Crenicichla gillmorlisi, Kullander, Sven O. & Santos De Lucena, Carlos A., 2013
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3641.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:CD620174-4898-4F8C-9200-E6D5CD9E7A9C |
|
DOI |
https://doi.org/10.5281/zenodo.5629962 |
|
persistent identifier |
https://treatment.plazi.org/id/92B3A9AF-6B20-43A4-884D-CE13C63CBD92 |
|
taxon LSID |
lsid:zoobank.org:act:92B3A9AF-6B20-43A4-884D-CE13C63CBD92 |
|
treatment provided by |
Plazi |
|
scientific name |
Crenicichla gillmorlisi |
| status |
sp. nov. |
Crenicichla gillmorlisi View in CoL , new species
Figs. 1−7 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 6 View FIGURE 7 , Tables 1–3
Holotype. MNHNP 126, adult female, 76.2 mm SL; Paraguay: Alto Paraná: río Acaray, dry arm below the reservoir. 15 May 1982. L. Naylor et al.
Paratypes. All from Paraguay, río Paraná drainage, río Acaray drainage.— Alto Paraná: MHNG 2237.036. 1, 105.8 mm SL. Lago Acaray. 3 May 1984. C. Dlouhy.—MHNG 2237.037. 1, 66.9 mm SL. Lago Acaray. 22 Jan 1983. C. Dlouhy.—MHNG 2237.066. 1, 110.7 mm SL. Lago Acaray. 25 Oct 1984. C. Dlouhy.— MHNG 2395.011. 6, 99.3–120.1 mm SL. Arroyo Yguazú at Juan E. O’Leary. 18 Oct 1987. C. Vaucher et al. — MHNG 2730.057. 2, 61.4–71.3 mm SL. Río Acaray, toll bridge. 12 Oct 1981. C. Dlouhy.—MHNG 2730.058. 1, 131.6 mm SL. Lago Acaray. 7 Sep 1982. C. Dlouhy.—MHNG 2730.059. 11, 40.6–94.8 mm SL. Río Acaray-cué below barrage. 15 May 1982. MHNG.—MHNG 2730.060. 1, ca 104 mm SL. Río Acaray below barrage. 9 Dec 1980. C. Dlouhy.—MHNG 2730.061. 1, 78.9 mm SL. Lago Acaray. Mar 1982. C. Dlouhy.—MHNG 2730.062. 2, 65.0– 78.8 mm SL. Lago Acaray. 16 Oct 1982. C. Dlouhy.—MHNIB 0 234, 2, 166- 172 mm SL. Yguazú Reservoir at José D. Ocampos. 29 Feb 2012. O. Romero.—MNHNP 3686. 29, 25.2–82.6 mm SL. Same data as holotype.— NRM 42452. 1, 174.3 mm SL; NRM 42461, 2, 19.7–35.1 mm SL. Hernandarías, arroyo Paso Itá, Colonia Acaray, at balneario Paso Itá. 26 Feb 1998. S. O. Kullander & S. Rolón.—Caaguazú: NRM 41828, 1, 30.4 mm SL. Arroyo crossing at about 35 km on road Caaguazú–Yhú. 20 Mar 1998. E. Åhlander et al.
Diagnosis. Crenicichla gillmorlisi is recognized as distinct from all other species of Crenicichla by the colour pattern which develops as a row of paired vertical bars in young, and which are further fragmented into an adult colour pattern composed of small dark spots all over the sides. Distinguished from species of the C. lugubris and C. acutirostris groups, including C. vittata Heckel , by low number of E1 scales (42–57 vs. 80 or more); from species of the C. wallacii group by smooth vs. serrated supracleithrum; from species of the C. saxatilis group by absence vs. presence of a humeral blotch; from C. hemera Kullander and C. chicha Varella, Kullander & Lima (Varella et al. 2012) by infraorbitals 3 and 4 separate vs. co-ossified; from C. macrophthalma Heckel by fewer E1 scales (44– 57 vs. 63–69), and cycloid vs. ctenoid cheek and predorsal scales; from the C. reticulata group, including C. semifasciata , by movable vs. rigidly implanted outer teeth, and nostril well separated from upper lip vs. close to upper lip (except in C. cyanonotus Cope in which nostril distinctly separated (Kullander 1986)); from the C. missioneira group by serrated vs. smooth preopercular margin. Similar in general aspect to other species of Crenicichla in the Paraguay and Paraná drainages and from the coast of Brazil, in particular to species with a juvenile or adult colour pattern composed of a row of narrow vertical bars along the middle of the side; among those, distinguished from Crenicichla “ PARANÁ” in presence (vs. absence) of numerous small dark spots on sides, and less scales in E1 row (44–57 vs. 56–65); from C. jaguarensis Haseman , in the upper río Paraná, by presence of numerous narrow vertical bars in young specimens (vs. absence) and small spots present on sides in adults (vs. absence); from C. jupiaensis Britski & Luengo , in the upper and middle río Paraná, by lower jaw prognathous, vs. jaws isognathous, preopercular margin serrated vs. smooth, and by colour pattern, C. jupiaensis not developing a lateral band; from C. haroldoi Luengo , in the upper río Paraná, by preopercle serrated vs. commonly not serrated, and black dots marking lateral line scales absent vs. present; from C. mucuryna von Ihering, in the rio Mucuri, by low number of scales in E1 row (44–57 vs. 57–63) and serrated vs. smooth preopercular margin.
HT N Min Max Mean SD a b r
Standard length (mm) 76.2 21 61.4 174.3 94.2 26.7
Head length 29.3 21 28.3 33.2 30.5 1.4 2.390 0.278 0.990 Snout length 6.8 21 6.8 9.3 8.1 0.6 -1.688 0.100 0.988 Head depth 14.8 21 13.8 16.6 15.1 0.8 0.046 0.150 0.982 Body depth 22.1 21 19.8 25.0 22.0 1.2 -2.160 0.244 0.977 Orbital diameter 9.1 21 6.5 10.8 8.5 1.3 4.235 0.327 0.906 Interorbital width 5.1 21 4.7 7.9 5.6 0.8 -3.148 0.092 0.982 Pectoral-fin length 21.3 21 18.2 22.8 20.7 1.3 4.375 0.158 0.987 Upper jaw length 9.3 21 9.3 13.9 10.7 0.9 -3.337 0.144 0.977 Lower jaw length 13.9 21 12.7 16.2 14.4 0.8 -1.179 0.157 0.981 Caudal-peduncle depth 11.2 21 10.8 12.2 11.5 0.4 -0.937 0.125 0.995 Caudal-peduncle length 14.6 21 12.2 16.2 14.4 0.8 0.912 0.134 0.981 Last dorsal-fin spine length 13.5 20 10.9 14.0 12.7 0.9 2.215 0.102 0.977 N 42 44 45 46 47 48 49 50 51 52 53
Crenicichla ”PARANÁ” 12
Crenicichla gillmorlisi 22 1 1 4 4 3 2 5 2 4 1 2 Crenicichla mandelburgeri 33 1 2 4 3 1 8 4 3 3 N 54 55 56 57 58 59 60 61 63 65 Crenicichla ”PARANÁ” 12 1 1 2 1 2 2 2 1 Crenicichla gillmorlisi 22 1
Crenicichla mandelburgeri 33 1 2 1
Distinguished from C. tesay (río Urugua-í, río Iguazú basin; Argentina and Brazil), C. iguassuensis Haseman (río Iguazú, Argentina and Brazil), C. jurubi Lucena & Kullander and C. igara Lucena & Kullander (upper río Uruguay in Brazil), which richly spotted, by narrow vertical bars or indistinct dark lateral band along the side vs. row of dark blotches along middle of side. Distinguished from C. yaha (río Urugua-í, río Iguazú) by lower jaw prognathous (vs. jaws isognathous), and colour pattern consisting of narrow vertical bars (young), or numerous dark spots on side, and variably expressed lateral band (vs. a row of large blotches close below upper lateral line); and from C. hu by light ground colour and narrow vertical bars (young), or numerous dark spots on side, and variably expressed lateral band (vs. dark grey or dark brown to black colour of body and fins, and 7–9 black irregular blotches on side).
Similar in spotted sides to coastal Brazilian species C. lacustris (Castelnau) , C. iguapina Kullander & Lucena, C. maculata Kullander & Lucena, and C. punctata Hensel , differing in presence of vertical stripes in young specimens, and absence of blotches along the middle of the side vs. presence of a continuous dark lateral band or row of blotches and absence of narrow vertical stripes on the middle of the side at all sizes; distinguished from C. lacustris , and C. iguapina also by less scales in E1 row, 42–57 vs. 60–75.
Most similar to C. mandelburgeri in proportional measurements and meristics, but distinguished by the dissolution of vertical bars into small spots, absent in C. mandelburgeri .
Description. Largest male 174.3 mm SL, largest female 120.1 mm SL. Refer to Figures 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 for general aspect.
Head about as wide as deep. Caudal peduncle longer than deep. Snout moderately long, rounded from above, triangular in lateral view. Lower jaw slightly prognathous, its articulation below middle of orbit; ascending premaxillary processes reaching to 1/4 of orbit; maxilla reaching to or slightly beyond vertical from anterior margin of orbit. Lips thick and wide, lower lip folds separate anteriorly; folds of upper lip not continuous but cutting into a symphyseal wide thickening. Postlabial skin fold margin truncate. Orbit supralateral, not visible from below, chiefly in anterior half of head. Interorbital area flat, narrower than mouth. Nostril dorsolateral, closer to orbit than to margin of postlabial skin fold, with low tubular margin but no anterior marginal membranaceous skin flap. Preopercle with short, dense, regular serrations along vertical margin. Lateralis pores on head simple or with two small openings in small specimens, with multiple openings in larger specimens.
Flank scales ctenoid. All scales cycloid on head, on dorsum above anterior 1/3 of upper lateral line, along dorsal-fin base, on chest, and on belly below line from lower edge of pectoral-fin base to anal-fin origin. Predorsal scales small, embedded in skin, extending forward to transverse frontal lateralis canal. Prepelvic scales very small, deeply embedded in skin. Cheek naked anteroventrally; below eye about 8 scales, embedded in skin. Interopercle naked. Scales in E1 row 42–57 ( Table 2). Transverse scale row counted from scale next to first anal-fin spine obliquely dorsad to dorsal-fin base with about 13–16 scales below and 5–6 scales above lateral line. Circumpeduncular scale rows 10–11 dorsally, 11–12 ventrally (23–25 including lateral lines).
Lateral-line scales 23/13 (1), 24/10 (1), 24/11 (4), 24.12 (7), 14/13 (1), 25/10 (1), 25/12 (1), 25/13 (1), 26/10 (2), 26/12 (1); 2 scales continuing lower line onto caudal fin; accessory lateral lines on caudal fin usually absent, occasionally one tubed accessory scale on caudal fin, between rays D3 and D4 or between rays V3 and V4, in one specimen three tubed scales between rays V3 and V4. Upper and lower lateral lines non-overlapping. Scales between upper lateral line and dorsal fin 10–12 anteriorly, 3½ posteriorly; scale rows between lateral lines 3. Anterior upper lateral-line scales not much larger than adjacent scales, remaining lateral-line scales nearly same size as adjacent scales; two scales impinging on each scale of anterior part, one on each scale of posterior part of upper lateral line; 1–1½ scales impinging on each scale of lower lateral line. Dorsal, anal, pectoral and pelvic fins without scales. Caudal-fin squamation extending to about 1/3–½ of fin, posterior margin of scaled area straight vertical.
First dorsal-fin spine about 1/4–1/3 length of last; spines subequal in length from 6th–9th. Soft part of dorsal fin with rounded or subacuminate tip, reaching to base of caudal fin or slightly beyond. Dorsal-fin rays XIX.12 (1), XX.12 (1), XXI.11 (4), XXI.12 (20), XXII.11 (8), XXII.12 (2). Soft anal fin with rounded tip, reaching to or slightly beyond base of caudal fin. Anal-fin rays III.7 (6), III.8 (21), III.9 (1). Caudal fin rounded. Pectoral fin rounded, 6th or 7th ray longest, reaching about halfway to spinous anal fin. Pectoral-fin rays 15 (1), 16 (18), 17 (2). Pelvic fin inserted well posteriorly to vertical from pectoral axilla, with subacuminate tip, second ray longest, reaching about halfway to soft anal fin.
All teeth pointed, recurved, teeth in outer row movable but not depressible, teeth in inner rows depressible. Outer row teeth slightly larger than inner teeth. Outer row of teeth in upper jaw extending for nearly the length of the alveolar ramus of the premaxilla. Upper jaw with 3–4 inner rows anteriorly, one inner row continued almost as long as outer row. Outer row of teeth in lower jaw extending along 3/4 of length of jaw. Lower jaw with 2–3, occasionally 4 inner rows anteriorly, one inner row continued posteriorly for half the length of the outer row.
Gill rakers externally on first gill arch 1 (1), 2 (19), 3 (2) epibranchial, 1 in angle, 5 (1), 8 (17), 9 (4) ceratobranchial. Gill rakers on lower pharyngeal tooth-plate 8 (2), 9 (4), 10 (4), 11 (6), 13 (3). Microbranchiospines present externally on 2nd to 4th arches.
Lower pharyngeal tooth-plate ( Fig. 3 View FIGURE 3 ) dissected from a 131.6 mm specimen (MHNG 2730.058), relatively compressed dorsoventrally, with moderately long posterior and anterior processes; tooth-plate length 84% of width; dentigerous area length 70% of width; 22 teeth in posterior row, 7–8 teeth in median row. All teeth compressed, except posteromedian, which almost circular in cross section. Anterior teeth slender, erect or slightly inclined, with recurved tip, shape changing gradually in symphysian rows, posterior teeth more stout and with posterior straight cusp. Teeth along margin much shorter than median teeth, bevelled; teeth along posterior margin of bone with antrorse posterior cusp and slightly cuspidate anterior bulge.
Vertebral counts 16–17+18–20=35–37 ( Table 3 View TABLE 3 ).
Coloration in preservative. Smallest specimen, 25.2 mm SL (MNHNP 3686), pale yellowish with five brown vertical bars extending from dorsal midline across side, and dull blotch on side of caudal peduncle; anteriormost bar narrow, entire; posterior bars broad, each split below upper lateral line by narrow light vertical stripe. Caudal ocellus present as small black spot surrounded by hyaline ring. Slightly larger juveniles (NRM 41828, 42461; Fig. View FIGURE 2
2A) whitish with brown stripe from lower jaw to orbit. Postorbital brown stripe from orbit horizontal to tip of opercle. Brown band paralleling postorbital stripe slightly dorsal to it. Two contiguous dark stripes on each side of midline of top of head. Brown band along middle of side, enforced at intervals by double vertical bars extending down from dorsal-fin base. Indistinct narrow dark band along abdominal side. Brown blotch at base of caudal fin, in 30.4 mm specimen followed by chevron shaped black mark and short brownish stripe along middle rays, in 35.1 mm specimen by small black spot surrounded by hyaline ring and narrow vertical stripes, representing incipient ocellated blotch. Suborbital stripe absent in juveniles.
Young specimens from about 40 mm SL ( Fig. 2 View FIGURE 2 B) with short or incipient suborbital marking. Lateral band absent, replaced by series of about 8–9 pairs of brown vertical bars. Ocellated, elongate blotch at caudal-fin base.
Adults ( Fig. 1 View FIGURE 1 , 2 View FIGURE 2 C–E), about 60 mm SL and larger, with off-white, pale grey, or light brownish ground colour. Dorsum not noticeably darker than sides. Chest whitish, abdomen, body close to anal-fin base, and ventral margin of caudal peduncle off-white to faint yellowish. Dorsal part of head, snout and lips greyish, nape slightly lighter, brownish with darker brown blotch on each side anterior to extrascapulars. Sides of head, below eye pale brownish, lighter ventrally. Postorbital dark brown stripe from orbit to opercular tip. Suborbital stripe present, narrow, always strongly contrasted, solid or composed of small spots, extending obliquely from lower margin of orbit to or almost to inner margin of lower limb of exposed preopercle. More or less distinct greyish or brownish preorbital stripe from orbit to upper lip. On each side, vertical bars in young and juveniles transformed into 3–5, usually four short segments, in the largest specimen about nine irregular horizontal rows of small spots. Along dorsum may be present five indistinct dark vertical blotches, first below origin of dorsal fin, last below soft dorsal fin. Lateral markings usually expressed as short horizontally extended blotches; in three specimens 110.7–174.3 (MHNG 2730.058, 2237.066, NRM 42452) relatively smaller and rounded ( Fig. 2 View FIGURE 2 E). Indistinct lateral band can be traced along middle of side in most specimens, but best expressed in MHNG 2395.11 ( Fig. 4 View FIGURE 4 D). Dorsal fin semihyaline; spinous portion with faint or distinct small dark spots arranged in two well defined rows; soft portion with 2–4 rows of small spots. Anal fin semihyaline to pale greyish with up to six rows of small dark spots on soft portion. Caudal fin brownish basally, semihyaline posterior to scaly portion, with 4–7 rows of small dark spots across fin and posterior margin greyish. Small round black blotch at base of caudal fin, immediately dorsal to lateral line scales, surrounded by yellowish ring. Caudal-fin blotch indistinct and not ocellated in largest specimen ( Fig. 2 View FIGURE 2 E). Pelvic fin whitish.
Only one specimen, 120.1 mm SL ( Fig. 2 View FIGURE 2 D) showing colour pattern characteristic of breeding female Crenicichla . Spots in fins absent or indistinct. A large indistinct brown, elongate blotch distally on dorsal fin between spines 14 and 17. In this specimen, spots apparently absent from sides, and middle of side with series of short vertical bars. Scales with highlighted pale bases and brownish margin. Black caudal-fin blotch not ocellated.
Live coloration. A freshly collected adult, probably male specimen ( Fig. 4 View FIGURE 4 ) shows colour pattern similar to preserved specimens, differing chiefly in olivaceous sheen on sides.
Comparative morphometrics. Bi-plots and PCA did not show any diagnostic differences between samples of C. gillmorlisi , C. mandelburgeri , and Crenicichla “PARANÁ”. Crenicichla gillmorlisi , however, has slightly wider interorbital space, especially in larger specimens, yielding a slope significantly different from that of Crenicichla “PARANÁ” and C. mandelburgeri , as showing clearly in comparison with head length (Fig. 5). Proportionally, there is considerable overlap, with 15.3–22.7 % of head length (N= 12 mean 18.1, standard deviation 2.0) in C. “PARANÁ”, 15.1–26.0 % of head length (N= 21, mean 18.5, standard deviation 3.3) in C. gillmorlisi , and 13.5– 19.3 % of head length (N= 28, mean 16.1, standard deviation 1.5) in C. mandelburgeri . Proportional measurements for C. mandelburgeri were reported by Kullander (2009), and are given here ( Table 4 View TABLE 4 ) for Paraguayan Crenicichla “PARANÁ”.
No significant differences were found in meristic data between C. gillmorlisi and C. mandelburgeri . Crenicichla “PARANÁ”, however, tends to higher scale and abdominal vertebral counts ( Tables 2–3) than both C. gillmorlisi and C. mandelburgeri .
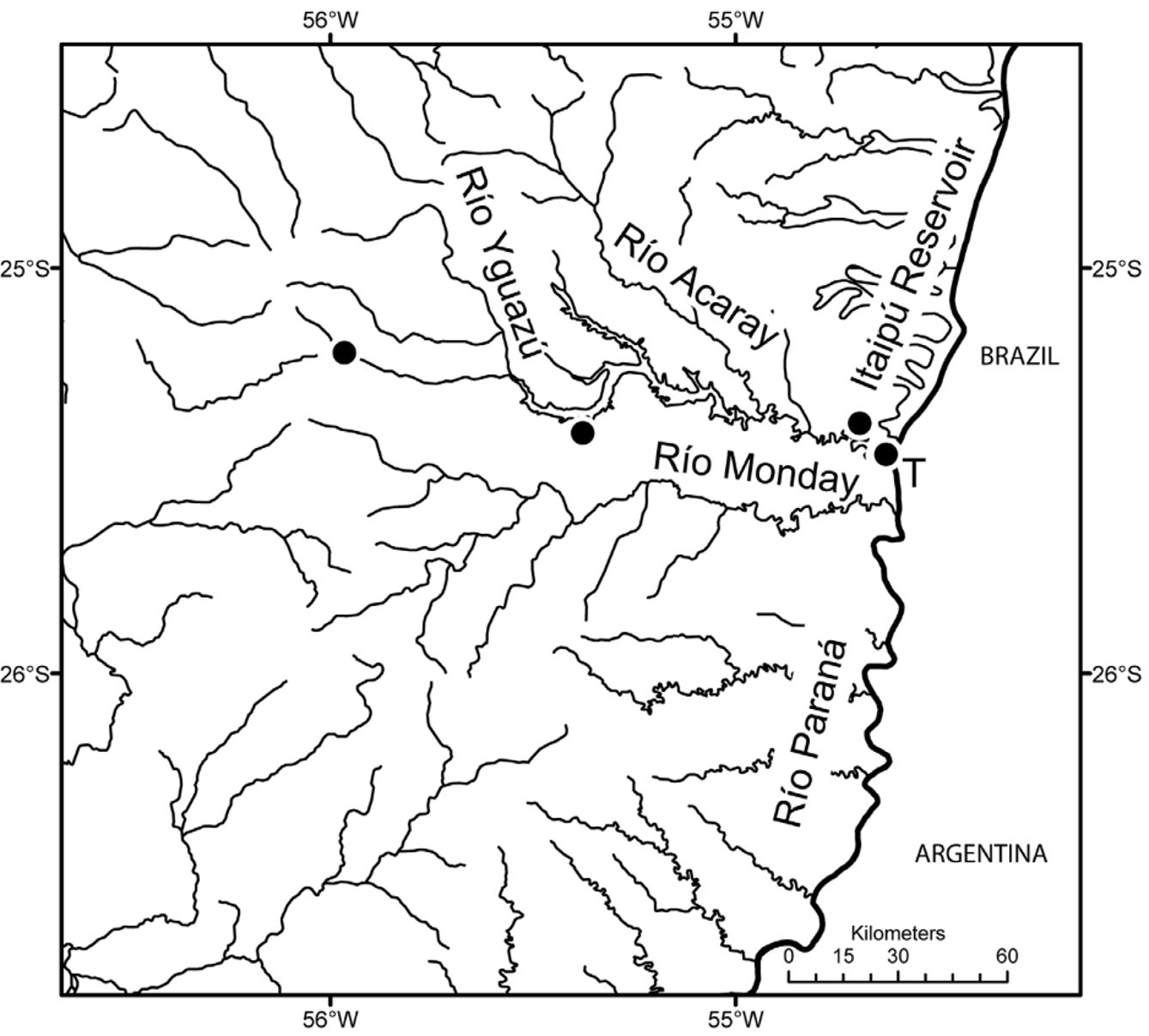
Geographical distribution and habitats. All localities of C. gillmorlisi are located in the río Acaray drainage in Paraguay ( Fig. 6 View FIGURE 6 ). The Acaray has two major branches, and the right branch is known as río or arroyo Yguazú. Habitat information is available only for the NRM samples obtained during high water levels. At Paso Itá near Hernandarías ( Fig. 7 View FIGURE 7 ) one adult and two young C. gillmorlisi were captured in a small stream with brownish very turbid water, with moderate current, about 4 m wide and 1–1.5 m deep. The landscape was open agriculture, with mainly grass vegetation. The bottom substrate consisted of rocks and stones. Fishing was made by seining along about 100 m of stream to 80 cm depth. Fishes were found only along overhanging banks. The most common associated species were Gymnogeophagus setequedas Reis, Malabarba & Pavanelli (Cichlidae) and Bryconamericus stramineus Eigenmann (Characidae) . Other species were identified as Astyanax sp. ( Characidae ), Hypostomus dlouhyi Weber , Hypoptopoma sp. ( Loricariidae ), Hoplias sp. ( Erythrinidae ), Leporinus lacustris Amaral Campos (Anostomidae) , Steindachnerina brevipinnis (Eigenmann & Eigenmann) (Curimatidae) , Eigenmannia virescens (Valenciennes) (Sternopygidae) , Crenicichla lepidota (Cichlidae) , and Pyrrhulina australis Eigenmann & Kennedy (Lebiasinidae) .
At Yhú a juvenile was collected in a small stream, more than 1.5 m deep with running, dark and turbid water, in open landscape with low trees and grass. Fishing was done with hand nets and seine in vegetation and on a small beach. The bottom consisted of sand. The most common associated species were Characidium sp. ( Crenuchidae ) and Bryconamericus stramineus (Characidae) . Other species were identified as Astyanax sp., Cheirodon sp., Hemigrammus sp., Mimagoniates microlepis (Steindachner) (Characidae) , Corydoras diphyes Axenrot & Kullander (Callichthyidae) , Bunocephalus sp. ( Aspredinidae ), Gymnogeophagus setequedas (Cichlidae) , Hypoptopoma sp., ( Loricariidae ), and Phallotorynus victoriae Oliveros (Poeciliidae) .
Crenicichla lepidota was also collected together with MHNG 2237.66 (lago Acaray) and MHNG 2395.11 (Juan E. O’Leary).
Etymology. This species is named for ichthyologist Walter A. Gill Morlis A., fisheries officer of the Itaipú Binacional, Ciudad del Este, Paraguay, who contributed considerably to the PROVEPA surveys of fishes in tributaries of the Paraná River, and in special recognition of his strong long term engagement in the inventory of the fishes of the río Paraná.
FIGURE 5. Biplot of interorbital width against head length in Crenicichla gillmorlisi (r2=0.957), C. “PARANÁ” (r2=0.944), and C. mandelburgeri (r2=0.897). The slope of C. gillmorlisi is significantly different from those of C. mandelburgeri (p=0.001), and C. “PARANÁ” (p=0.033).
TABLE 3. Frequency of vertebral counts in Crenicichla gillmorlisi, Crenicichla “ PARANÁ ” and C. mandelburgeri.
| Abdominal | 16 | 17 | 18 | N | ||||
|---|---|---|---|---|---|---|---|---|
| Caudal | 19 | 20 | 18 | 19 | 20 | 18 | 19 | |
| Crenicichla “PARANÁ” | 1 | 1 | 4 | 2 | 5 | 13 | ||
| Crenicichla gillmorlisi | 3 | 2 | 4 | 6 | 3 | 18 | ||
| Crenicichla mandelburgeri | 5 | 10 | 2 | 1 | 18 |
TABLE 4. Standard length (in millimeters) and proportional measurements in percents of standard length of Crenicichla “ PARANÁ ”. SD = standard deviation. Regression line parameters, a (intercept), b (slope), and r (Pearson’s correlation coefficient) are calculated from measurements expressed in millimeters; shown when p <0.05.
| N Min | Max | Mean | SD | a | b | r | |
|---|---|---|---|---|---|---|---|
| Standard length (mm) | 12 71.9 | 198.7 | 128.6 | 30.0 | |||
| Head length | 12 28.7 | 32.8 | 30.4 | 1.1 | -0.854 | 0.311 | 0.987 |
| Snout length | 12 8.3 | 11.3 | 9.5 | 0.9 | -3.694 | 0.125 | 0.975 |
| Head depth | 12 13.1 | 17.5 | 14.5 | 1.2 | -2.128 | 0.163 | 0.950 |
| Body depth | 12 19.1 | 24.0 | 21.1 | 1.4 | -1.117 | 0.220 | 0.961 |
| Orbital diameter | 12 5.8 | 9.0 | 7.2 | 0.8 | 3.591 | 0.042 | 0.930 |
| Interorbital width | 12 4.7 | 6.9 | 5.5 | 0.6 | -3.305 | 0.082 | 0.977 |
| Pectoral-fin length | 12 17.0 | 20.3 | 18.8 | 1.0 | 0.523 | 0.182 | 0.978 |
| Upper jaw length | 12 10.2 | 13.9 | 11.7 | 1.2 | -5.826 | 0.165 | 0.980 |
| Lower jaw length | 12 14.4 | 17.6 | 15.9 | 0.9 | -3.042 | 0.184 | 0.982 |
| Caudal-peduncle depth | 12 10.0 | 12.0 | 10.7 | 0.6 | -1.812 | 0.121 | 0.981 |
| Caudal-peduncle length | 12 12.9 | 14.7 | 13.9 | 0.6 | 1.932 | 0.124 | 0.980 |
| Last dorsal-fin spine length | 11 10.5 | 13.5 | 11.6 | 0.8 | 3.429 | 0.088 | 0.983 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.