Characidium krenak, Oliveira-Silva & Santos & Lopes & Zanata, 2022
|
publication ID |
https://doi.org/ 10.1590/1982-0224-2021-0125 |
|
publication LSID |
lsid:zoobank.org:pub:EA04D0B1-C153-4147-9318-F245E77CE0AA |
|
DOI |
https://doi.org/10.5281/zenodo.11118937 |
|
persistent identifier |
https://treatment.plazi.org/id/52661736-F921-FFD8-F10D-05C34688F9D8 |
|
treatment provided by |
Felipe |
|
scientific name |
Characidium krenak |
| status |
sp. nov. |
Characidium krenak , new species
urn:lsid:zoobank.org:act:4DC24B3B-8994-44CF-B536-C5456F8B5597
( Figs. 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 ; Tab. 1 View TABLE 1 )
Characidium sp. —Sales et al., 2018:271 (DNA barcode analysis).
Characidium sp. B . —Santos, Britto 2021:37 (list of species).
Holotype. MZUSP 125896 View Materials , 57.6 mm SL, Brazil, Minas Gerais, Caranaíba, rio dos Costas, tributary of rio Piranga sub-basin, upper rio Doce basin, 20º51’37”S 43º43’11”W, 9 July 2007, O. T. Oyakawa, E. Baena & M. Loeb. GoogleMaps
Paratypes. All from Brazil, Minas Gerais State, rio Doce basin. Rio Piranga sub-basin: MZUSP 94501 View Materials *, 2, 35.2–36.1 mm SL mm SL, collected with holotype. GoogleMaps Rio do Peixe , Santo Antônio sub-basin: MCNIP 976 *, 3, 42.7–51.1 mm SL, GoogleMaps Conceição do Mato Dentro , unnamed tributary, upstream from its confluence with córrego Ponte Nova , 18º59’23”S 43º23’01”W, 1 Nov 2011, S. A. Santos & B. C. Ramos. MCNIP 1026 *, 3, 28.4–52.6 mm SL, GoogleMaps Alvorada de Minas , ribeirão das Pedras, 18º45’16”S 43º26’50”W, 27 Oct 2012, S. A. Santos & B. C. Ramos. MCNIP 1993 *, 1, 51.8 mm SL, GoogleMaps Conceição do Mato Dentro , córrego Antonieta , 19º00’40”S 43º22’19”W, 23 Oct 2015, R. Ferreira. MCNIP 1994 *, 1, 60.3 mm SL, GoogleMaps Conceição do Mato Dentro , ribeirão Passa Sete, 18º52’10”S 43º20’03”W, 24 Oct 2015, R. Ferreira. MCNIP 2025 *, 3, 37.4–48.3 mm SL, GoogleMaps Conceição do Mato Dentro , córrego Antonieta, 18º58’27”S 43º22’19”W, 23 Oct 2015, R. Ferreira & S. Santos. MCNIP 2268 , 2 , 33.3–39.5 mm SL, GoogleMaps Alvorada de Minas , ribeirão das Pedras, 18º45’10.04”S 43º26’05.03”W, B. Ferreira & L. Nogueira. MCNIP 3014 *, 5, 43.3–51.1 mm SL, GoogleMaps córrego Vermelho, tributary of right bank of ribeirão das Pedras, 18º46’27.0”S 43º26’16”W, 10 Aug 2013, S. Santos, T. Souza & D. Santana. MCNIP 3029 *, 2, 38.7–41.4 mm SL, GoogleMaps Conceição do Mato Dentro , córrego Antonieta, 19º00’40”S 43º22’19”W, 7 Aug 2013, S. A. Santos, T. Souza & D. Santana. MCNIP 3030 *, 1, 34.2 mm SL, GoogleMaps Conceição do Mato Dentro , córrego Trote Velho, 18º58’40”S 43º22’26”W, 8 Aug 2013, S. A. Santos, T. Souza & D. Santana. MCNIP 4889 , 1 , 33.3 mm SL, ( LGC RD161 , BOLD: ACS9348 ), GoogleMaps Alvorada de Minas , córrego Escadinha, 18°45’14.4”S 43°26’49.2”W, 5 Nov 2013, S. A. Santos. MNRJ 48960 View Materials , 5 View Materials , 28.1–54.6 mm SL, GoogleMaps Conceição do Mato Dentro , ribeirão Santo Antônio, 18º47’0.0”S 43º33’37”W, 30 Jul 2016, S. A. Santos, M. Britto & D. Moraes-Jr. MZUSP 104678 View Materials , 4 View Materials , 19.7–24.3 mm SL, GoogleMaps Conceição do Mato Dentro , córrego Antonieta Norte, 19°00’40.3”S 43°22’19.7”W, I. Fichberg & M. Loeb. MZUSP 104679 View Materials *, 3, 22.6–30.7 mm SL, GoogleMaps Conceição do Mato Dentro , stream on the urban area of the district of Meloso, 19°04’33.3”S 43°20’25.7”W, I. Fichberg & M. Loeb. MZUSP 104694 View Materials *, 9, 18.5–36.2 mm SL, GoogleMaps Conceição do Mato Dentro , córrego Faia, tributary of ribeirão São João, 19°01’20.1”S 43°20‘39.0”W, I. Fichberg & M. Loeb. MZUSP 104697 View Materials , 3 View Materials , 21.8 View Materials –37.0 mm SL, GoogleMaps Conceição do Mato Dentro , ribeirão São João, 19°2’29.1”S 43°20’33.8”W, I. Fichberg & M. Loeb. MZUSP 104698 View Materials , 3 View Materials , 19.4–35.7 mm SL, GoogleMaps Conceição do Mato Dentro , ribeirão São João, 19°3’33.0”S 43°10’21.3”W, I. Fichberg & M. Loeb. MZUSP 109301 View Materials *, 4 (1 cs), 38.6–42.4 mm SL, GoogleMaps Conceição do Mato Dentro , tributary of right bank of rio do Peixe, 18°43’50.0”S 43°26’8.0”W, T. C. Pessali. MZUSP 112265 View Materials *, 6 (1 cs), 36.5–49.2 mm SL, GoogleMaps Conceição do Mato Dentro , ribeirão São João, 19°02’30.6”S 43°20’24.0”W, 11 Jan 2011, M. Loeb. MZUSP 112275 View Materials , 2 View Materials , 35.2–41.1 mm SL, GoogleMaps Conceição do Mato Dentro , tributary of ribeirão São João, 19°01’21.5”S 43°20’38.6”W, 11 Jan 2011, M. Loeb. MZUSP 112284 View Materials *, 1, 47.3 mm SL, GoogleMaps Conceição do Mato Dentro , córrego Meloso, 19°04’34.5”S 43°20’28.5”W, 12 Jan 2011, M. Loeb. UFBA 9129 , 14 , 35.7–50.7 mm SL, GoogleMaps Serro, ribeirão das Pedras, 18°43’50.6”S 43°26’08.2”W, 12 Dec 2021, A. Zanata, L. Oliveira-Silva, T. Quadros & R. Burger. UFBA 9130 , 3 , 38.6–40.3 mm SL, GoogleMaps Alvorada de Minas , ribeirão São José, 18°48’36.4”S 43°24’20.9”W, 12 Dec 2021, A. Zanata, L. Oliveira-Silva, T. Quadros & R. Burger GoogleMaps .
Diagnosis. Characidium krenak can be diagnosed from congeners, except C. alipioi Travassos, 1955 , C. amaila Lujan, Agudelo-Zamora, Taphorn, Booth & López- Fernández, 2013, C. boavistae Steindachner, 1915 , C. bolivianum Pearson, 1924 , C. crandellii Steindachner, 1915 , C. cricarense , C. declivirostre Steindachner, 1915 , C. duplicatum Armbruster, Lujan & Bloom, 2021 , C. fasciatum Reinhardt, 1867 , C. gomesi Travassos, 1956 , C. grajahuense Travassos, 1944 , C. hasemani Steindachner, 1915 , C. helmeri Zanata, Sarmento-Soares & Martins-Pinheiro, 2015 , C. iaquira Zanata, Ohara, Oyakawa & Dagosta, 2020 , C. japuhybense Travassos, 1949 , C. kalunga Melo, Bouquerel, Masumoto , França & Netto-Ferreira, 2021, C. kamakan Zanata & Camelier, 2015 , C. lauroi Travassos, 1949 , C. macrolepidotum (Peters, 1868) , C. nambiquara Zanata & Ohara, 2020 , C. oiticicai Travassos, 1967 , C. pterostictum Gomes, 1947 , C. purpuratum Steindachner, 1882 , C. schubarti Travassos, 1955 , C. summus Zanata & Ohara, 2015 , C. tatama Agudelo-Zamora, Tavera, Murillo & Ortega-Lara, 2020 , C. timbuiense Travassos, 1946 , C. travassosi Melo, Buckup & Oyakawa, 2016 , C. vidali Travassos, 1967 and C. wangyapoik Armbruster, Lujan & Bloom, 2021 by having the isthmus scaleless (vs. isthmus scaled). The new species differs from the aforementioned species, except C. cricarense , C. hasemani , C. helmeri , C. kalunga , C. pterostictum , C. schubarti , C. summus and C. travassosi , by lacking scales on the ventral surface only in the area between the anterior limit of the isthmus and the anterior margin of the cleithrum ( Fig. 2 View FIGURE 2 ) (vs. lacking scales on the isthmus and a large area surrounding the bases of the pectoral fins, or with the area between the contralateral pectoral-fin bases largely unscaled, or even the belly unscaled). Characidium krenak differs from C. helmeri , C. summus and C. travassosi by the presence of the adipose fin (vs. absence), from C. cricarense by having 2–4 scales between the anus and anal-fin and a distance between the anus and anal-fin origin shorter than 9% of SL (vs. 5–7 scales between the anus and anal-fin and a distance from the anus to anal-fin origin longer than 11.0% of SL) and vertically elongated dark blotches on body or bars not distinctly wider dorsally nor connected across the dorsal midline (vs. 8 to 13 triangular bars, wider dorsally, thinner ventrally, with bars from opposite sides united across the dorsal midline). The new species differs from C. kalunga by having two rows of dentary teeth (vs. a single row) and dorsal and caudal fins with conspicuous dark dashes on the rays (vs. dorsal and caudal fins mostly hyaline, without dark dashes on the rays), from C. schubarti by having the parietal branch of the supraorbital canal (vs. parietal branch absent) and absence of small and rounded dark spots on laterals of body (vs. present). It differs from C. pterostictum by having 4 scale rows above the lateral line (vs. 5), 33–34 perforated scales in the lateral line (vs. 35–38) and lacking a conspicuous dark band crossing the proximal third or midpoint of all dorsal-fin rays and membranes (vs. presence of a conspicuous dark band below the midpoint of all dorsal-fin rays and membranes). The new species differs from C. hasemani by lacking two inclined dark bands crossing each caudal-fin lobe (vs. possessing such bands) and by having 14 circumpeduncular scales (vs. 12).
Description. Morphometric data of holotype and paratypes in Tab. 1 View TABLE 1 . Body elongate. Highest body depth at vertical through dorsal-fin origin. Anterior portion of head convex in lateral view; dorsal profile convex from snout to vertical through anterior border of eyes, slightly convex from this point to posterior tip of supraoccipital, slightly convex from supraoccipital to end of dorsal-fin base, straight from that point to adipose fin and straight or slightly concave from adipose fin to origin of dorsal procurrent caudal-fin rays. Ventral profile of body nearly straight near dentary symphysis, straight or slightly convex from that point to anal-fin origin, slightly concave along anal-fin base and straight or slightly concave from end of anal-fin base to ventral procurrent caudal-fin rays. Snout triangular-shaped in lateral view. Mouth subterminal, aligned or slightly lower than ventral margin of orbit. Distal tip of maxilla barely reaching anterior margin of orbit. Orbit approximately round, similar in size to snout length. Cheek somewhat broad, its depth approximately a third of orbit diameter. Nares separated, without distinctly raised margins; posterior naris approximately equidistant from anterior naris and from orbit. Supraorbital small; inner and outer border nearly parallel to each other. Nasal bones restricted to ossified canal. Parietal fontanel limited anteriorly by frontals or by parietals. Parietal branch of supraorbital canal present, extending well beyond limit between frontal and parietal bones.
Dentary teeth in two rows; outer row with 7 (6), 8 (18) or 9* (5) teeth, uni- or tricuspid, rarely bicuspid; teeth decreasing in size and number of cusps from symphysis; inner row with several minute conical teeth inserted on edge of replacement tooth trench. Premaxilla with single row of 5 (2), 6* (15) or 7 (1) teeth decreasing in size from symphysis; larger teeth tricuspid with almost imperceptible lateral cusps, followed by bicuspid and posteriorly unicuspid teeth. Maxillary edentulous. Ectopterygoid with one row, with 10 (1), or 11 (1) teeth, minute and conical. Endopterygoid teeth absent. Branchiostegal rays 5 (2), 4 connected to anterior ceratohyal, 1 connected to posterior ceratohyal.
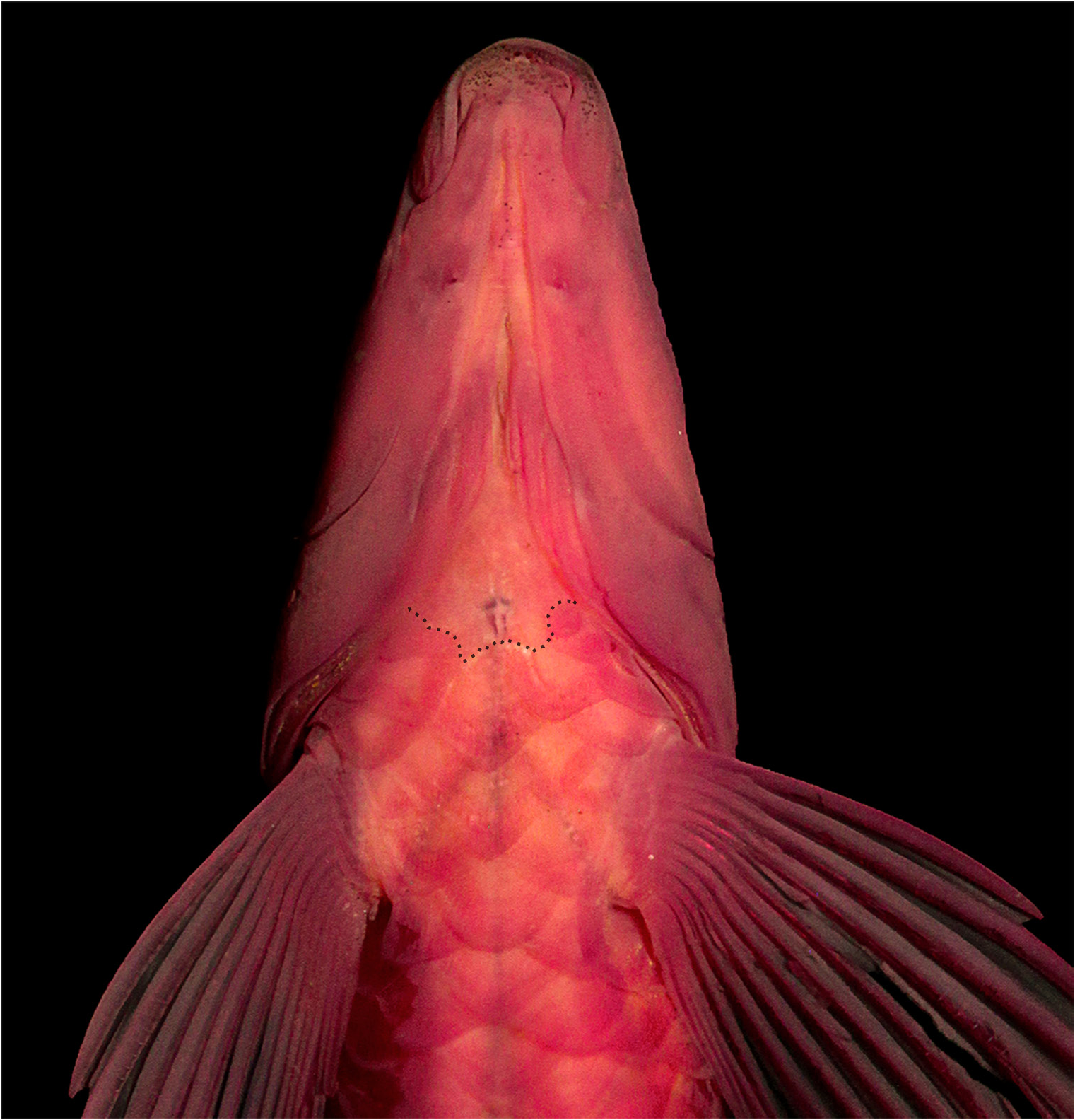
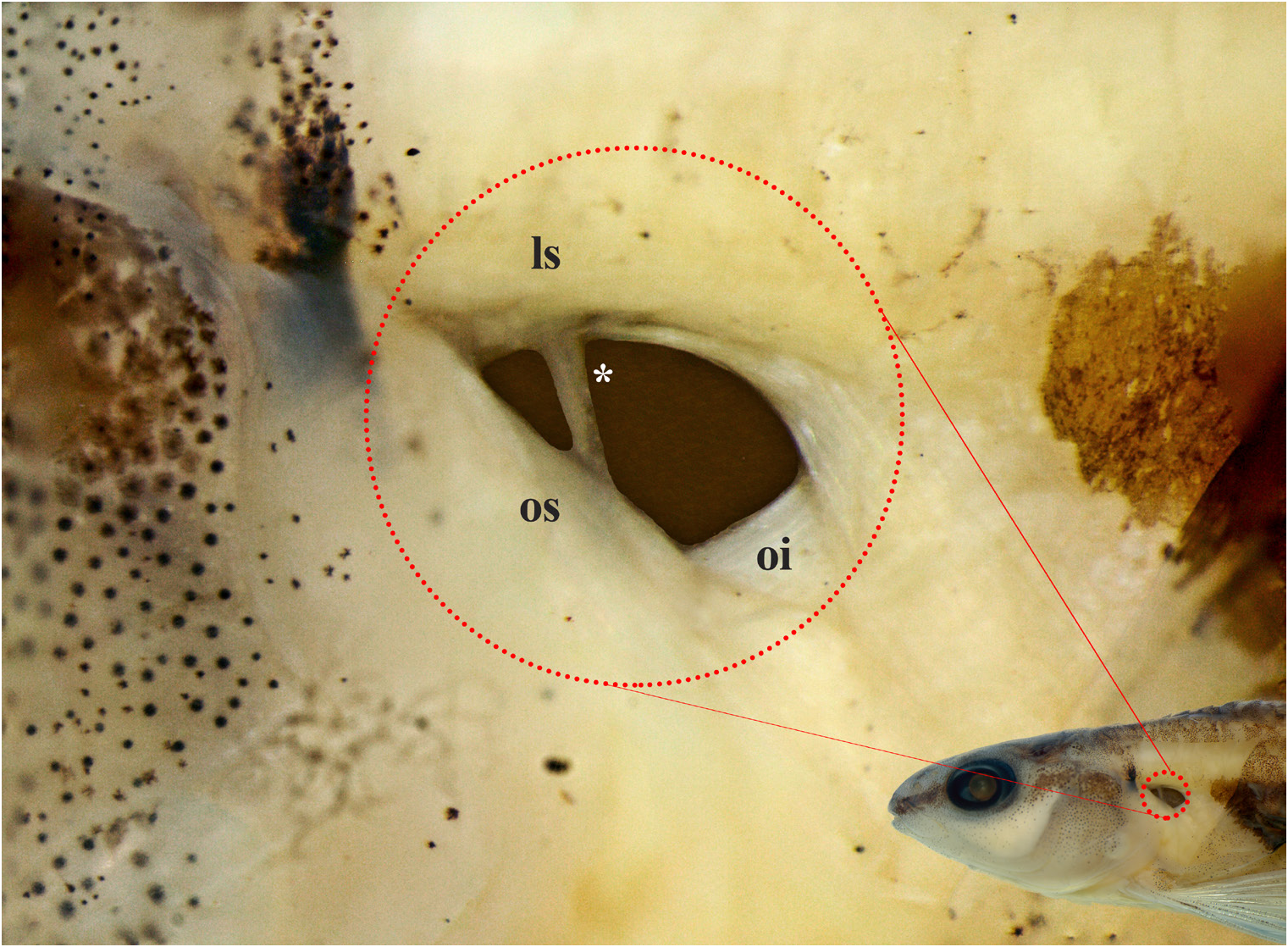
Scales cycloid; circuli absent on exposed portion of scales; up to 18 slightly divergent radii present on exposed portion of scales. Lateral line completely pored, with 33 (7) or 34* (25) scales; horizontal scale rows above lateral line 4 (30); horizontal scale rows below lateral line 4 (30). Scales along middorsal line between supraoccipital and origin of dorsal fin 8* (9), 9 (18), or 10 (5). Scale rows around caudal peduncle 14 (31). Two (3), 3* (27) or 4(2) scales between anus aperture and anal-fin insertion. Unscaled portion of body’s ventral surface restricted to area between anterior limit of isthmus and anterior margin of cleithrum. Pseudotympanum present, limited dorsally by lateralis superficialis, anteriorly and posteriorly by obliquus inferioris, and ventrally by obliquus superioris. Humeral hiatus divided into anterior and posterior chambers by pleural rib of fifth vertebra ( Fig. 3 View FIGURE 3 ).
Dorsal-fin rays ii,9* (30) or ii,10 (1); distal margin of dorsal fin nearly straight or somewhat convex. Adipose fin well developed. Pectoral-fin with 12–13 total rays; iii,7,ii (7), iii,8,i* (15), iii,8,ii (5) or iii,9,i* (4); second and third branched pectoral-fin rays usually longest; posterior tip of pectoral fin reaching or almost reaching pelvic-fin origin in small specimens (approximately 30.0 mm SL) and pectoral-fin tip falling short of pelvic-fin insertion in larger specimens. Postcleithrum 1 absent. Pelvic-fin rays i,6,i (3), i,7 (3), i,7,i* (22); second to third branched pelvic-fin rays longest; posterior tip of pelvic fin falling short of anal-fin origin. Anal-fin rays ii,5 (2), ii,6* (27), ii6,i (3); posterior margin of anal fin slightly rounded, with second branched ray usually longest; last ray adnate (2). Caudal-fin rays i,9,8,i* (29). Dorsal procurrent caudal-fin rays 9 (2); ventral procurrent caudal-fin rays 7 (2). Total vertebrae 31 (2); precaudal vertebrae 17 (2); caudal vertebrae 15 (2). Supraneural bones 5 (2). Epural bones 2 (2). Uroneural bone 1 (2).
Coloration in alcohol. Ground color of head and body pale brown ( Fig. 1 View FIGURE 1 ). Dorsal surface of head with dark patches of pigmentation, usually not forming conspicuous blotches; area between nares and slightly anterior to them usually clearer and area posterior to eyes darker. Dorsal half of head darker than ventral half, except in a few more darkly pigmented specimens. Dorsal half of head with sparsely distributed melanophores of with patches of dark pigmentation bordered by clear areas; a dark stripe present from anterior margin of snout to anterior margin of eye, less conspicuous in largest specimens examined; an inconspicuous dark blotch posterior to eye, somewhat aligned with stripe of snout, but usually with its anterior portion slightly elongated ventrally. Ventral half of head with variable pigmentation; melanophores sparsely distributed and not forming spots or completely absent in a few specimens; skin covering laterosensory canal on infraorbital 3, 4 and 5 usually without dark pigmentation, resulting in pale curved line posterior to eye. Ventral surface of head with sparsely distributed melanophores; isthmus pale or with a few melanophores. Opercle mostly dark, with pale bordering membranes. Humeral region with rounded humeral blotch, somewhat merged with midlateral stripe and imperceptible in some specimens. A continuous narrow (approximately half scale wide) dark stripe extending from humeral blotch to end of caudal peduncle; stripe usually more conspicuous anterior to last blotch or bar on caudal peduncle. Dorsal half of body usually with up to three horizontal series of dark spots formed by concentration of melanophores on dorsal half of exposed portion of scales. Occurrence, position and form of blotches or bars on flanks highly variable; variation apparently not ontogenetically influenced or sexually dimorphic. Majority of specimens with vertically elongate irregular blotches or bars, without sharply defined borders ( Figs. 1A–E View FIGURE 1 ); blotches or bars usually not solidly dark, resulting in chain-like pattern. Blotches with variable height, usually not crossing entire body, restricted to its dorsal half in some specimens, limited to central portion or ventral half of body in others. When present, bars number up to 10, variable in form and position, inclined or not; some specimens with bars divided in two inconspicuous branches (MZUSP 104694, 30.0 mm SL; MCNIP 3014, 51.1– 43.3 mm SL). Number of bars not necessarily equal on contralateral sides of specimen; one side may have bars while other side displays diffuse blotches; bars not connected dorsally to each other. Bars present in small (MZUSP 104679, 19.7–24.3 mm SL) and large (MCNIP 1993, 51.8 mm SL) specimens. A few specimens with homogeneous coloration on body (MZUSP 109301, 40.0– 42.5 mm SL; MCNIP 1026, 28.4 mm SL), with melanophores equally distributed or concentrated on border of scales and without conspicuous dark blotches or bars on flanks ( Fig. 1F View FIGURE 1 ). Basicaudal spot usually well marked; spot poorly visible in some specimens (MZUSP 109301, 40.0– 42.5 mm SL). Ventral surface of body usually with melanophores sparsely distributed throughout, more conspicuous posterior to anus; some specimens with preanal region completely pale. All fin rays display dark pigmentation, represented mainly by black dashes, more evident on dorsal and caudal fins. Dorsal fin with dark pigment on basal portion of rays and interradial membranes; some specimens with dark dashes arranged in one inconspicuous band at midlength of fin and others with up to three series of dashes restricted to rays. Caudal fin usually with conspicuous dark dashes on rays, loosely organized in up to three series following contour of fin; some specimens with caudal fin homogeneously pigmented and without well-defined dark dashes. Anal fin usually devoid of dark pigmentation in smaller specimens; fin of medium and large specimens with inconspicuous concentration of melanophores on its anterior half or with a series of dashes at its midlength. Pectoral and pelvic fins with dark dashes on dorsal surface of rays, not forming bands, or somewhat homogeneously darkened. Adipose fin usually with melanophores on its central portion.
Coloration in life. Ground color slightly olivaceous dorsally and white ventrally ( Fig. 4 View FIGURE 4 ). Conspicuous black stripe on snout continuous with narrow black midlateral stripe on flanks; golden line extending along dorsal portion of black midlateral stripe. Parts of iris, infraorbitals, and opercle with silvery and golden hues. Black rounded and vertically elongate blotches distributed along midlateral line of body of some individuals ( Fig. 4A View FIGURE 4 ), with that coloration less conspicuous in others ( Fig. 4B View FIGURE 4 ). Pigmentation on fins similar to that of preserved specimens.
Sexual dimorphism. No sexually dimorphic traits were observed.
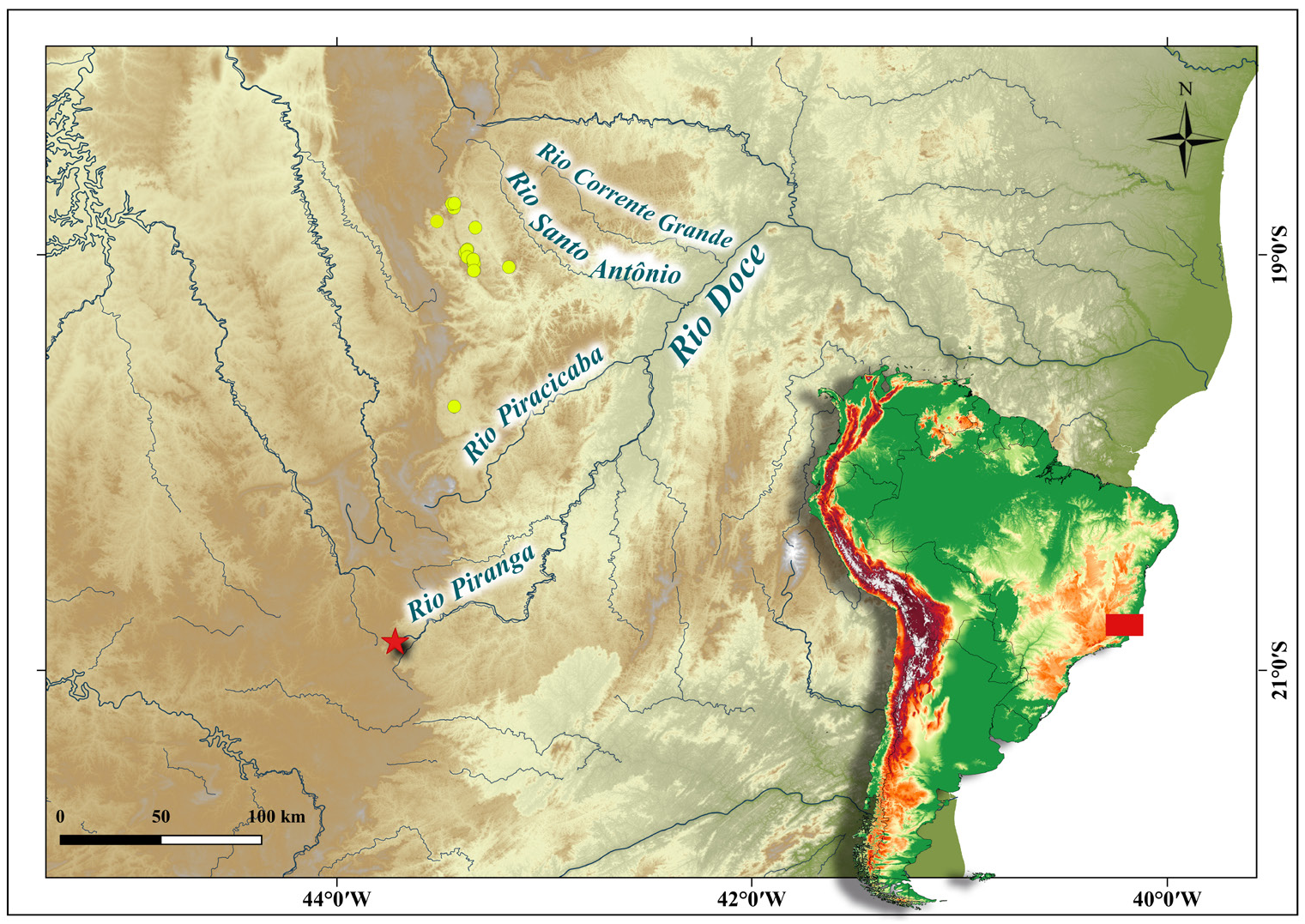
Geographical distribution. Characidium krenak is known to occur in tributaries of the upper and middle rio Doce basin, Minas Gerais State, Brazil ( Fig. 5 View FIGURE 5 ).
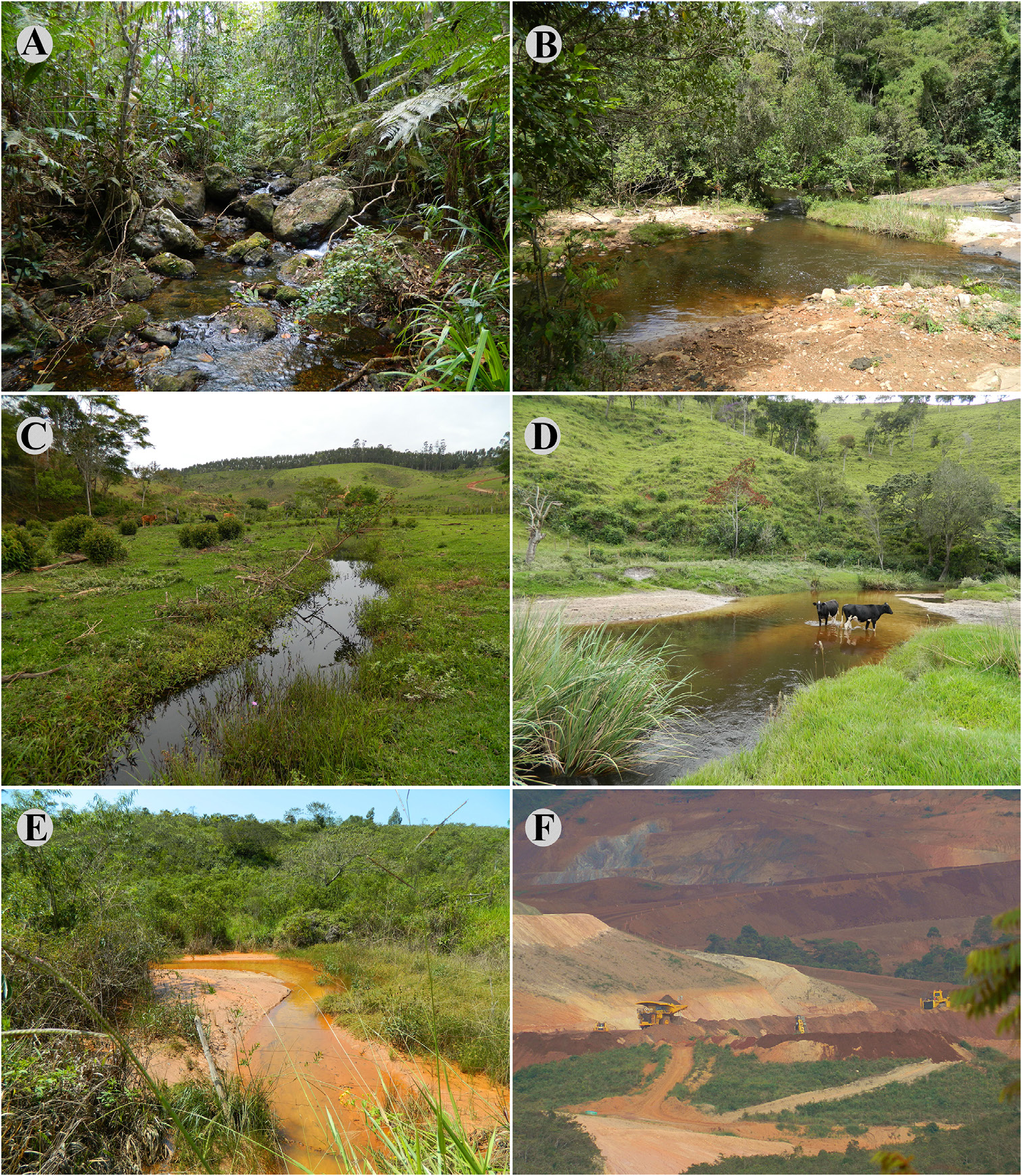
Ecological notes. Characidium krenak was collected in clear and dark water tributaries at 600 to 900 m above sea level. It inhabits slow to turbulent flowing streams, with rocky, sandy or clayish substrate ( Figs. 6A, C View FIGURE 6 ). Those environments are in different degrees of preservation, ranging from sites with intact riparian vegetation ( Figs. 6A, B, E View FIGURE 6 ) to areas highly impacted by direct anthropogenic interference, such as mining and deforestation for agriculture, eucalyptus plantations and livestock ( Figs. 6C, D, F View FIGURE 6 ).
Etymology. The specific name krenak honors the Krenák indigenous people (also known as Aimorés, Grén or Krén), that nowadays inhabit a small area on the left margin of the rio Doce. The Krenák were victims of constant massacres in the past and are currently impacted by severe environmental alterations. A noun in apposition.
Conservation status. The rio Doce basin has been considered highly impacted in the last decades due to a series of anthropogenic activities. Mining is one of the most striking, and occurs throughout most of the known range of C. krenak . One of the major recent catastrophes reported in the rio Doce basin was a burst of the oredisposal dam in November 2015, in the municipality of Mariana, Minas Gerais State, with incalculable impacts on the whole aquatic biota that critically endanger all of its biodiversity (Reis et al., 2019). Though the known range of occurrence of C. krenak does not include the portion of the main channel of the rio Doce impacted directly by the aforementioned burst of the dam, the species is present in tributaries that have been impacted indirectly. Deforestation due to agriculture and livestock ranching generate different environmental impacts in streams where C. krenak occurs. Such streams drain the municipalities of Alvorada de Minas and Conceição do Mato Dentro and include córrego Ponte Nova, ribeirão das Pedras, córrego Antonieta, ribeirão Passa Sete, Córrego Vargem Grande, córrego Vermelho, córrego Trote Velho and other tributaries of the rio Santo Antônio. In particular, streams from the rio Santo Antônio sub-basin near Conceição do Mato Dentro are more likely to suffer from agricultural or ranching impacts than are the tributaries of the rio Piranga sub-basin. Siltation can be observed in some tributaries of the rio Santo Antônio sub-basin ( Fig. 6E View FIGURE 6 ), in the surroundings of urban areas and downstream from ongoing mining projects. Dam construction is also particularly damaging to the rheophilic fauna (Agostinho et al., 2008; Hrbek et al., 2018), and such enterprises exist within the area of occurrence of C. krenak ( ANA 2015; fig. 10). Although one of the authors ( SAS) has witnessed a decline in abundance of C. krenak in recent years, no adequate information is available to make direct or indirect assessments of extinction risk based on the distribution and/or population status of the species. Therefore, we recommend that C. krenak be classified as Data Deficient (DD), according to the guidelines published by the International Union for Conservation of Nature Standards and Petitions Committee ( IUCN, 2019).
Remarks. The new species shares all putative synapomorphies of the Clade C1 proposed by Buckup (1993b), including the absence of postcleithrum 1, the naked area of ventral squamation and the reduced cranial fontanel limited antero-laterally by the parietals. However, in C. krenak the bordering of the cranial fontanel is variable, being limited anteriorly by the frontals in one cs specimen examined herein and by the parietals in the other. According to Buckup (1993b), the size of the fontanel is variable among species included in the Clade C1, but no intraspecific variation was cited by the author. Such variation is almost always absent in original descriptions of species of Characidium , possibly due to the small number of conspecific specimens typically examined for the feature. The recent description of C. onca Melo, Ribeiro & Lima (i.e., 2021), provides an exception, and those authors report two specimens in which the cranial fontanel is limited antero-laterally by the parietals and posteriorly by the supraoccipital in two specimens, and one specimen in which the fontanel extends anteriorly to contact the frontals (Melo et al., 2021b). This variation is similar to that observed here in C. krenak . Examination of this feature in a large number of conspecific specimens is desirable in future species descriptions in order to provide an overview of its variation within Characidium .
Variation in color pattern within Characidium has been recently discussed (see Zanata, Ohara, 2020), including examples of variation putatively linked to sexual dimorphism (e.g., C. satoi Melo & Oyakawa, 2015 ; Melo, Oyakawa, 2015), ontogenetic variation (e.g., C. tapuia Zanata, Ramos & Oliveira-Silva, 2018 ; Zanata et al., 2018) and phenotypic plasticity [e.g., C. cacah Zanata, Ribeiro, Araújo-Porto, Pessali & Oliveira-Silva, 2020 (Zanata et al., 2020) and C. nambiquara (Zanata, Ohara, 2020) ]. The variation observed in C. krenak is apparently not related to ontogeny nor to sexual dimorphism, with similar-sized specimens of the same sex having distinct color patterns. The variation of coloration in the species is more likely due to phenotypic plasticity, similar to that observed C. cacah and C. nambiquara .
Two other species of Characidium are known to occur in the rio Doce basin: C. cricarense and C. cf. timbuiense (Sales et al., 2018; Malanski et al., 2019). The presence of C. cricarense in the basin is unquestionable (Malanski et al., 2019), and is syntopic with C. krenak , from which it differs by a series of morphological features (see diagnosis). Conversely, the occurrence of C. timbuiense in the rio Doce has yet to be confirmed, and will depend on a detailed study that clearly delimits the species taxonomically and geographically. Aside from the distinct ventral squamation that C. krenak and C. timbuiense share (see diagnosis), the former differs from the congener by the presence of 4 scale rows above the lateral line (vs. 5), lacking of a conspicuous dark band crossing the proximal third to midlength of all dorsal-fin rays and membranes (vs. possessing such a band) and possessing conspicuous dark dashes on the caudal-fin rays that do not form continuous bands, or uniformly pigmented caudal-fin rays (vs. possessing dark bands or blotches on the caudal fin formed by dark pigmentation on the rays and membranes).
The new species possesses morphological adaptations (e.g., streamlined body, distal portion of the four first pectoral-fin rays protruded beyond the margin of the interradial membrane and scaleless isthmus) present in congeners that inhabit fast-flowing water environments, as described originally in C. cf. timbuiense (Buckup et al., 2000) and recorded later in various congeners [e.g., C. iaquira (Zanata et al., 2020) , C. kamakan (Zanata, Camelier, 2015) and C. nambiquara (Zanata, Ohara, 2020) ]. These modifications possibly provide adherence, resistance and strength to avoid being swept downstream in the fast-flowing water (Buckup et al., 2000). So far, C. krenak is known from tributaries of the upper and middle rio Doce basin on the Serra do Espinhaço, where it occurs most commonly in middle- to fast-flowing habitats. The literature mentions this region as poorly known despite its species richness, and as capable of harboring several endemic and/or endangered species (Alves et al., 2008; Santos, Britto, 2021). The presence of a newly discovered and apparently endemic species of Characidium in the rio Doce highlights the need to include specimens from this region in other taxonomic studies. Furthermore, the extensive anthropogenic activities that have altered the environment of this basin potentially threaten the endemic fish species that occur there and highlight the need for focused conservation policies.
Comparative material examined. Comparative material was obtained from the list of species provided by Zanata et al. (2018), with addition of Characidium amaila : Guyana: MZUSP 109096, 3, 36.2–68.4 mm SL. Characidium cacah : Brazil: MZUSP 125765, 1, 36.5 mm SL, holotype. Characidium hasemani : Brazil: MPEG 4568, 10, 35.5–41.4 mm SL; MZUSP 97283, 10, 37.9–59.6 mm SL. Characidium iaquira : Brazil: MZUSP 125780, 1, 64.6 mm LS, holotype. Characidium mirim : Brazil: MZUSP 111123, 1, 20.2 mm SL, holotype. Characidium nambiquara : Brazil: MZUSP 118566, 1, 67.7 mm LS, holotype. Characidium pterostictum : Brazil: MCP 54059, 5, 52.4–63.3 mm SL. Characidium satoi : Brazil: MZUSP 115059, 17, 26.2–44.3 mm SL, paratypes. Characidium summus : Brazil: MZUSP 116105, 11, 14.0– 42.3 mm SL, paratypes. Characidium tapuia : Brazil: UFBA 8511, 3, 29.8–32.1 mm SL, 1 cs, 30.8 mm SL, paratypes.
| T |
Tavera, Department of Geology and Geophysics |
| R |
Departamento de Geologia, Universidad de Chile |
| ANA |
Orange County Department of Agriculture |
| SAS |
Sammlung Arnhardt des Museums Schloss Wilhelmsburg Schmalkalden |
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
| MPEG |
Museu Paraense Emilio Goeldi |
| LS |
Linnean Society of London |
| MCP |
Pontificia Universidade Catolica do Rio Grande do Sul |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.