Careopalpis akko Dorchin and Freidberg
|
publication ID |
https://doi.org/ 10.5281/zenodo.184984 |
|
DOI |
https://doi.org/10.5281/zenodo.6231592 |
|
persistent identifier |
https://treatment.plazi.org/id/03FF87F2-E273-BF5A-FF58-6E5F79ACFDD4 |
|
treatment provided by |
Plazi |
|
scientific name |
Careopalpis akko Dorchin and Freidberg |
| status |
sp. nov. |
Careopalpis akko Dorchin and Freidberg View in CoL , new species
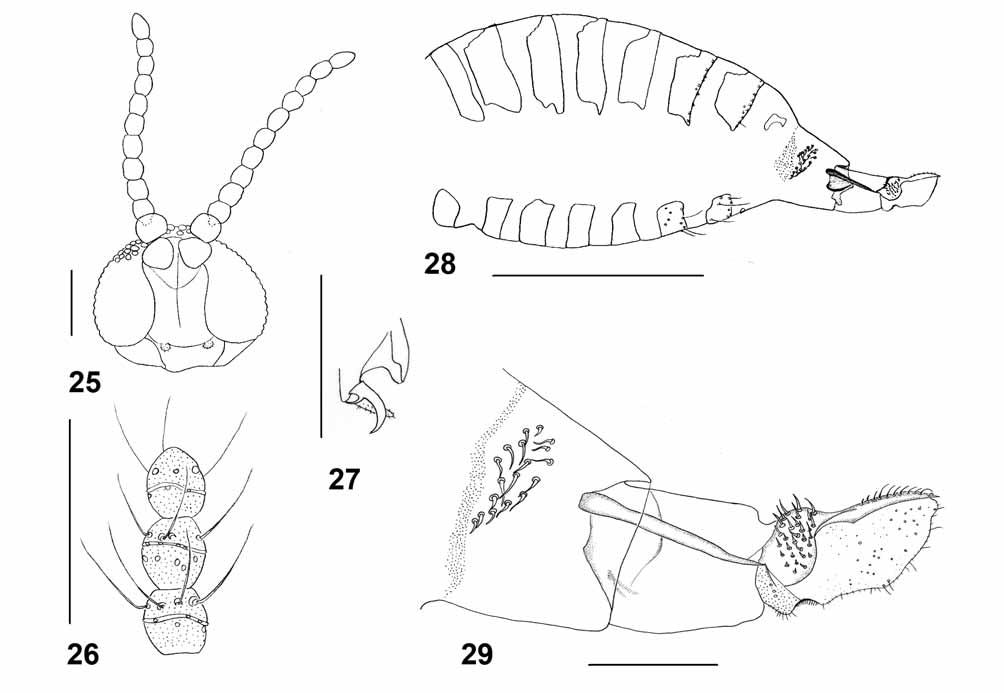
Adult. – Head ( Fig. 25 View FIGURES 25 – 29 ): Eye facets circular, gap between eyes on vertex 0.5 times as wide as facet in male, 0– 0.5 in female (one female with two merged facets on vertex, forming one large facet). Palpus 1-segmeted, tiny, hyaline, barely visible. Antenna ( Fig. 26 View FIGURES 25 – 29 ): similar in both sexes; scape conical, widest distally, pedicel globular, flagellomeres: 10, barrel-shaped, about 1.5 times as long as wide, each with whorl of circumfila split in two on one side of flagellomere and two whorls of setae – distal and proximal of circumfilum. Apical flagellomere slightly tapered, in some specimens seems to be “budding” to produce additional flagellomere; one male with tiny 11th flagellomere.
Thorax: Wing: length 1.1–1.4 mm in female (n=16), 0.9–1.3 mm in male (n=11); transparent, veins other than Sc and R5 barely visible, R5 joins C anterior to wing mid length, M present, Cu unforked. C, Sc and R5 brownish, with sparse hairs. Legs ( Fig. 27 View FIGURES 25 – 29 ): Tarsal claws toothed, evenly curved, tooth thin and strongly curved, empodia extend beyond bend of claw, pulvilli about 0.3 times as long as claw.
Female abdomen ( Fig. 28 View FIGURES 25 – 29 ): General color pinkish; scale pattern on dorsum forming three black spots on white background on anterior part of each tergite. Tergites 1–7 less sclerotized anterodorsally, with posterior row of long setae and evenly scattered scales on entire surface; tergite 8 largely unsclerotized, without setae; trichoid sensilla not detectable. Sternites 2–6 with posterior and median rows of long setae; sternite 7 weakly sclerotized medially; sternite 8 undifferentiated from surrounding membrane. abdomen laterally rugose between tergite 8 and ovipositor. Ovipositor ( Fig. 29 View FIGURES 25 – 29 ): Lateral group of setae on segment 8 comprising 18–22 setae, slightly and evenly curved, sometimes curved like the letter S, usually forming more or less three transverse rows. Sclerotized rods narrowed apically, joining lateral plate and weakly sclerotized lateroventral plate at base of cercus; lateral plate bearing 30–33 straight or slightly curved fine setae. Aculeus thick, curved posteroventrally, almost of same width throughout length, with row of posteriorly curved fine setae; apical lamella ovoid, as long as aculeus.
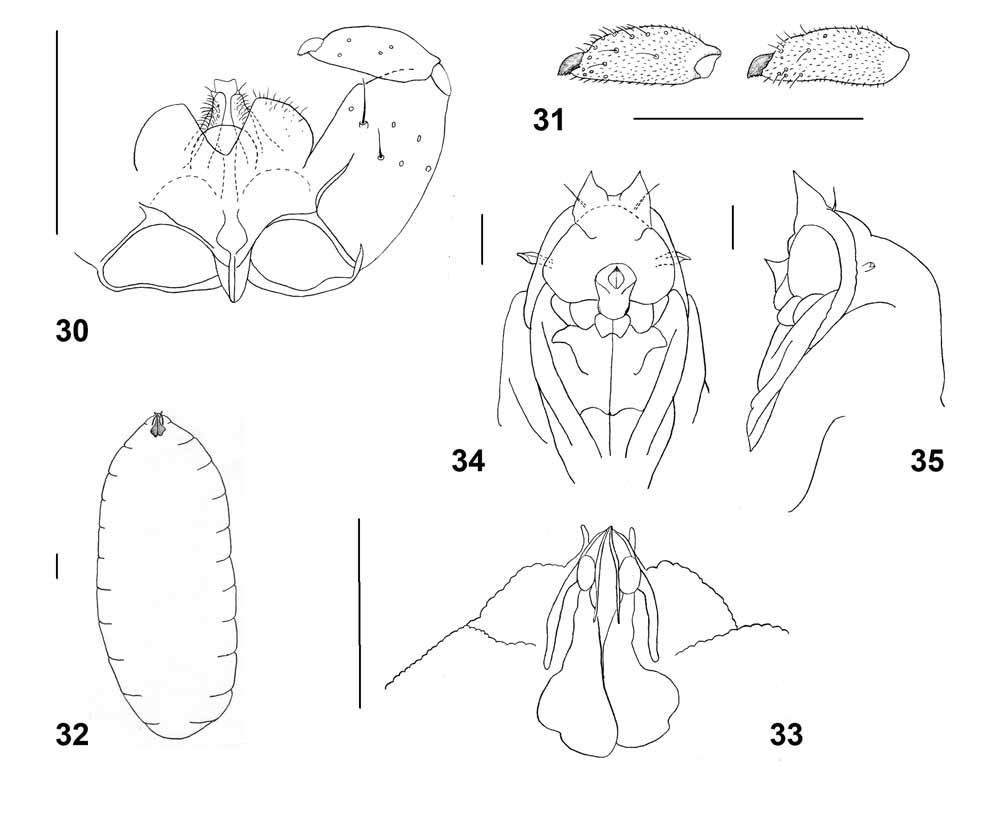
Male abdomen: General color brownish-orange; scale pattern as in female but much duller. Tergites 1–7 entire, with posterior row of long setae and evenly scattered scales; tergite 8 almost entirely undifferentiated from surrounding membrane; trichoid sensilla not detectable. Sternites 2–4 with randomly scattered setae, sternites 5–7 with median and posterior rows of long setae; sternite 8 sclerotized only in posterior third, with posterior row of setae. Terminalia ( Figs. 30–31 View FIGURES 30 – 35 ): Gonocoxite stout, same width throughout length, with several strong setae on distal half; mediobasal lobe prominent, evenly setulose. Gonostylus cylindrical, narrowed from mid length toward apex, evenly setulose on entire surface, with several longer setae mostly around tooth. Fused cerci much wider than hypoproct, deeply separated, evenly setose and setulose. Hypoproct entire, rounded. Paramere narrowed apically with longitudinal groove, with an apical seta on elevated lobe; evenly covered by strong, curved setae. Aedeagus longer than parameres, rectangular, sometimes with shallow apical notch.
Larva (third instar) ( Figs. 32–33 View FIGURES 30 – 35 ) – Cylindrical, yellowish-white; length: 1.44–1.60 mm (n=3). Posterolateral apodemes 2.4 times as long as head capsule. Salivary glands enlarged to form sclerotized sacs extending into prothorax. Integument smooth, ventrally with rows of spicules on mid part of thoracic segments and on proximal third of abdominal segments. Venter of terminal segment without spicules; dorsum of thoracic segments mostly smooth, with rows of spicules around stigma; dorsum of abdominal segments covered by spicules, with bare median sections. Spatula absent. Lateral papillae asetose; pleural papillae with short, blunt setae; terminal papillae two on each side of anus, asetose.
Pupa ( Figs. 34–35 View FIGURES 30 – 35 ) – Orange. Antennal horns prominent, straight and tapered. Cephalic seta short and fine. Frons with one prominent horn, slightly pointed anteriorly. Prothoracic spiracle wide at base, abruptly narrowed toward apex. Dorsal part of abdominal tergites evenly covered by tiny bumps.
Holotype – Ψ, Israel, Akko , 12.VIII.2002, N. Dorchin and A. Freidberg, reared from Suaeda splendens leaf gall.
Paratypes – All material from Israel, Akko , reared by N. Dorchin from Suaeda splendens leaf galls unless otherwise noted. 9 Ψ, 5 ɗ, same data as holotype; 5 Ψ, 3 ɗ, 3 larvae: 21.X.1996; 3 Ψ, 2 ɗ, 26.VII.1997; 1 Ψ, 2 ɗ, 29.VIII.1998.
Distribution. – Israel ( Akko salt marsh).
Etymology – This species is named after its type locality ( Akko is the Hebrew name of Acre).
Biology. – This species induces single-chambered leaf galls on Suaeda splendens ( Fig. 7 View FIGURES 5 – 10 ). Galls are yellowish, grain-like, slight swellings on the leaf blade, usually one per leaf, each containing a single larva. The gall is relatively thin-walled and semi-transparent. Although galls are somewhat inconspicuous, they form rigid capsules that are easily distinguished from the normal, soft tissues of the leaf and are usually found in clusters on adjacent leaves. The host plant is an annual, and was found in a restricted area of the salt marsh from June to November. Adults were reared in great numbers from July to mid November with a peak in August. Thus, the species appears to complete at least two generations within this period of time, and larvae of the last generation possibly overwinter in the ground or in shed leaves.
Remarks. – Careopalpis akko fits the genus in having characteristic small and stout body shape and ovipositor morphology as described above. All currently described species are of very uniform morphology and all develop in leaf or stem galls on Chenopodiaceae in Eastern Europe and central Asia ( Russia, Kazakhstan, Uzbekistan, Azerbaijan, Armenia, Turkmenistan, and Iran). Three of these species ( C. suaedae Fedotova , C. suaedicola Fedotova , and C. suaediphila Fedotova ) develop in Suaeda , and we therefore assume they are more closely related to C. akko than species that develop in other Chenopodiaceae . Both C. suaedae and C. suaedicola induce multi-chambered leaf swellings on Suaeda physophora , which occupy the entire diameter of the leaf ( Fedotova 1983, 1985). This is in contrast to the inconspicuous leaf infestations of C. suaediphila in Suaeda acuminata ( Fedotova 1992) and to the single-chambered, rice-grain-like galls of C. akko . Males of C. sauedicola have gonostylus and gonocoxite that are more elongate and slender than those of C. akko and C. suaedae . Females of C. akko could not be distinguished from those of C. suaedae based on a single paratype we examined of the latter. Unavailability of type material of C. suaedicola and C. suaediphila and the lack of sufficient details in the drawings that accompany their descriptions precluded a comparison to their females. Notwithstanding, the information we have about the morphology, life-history, and distribution of the relevant species supports the validity of C. akko as a new species.
We reared adults of Careopalpis from normal-appearing leaves of three more Suaeda species in Israel ( S. aegyptiaca , S. fruticosa , and S. asphaltica ), which cannot be distinguished morphologically from adults of C. akko . Further study, including genetic, is needed in order to establish whether the populations from the different hosts represent separate species.
Despite the overall uniformity of Careopalpis species, clear morphological differences can be found among some of them. For example, the palpi in C. roborovskyi Marikovskij are larger and more conspicuous than in either of the species from Suaeda , and the lateral group of setae at the base of the female ovipositor in this species has about twice the number of setae than in C. akko . Nevertheless, galls still offer the best attributes for distinguishing among species in this genus. For example, C. roborovskyi and C. balkhashensis Marikovskij induce large and complex stem deformations, whereas other Careopalpis species induce simple or barely conspicuous galls.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.