, f); Holthuis & Manning 1990: fig. 13g; Dai & Yang 1991: fig. 25.2, as D. (D.) sinica ; Chen & Sun 2002: fig. 90.4, 5; Hayer et al. 2016a: figs 2, 3A; Vehof 2020: fig. 10A-C. D. tenuipes: Chen 1980 : fig. 2.5, as D. (D.) tenuipes (reproduced by Holthuis & Manning 1990: fig. 18e); Holthuis & Manning 1990: fig. 17i; Dai & Yang 1991: fig. 26, as D. (Dorippe) tenuipes ; Chen & Sun 2002: fig. 91.4. D. trilobata: Manning 1993 : fig. 2g.
Illustrations: Dorippe quadridens: Stephensen 1946 : fig. 4B, as D. dorsipes ; Guinot et al. 2013: 102, fig. 16E.
G2 straight.
Illustrations: D. quadridens: Guinot et al. 2013 : fig. 16E; D. sinica: Vehof 2020 : fig. 10D.
Vulvae ( Figs 12D, E View FIG ; 13D View FIG ; 14G View FIG ; 32A View FIG )
Vulva at the summit of elevated portion of sternite 6 and at extremity of setose raised sternal ridge; opening quite large, rounded, not recessed, well exposed, covered by operculum leaving inverted V-shaped opening.
Illustrations: Dorippe glabra: Manning 1993 : fig. 1f. Dorippe quadridens: Holthuis & Manning 1990 : fig. 6g. Dorippe sinica: Hayer et al. 2016a : figs 1, 2, 3A; Vehof et al. 2017: fig. 1D, E. Dorippe quadridens and D. sinica: Vehof et al. 2017 : fig. 2A.
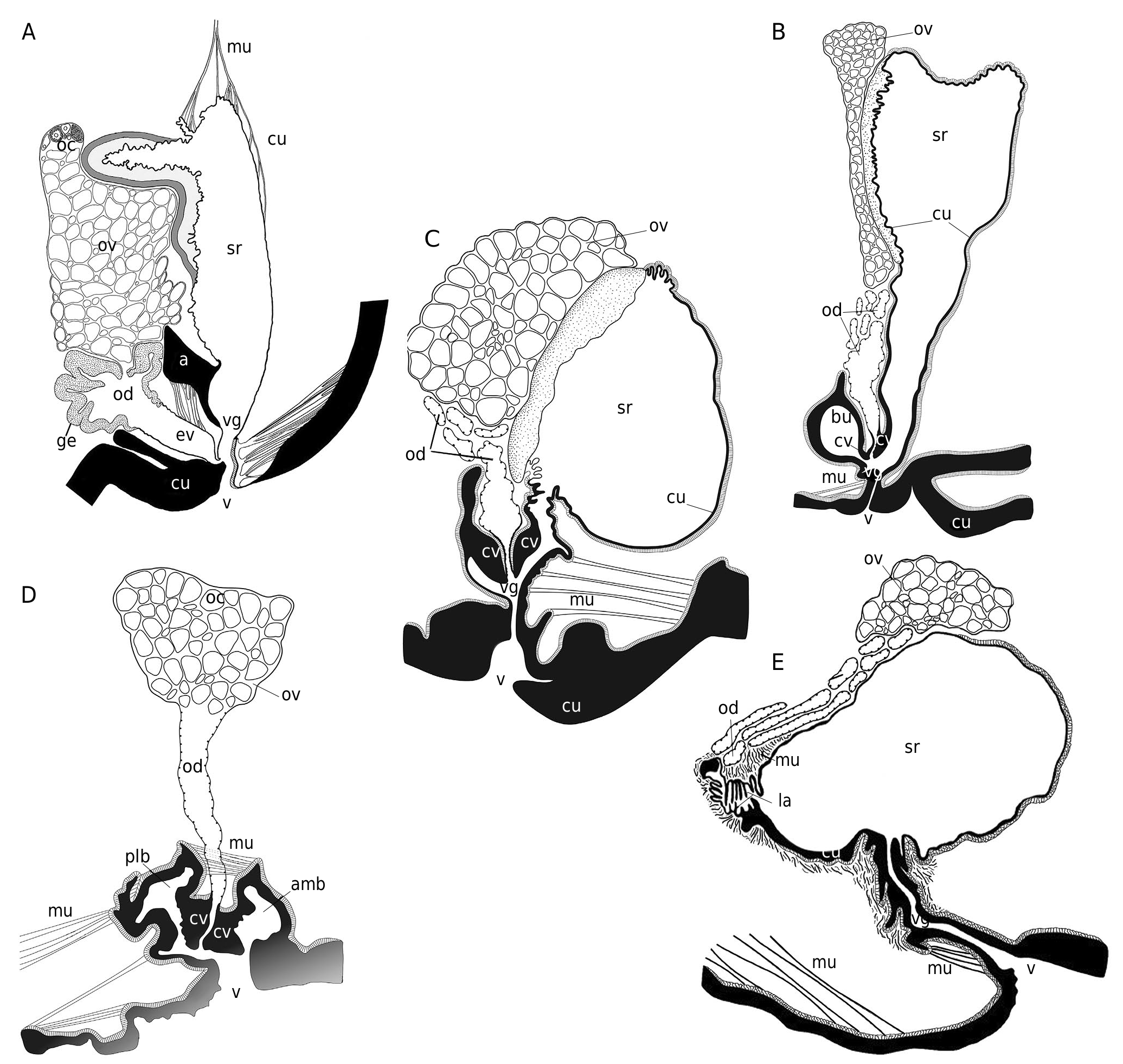
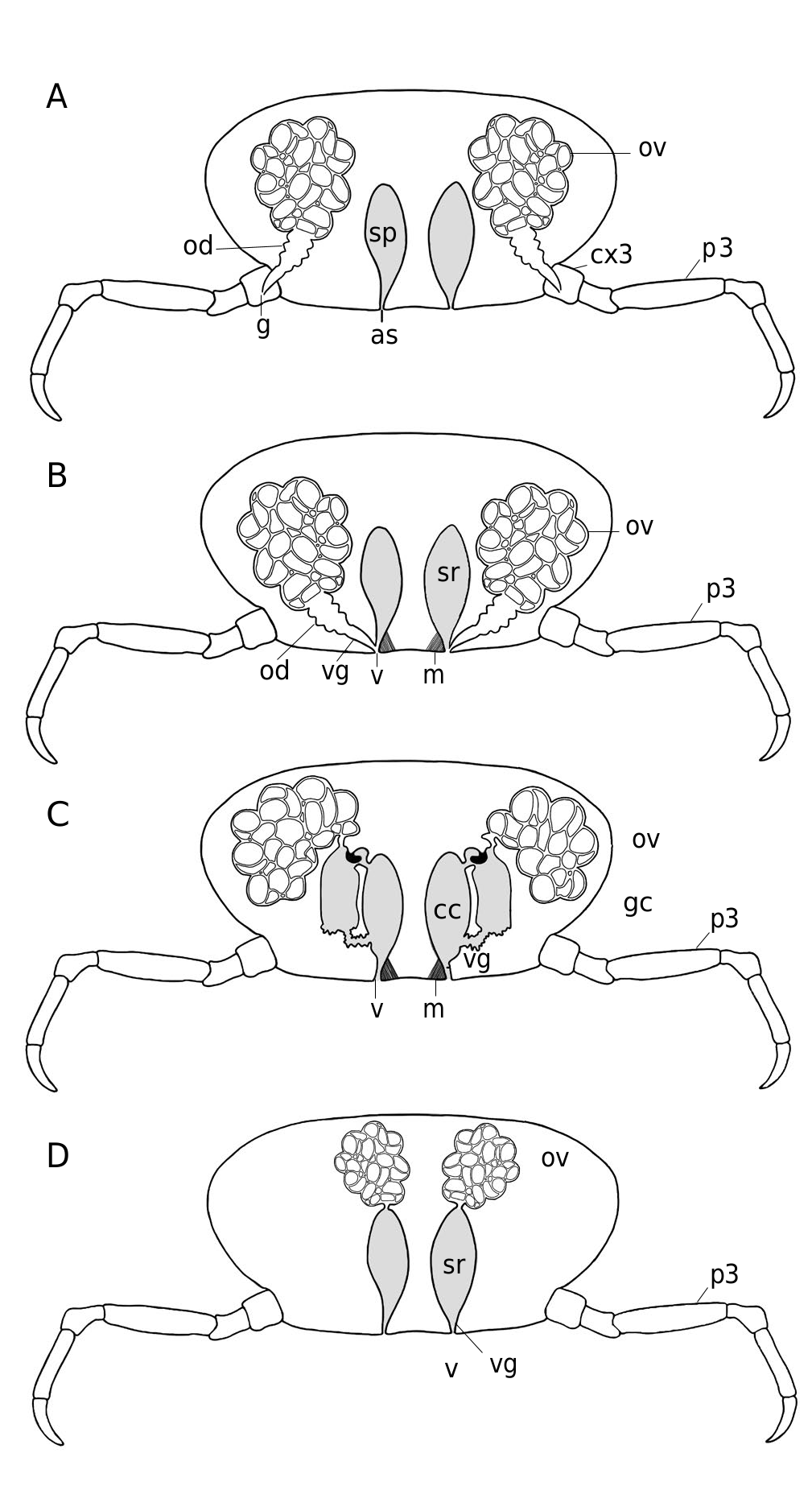
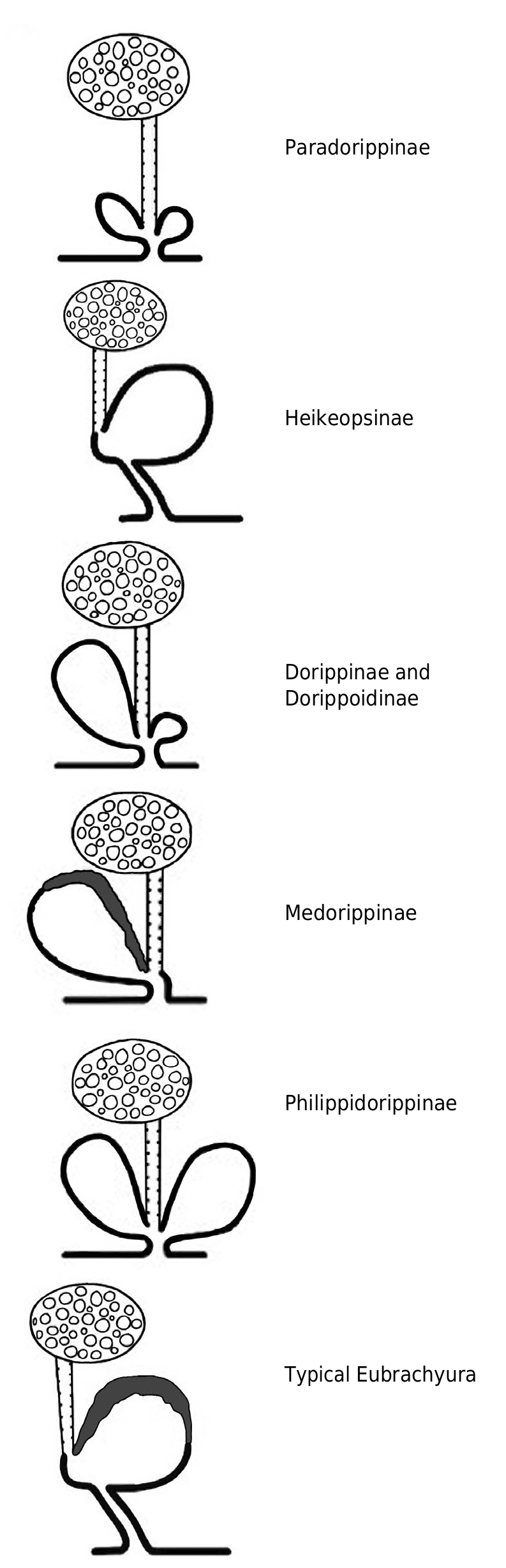
Female reproductive system
Studied in Dorippe quadridens by Vehof et al. (2017) and in D. sinica by Hayer et al. (2016a), Vehof et al. (2017) and Vehof (2020). See Figs 35A, B View FIG ; 36B View FIG ; 37 View FIG and below, The female reproductive system in Brachyura , its evolution and unique disposition in Dorippidae .
Callosities
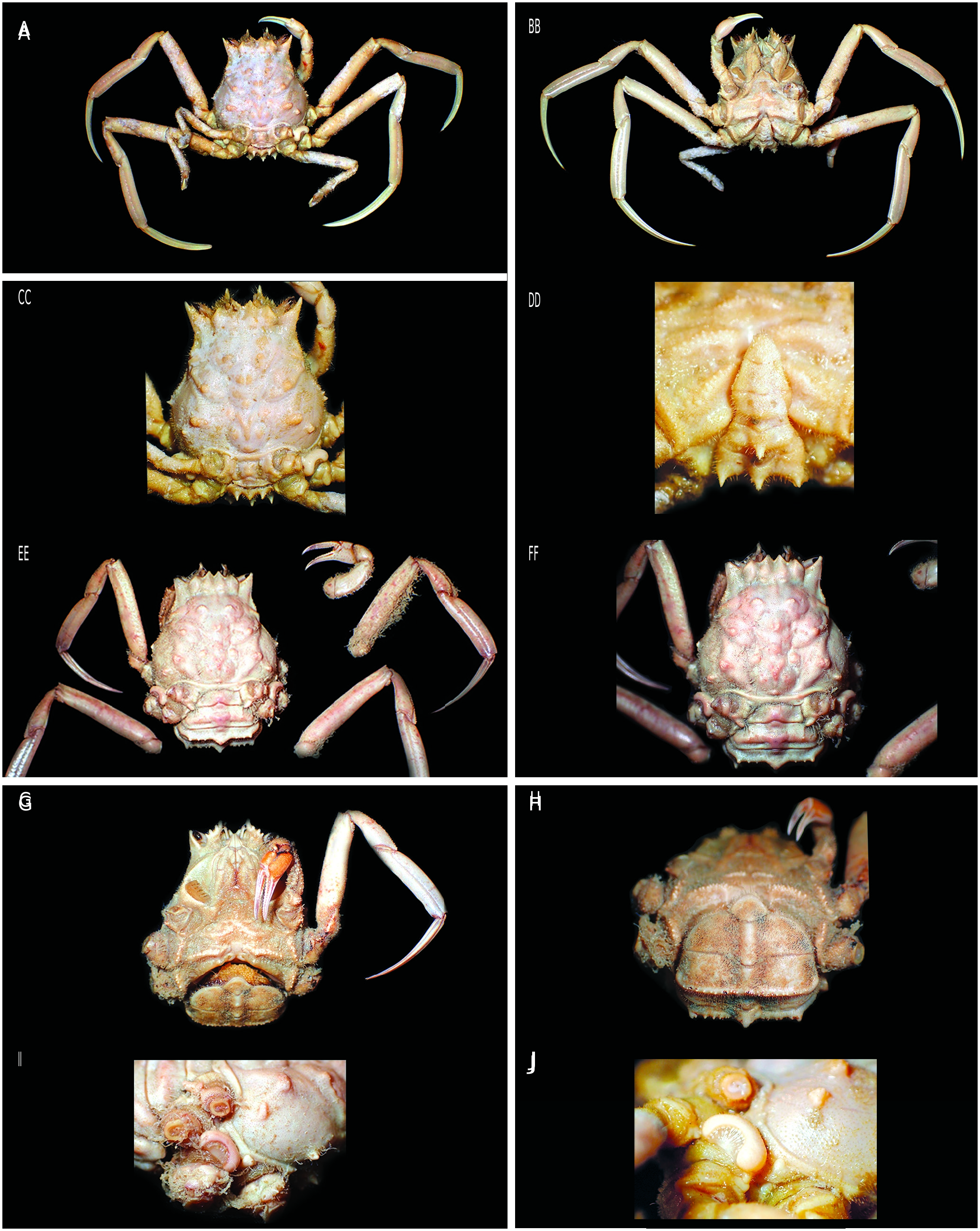
In both sexes, dorsal part of P3 coxa bearing a callosity, variously developed according to the species: a simple thickening and elongated calcified bulge in Dorippe quadridens ( Fig. 33C View FIG ); or in the form of a hemicircular structure in D. sinica ( Figs 9C View FIG ; 10C, D View FIG ; 33D, E View FIG ), D. frascone ( Fig. 33F View FIG ), D. trilobata ( Fig. 15A, C, J View FIG ), D. glabra ( Fig. 15E, F, I View FIG ); or more complex and taking the form of a double calcified bulge with a central part showing a special texture in D. tenuipes ( Figs 9D View FIG ; 33G, H View FIG ). The callosity, partially concealed by the P5, is visible in the photograph by Takeda & Manuel-Santos (2006: fig. 6B). It was not possible to detect whether a callosity is present in D. irrorata ( Fig. 14H View FIG ).
DISTRIBUTION AND HABITAT
Dorippe quadridens is the most widely distributed dorippid in the Indo-West Pacific. It is a Lessespian species introduced into the Mediterranean Sea through the Suez Canal ( Monod 1937, as D. dorsipes ), in Egyptian waters (Timsah Lake and Port Said), along the coasts of Israel ( Galil 2005, 2011; Galil & Shlagman 2010; Brockerhoff & McLay 2011), perhaps Syria ( Hasan 2008). Its range extends from the Red Sea, Persian Gulf ( Apel 2001), Gulf of Aden ( Zarenkov 1971), Gulf of Oman, the Seychelles, southeast Africa to the west and east coasts of India ( Dev Roy & Nandi 2007, 2008; Dev Roy 2008; Dev Roy & Bhadra 2011: 117; Varadharajan & Soundarapandian 2014; Dev Roy & Rath 2017; Vidhya et al. 2017; Beleem et al. 2019: 20, fig. 1a; Gosavi et al. 2021: table 3), the Andaman and Nicobar Islands ( Venkataraman et al. 2004: 312, as Dorippe dorsipes ), the Philippines, Singapore, Indonesia, Vietnam ( André 1931: 638, as Dorippe dorsipes ), Peninsular Malysia ( Razak et al. 2022: fig. 6.11), Thailand, China and Hong Kong ( Chen & Sun 2002; Wong et al. 2021), Taiwan ( Wang et al. 2017; Ng et al. 2017), Australia, and also southern Japan ( Takeda et al. 2019: 13, pl. 3, fig. E, F). Many records of Dorippe quadridens with its synonyms cannot reliably be referred to this species, and all Japanese records probably refer to D. sinica ; the ranges of the two species (both often incorrectly identified as Dorippe frascone in early publications) overlap over most of southern China and probably Taiwan (Holthuis & Manning 1990; Ng et al. 2017). Recent data on the distribution and ecology of D. quadridens and D. sinica have been provided by Osman et al. (2015); Ng et al. (2001, 2017); Ng & Davie (2002); Thoma (2007); Beleem et al. (2019); also Zairion et al. (2018). The depth from which D. quadridens has been reported varies from 1 to 73 m, with most records between 1 and 30 m. It is found on rather flat bottoms of mud and/or sand, sometimes with weeds, corals, or sponges; it has also been reported on coral reefs and oyster beds (Holthuis & Manning 1990).
Recent records of Dorippe quadridens in the Middle East do not reliably refer to this species. It appears that the carapaces of D. quadridens and Dorippoides nudipes (both previously cited in the “Annotated checklist of the decapod crustaceans of the Gulf of Oman ” by Naderloo et al. 2015: table 2) studied and represented in the Atlas of crabs of the Persian Gulf by Naderloo (2017: figs 7.1 and 7.3, respectively) were mistakenly interchanged, whereas the keys to both species, the figures of the G1s and the distribution maps ( Naderloo 2017: 47, fig. 4.2.e and 4.2.f, respectively, and fig. 7.2) are correct. Subsequently, crabs from the northwestern Persian Gulf, Iraq, identified as Dorippe quadridens by Yasser & Naser (2019: fig. 2) and Al-Khafaji et al. (2019: fig. 2a, table 2) are Dorippoides nudipes instead of Dorippe quadridens (see under Dorippoides nudipes ).
Most of the specimens reported in the literature as Dorippe frascone are either not recognisable from the available data or belong to D. quadridens or to D. sinica . Dorippe frascone is known with certainty only from the Philippines, Indonesia and Papua New Guinea (present paper), at depths between 1-10 m on a sandy bottom, and questionably from southern China ( Dai & Yang 1991, as Dorippe (Dorippe) frascone ). Records of D. frascone by Jeyabaskaran et al. (2000: 46, pl. 31c, as D. (D.) frascone ) from India in the Gulf of Mannar, by Venkataraman et al. (2004: 312) in Tamil Nadu, and by Krishnamoorthy (2007: 90) on the Chennai Coast probably correspond to D. quadridens . So far, Dorippe sinica reported from East Asia ( Chen & Sun 2002; Ng et al. 2017; Wong et al. 2021) is the only species of the genus known with certainty from Japan ( Minemizu 2000: 189; Takeda et al. 2006, 2011, 2019); the ranges of D. sinica and D. quadridens overlap in southern China. Dorippe sinica is probably present in Taiwan ( Ng et al. 2017; Wong et al. 2021). The species is reported from the shoreline, on the tidal flat ( Yamaguchi et al. 1987) and at depths between 15-50 and 118 m; specimens are collected from mud, sand bottoms, and from “volcanic sand, shells, and rock” (Holthuis & Manning 1990).
Dorippe tenuipes is known from Vietnam, the Philippines (including Balicasag Island, Bohol, see Takeda & Manuel-Santos 2006: fig.6B), eastern Indonesia and southern China, at depths ranging from 15-20 m ( Serène 1982), 52-92 m ( Chen 1980), 76- 70 m ( Serène & Vadon 1981), 49-53 m (Holthuis & Manning 1990) and 33-128 m ( Chen 1986b), and is found on muddy sand, sand and shell bottoms. According to Trivedi et al. (2018), as D. tenuipes is known with certainty only from the abovementioned regions, the report of D. tenuipes from the Gulf of Mannar region, southeast coast of India by Vidhya et al. (2017) is most likely a misidentification with another Dorippe species.
Three species of Dorippe are so far only known from their type locality and have never been found since. Two are endemic to Australia: D. glabra from the Northern Territory on the north coast at 38 m, and D. trilobata from Western Australia (Admiralty Gulf) at 18 m depth ( Manning 1993; Davie 2002). The third is Dorippe irrorata from the east Andaman Sea, at depths of 62 and 73 m (see below, Remarks).
CARRYING BEHAVIOUR
Dorippe quadridens has been observed carrying a sponge ( Borradaile 1903: 439), the scutellid sea urchin Echinodiscus Leske, 1778 ( Macnae & Kalk 1958; 1969: 44, 71; Kalk 1995) and starfish ( McNeill 1923), pieces of shell or debris ( Ng 1987: 15), broken or intact valves of lamellibranchs ( Ng & Tan 1986), and accumulated amounts of silt and detritus ( Guinot et al. 1995). The sculptured carapace with its different microstructures and dense setae likely assists the species to acquire a massive coat of detritus ( Osman et al. 2021). According to Holthuis & Manning (1990: 33), it is not clear whether the species camouflaged under the umbrella of a jellyfish reported as D. quadridens by Estampador (1937: 514; 1959: 65, footnote) is really this species or rather represents D. frascone instead, since these authors reported a D. frascone from the Philippines “taken from a jellyfish”. Several online field videos show amazing images of a crab on the sea floor holding the toxic ‘fire urchin’ Asthenosoma varium Grube, 1868 with its P4 and P5, running on the bottom and then burying forward until most of the body is covered by the sediment. The crab in situ, called ‘sea urchin carrier crab’ and alternatively identified with D. frascone or D. quadridens , is probably the latter because of the tuberculate carpus of the cheliped recognisable on the videos. Laboratory experiments on D. quadridens collected in Thailand (Rayong Province) and tested by Wisespongpand et al. (2014) provide some summary data: about 45% of the crabs tested selected the green urchin Salmacis sphaeroides ( Linnaeus, 1758) as the first item for carrying, whereas 60% selected the majoid Chlorinoides sp. as the first item from five crab species. According to Sakai (1937: 74, as D. dorsipes ), Dorippe sinica was protected by a dead shell, but there is no other record of the species carrying a mollusc. According to Quintana (1987: 285, figs 3B-F, 5L, M, m, 7F, G, 24A, as Dorippe frascone ), both the megalopa and the first crab stages of D. sinica observed in the laboratory normally used their P4 and P5 to carry small objects dorsally over the carapace, so they were not active swimmers.
REMARKS
The subfamily Dorippinae n. stat. is monotypic, with the genus Dorippe known from seven species, of which three, very poorly known, are photographed here for the first time (see below).
Dorippe callida of White (1847: 54) (not Fabricius 1798), based on two specimens from the Philippine Islands, was assigned to D. dorsipes , actually D. quadridens , by Miers (1884: 258) despite the elongated legs and weakly sculptured carapace surface that distinguish it. Serène (1982: 1128, figs 1-3, pl. 1, figs 1, 2, pl. 2, figs 1-4) regarded this species as new and established it as Dorippe miersi (type locality: Vietnam, Nhatrang), unaware that Chen (1980: 156. fig. 2, pl. 2, figs 3, 5, 7, 8) had described one year earlier Dorippe tenuipes (type locality: South China Sea, off Guangdong Province), a specific name meaning ‘thin-legged’. Comparison of the descriptions provided by these two authors and examination of their type material convinced Holthuis & Manning (1990: 47) of their synonymy. They consider that D. tenuipes “is distinguished from all other species of Dorippe by the very long and slender legs” (a character already noted by Serène: “length of the merus of P2 six times its width”) and by the sculpture of the body surface, especially that of the pleon “far less pronounced” than in most other species of the genus. Serène (1982: 1129) speculated that D. miersi (in fact D. tenuipes ) might be a much smaller species than D. quadridens (which he named D. frascone ) and in which the shape of the adult cheliped would appear at a smaller size, e.g. 28 × 30 mm, with the conclusion that D. miersi in relation to D. quadridens seemed to be “in a similar situation to that of Neodorippe taiwanensis in relation to Neodorippe japonica ”. Dorippe tenuipes is distinguished from all other Dorippe by the most developed callosity ( Figs 9D View FIG ; 33G, H View FIG ).
The female of Dorippe frascone measuring 23.3 × 24.4 mm figured here ( Fig. 14E, F View FIG ) is prepubertal, as evidenced by its not yet enlarged triangular pleon ( Fig. 14F View FIG ), compared to the one shown by Chen (1980: pl. 2, fig. 4): the latter is characterised by the shape of somites 4-6 that differs from that of other Dorippe .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.