Acanthemblemaria aceroi, Hastings & Eytan & Summers, 2020
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4816.2.5 |
|
publication LSID |
lsid:zoobank.org:pub:A651DF06-FC88-419F-86C3-A677CB16E809 |
|
persistent identifier |
https://treatment.plazi.org/id/03B787EB-FFC2-5300-A6A3-40B9FCBDAA58 |
|
treatment provided by |
Plazi |
|
scientific name |
Acanthemblemaria aceroi |
| status |
sp. nov. |
Acanthemblemaria aceroi sp. nov.
“Blue-spotted Barnacle Blenny”
( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 )
Synonymy: Acanthemblemaria rivasi (in part): Acero 1984.
Holotype. SIO 02-106 View Materials (40.8 mm SL), Mochima National Park , Venezuela; approximately 10° 18.62’ N, 64° 33.7’ W; found within vacant barnacle tests. GoogleMaps
Paratypes. Venezuela: AMNH 241424 View Materials (1, 27.9 mm SL) , La Cienga, Ocumare de la Costa, Aragua, 10° 29.3’ N, 67° 48.2’ W; AMNH 247576 View Materials (12, 22.1–29.1) GoogleMaps , Taguarumo, Isla Caracas, Parque Nacional Mochima, Sucre, 10° 15.83’ N, 64° 28.85’ W; AMNH 249352 View Materials (1) GoogleMaps Islas Los Monjes del Sur , 12° 21.63’ N, 70° 54.17’ W; AMNH 249362 View Materials (3, 17.5–29.7) GoogleMaps , Isla Los Monjes del Norte , 12° 29.01’ N, 70° 54.96’ W; ANSP 147650 View Materials (5, 20.7–27.8) GoogleMaps , Punta Morón, approximately 10° 30’ N, 68° 8.99’ W; SIO 02-6 View Materials (1, 34) GoogleMaps , Mochima National Park near Puerto La Cruz , approximately 10° 18.62’ N, 64° 33.7’ W; SIO 02-106 View Materials (3, 28–33) GoogleMaps , collected with the holotype; SIO 06-277 View Materials (2, 25–27) , Isla Caracas, Parque Nacional Mochima, approximately 10°15.8’ N, 64° 28.8’ W; SIO 08-182 View Materials (4, 24–28) GoogleMaps , Los Monjes, 12°29.1’ N, 70°54.93’ W.
Diagnosis. Unique within the hancocki group of the genus Acanthemblemaria (sensu Hastings & Robertson, 1999) in having a combination of the following: a short posterior extent of the infraorbitals ending well before the posterior tip of the maxilla, a smooth supraorbital margin, field of frontal wedge spines intersecting orbit at the anteriormost supraorbital sensory pore well anterior to the junction of the upper infraorbital and frontal, and a profusion of fine blue dots on head and anterior body.
Description. Dorsal fin with XXI–XXIII spines and 12–14 rays, 33–36 total elements ( Table 1). Anal fin with II spines and 21–25 rays. Pectoral fin with 13 rays. Eleven to twelve precaudal vertebrae, 29–30 caudal vertebrae and 41–42 total vertebrae (holotype 11+30=41).
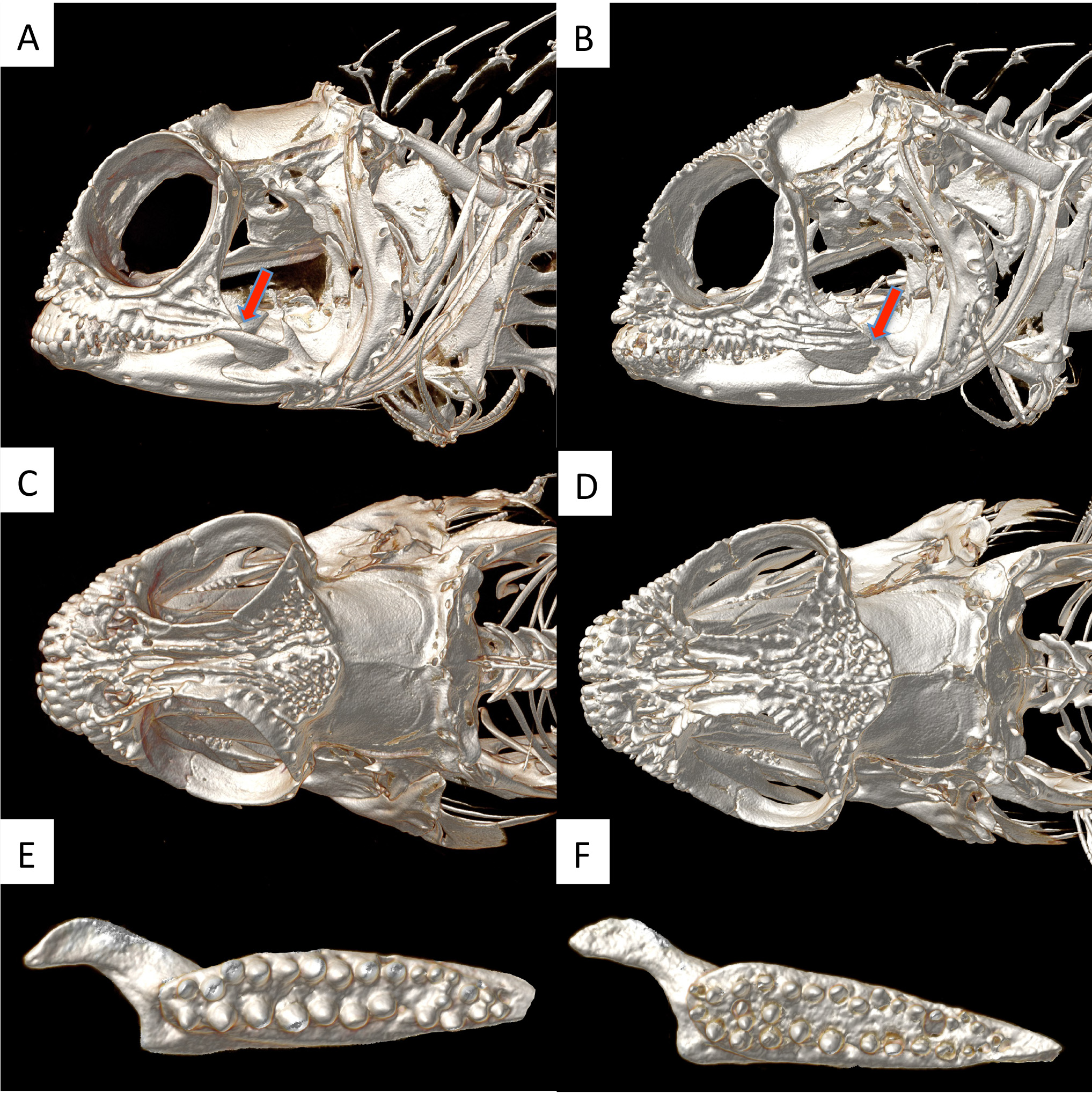
Head with well-developed spines on several cranial bones of adults; spines smaller and less numerous in juveniles. Adults with postero-dorsal portion of frontals covered with short rounded spines extending in a wedge from well anterior of junction with dorsalmost infraorbital. Holotype (largest known specimen) with two spines on right side fused into a ridge perpendicular to posterior margin of wedge, otherwise all spines on frontal wedge separate. Supraorbital margin of frontal smooth to slightly crenulate anteriorly in holotype. Interorbital region of frontal with two well-developed central rows of 5–6 spines on raised ridge; lateral interorbital row absent. AFO spine large and bladelike. Nasal bones fused, each with a row of 4–6 well-developed, blunt spines that curve laterally around medial margin of posterior nostril. Anterior margin of nasals above upper lip with three blunt spines on each side. Orbital margin of lateral ethmoid with a series of 3–4 blunt spines and a single enlarged spine ventrally. Orbital margin of anterior infraorbital with ridges and a single enlarged spine anteriorly; ventral margin with 15–16 well-developed knob-like spines along anterior two thirds of its length, posterior one third smooth, lacking spines. Central area of anterior infraorbital with a few blunt spines anteriorly and ridges posteriorly. Orbital margin of posterior infraorbital smooth. Lower and upper infraorbitals with a posterior extension ending well before posterior end of jaw ( Fig. 2A View FIGURE 2 ); posterior extension with a prominent central ridge but no spines.
Body relatively deep, body depth 5.6 times in standard length. Head large, robust, its length approximately four times in standard length. One pair of typically unbranched supraorbital cirri, length one half to two thirds orbital diameter; occasionally shallowly branched in about 10 percent of specimens ( Acero 1984). Nasal cirri on anterior nostrils branched. Posterior nostrils with a raised rim along anterior margin.
Dorsal fin single, slightly notched between spinous- and soft-rayed portions. Caudal fin truncate. Upper jaw large (length about 1.8 times in head length), extending beyond level of posterior margin of orbit. Palatine with a double row of teeth, about 13 in inner row and 11 in outer row ( Fig. 2E View FIGURE 2 )
Measurements (mm) of holotype: standard length 40.8; maximum head length 10.5; snout to opercular membrane insertion 9.0; upper jaw length 5.5; bony orbital diameter 2.5; iris diameter 2.2; snout length 2.0; interorbital width 1.2; predorsal length 8.0; preanal length 18.6; caudal peduncle depth 3.0; maximum body depth 7.3; body depth at anal-fin origin 6.6; supraorbital cirrus length 1.6 (left side, right side missing).
Cephalic pore counts (left side only except where noted for holotype; number of specimens in parentheses; * holotype): mandibular: 4(21*); common: 1(2*), 2(19*; holotype with 1 on right side); preopercular: 5(21*); posttemporal: 4(21*); lateral supratemporal: 3(16*), 4(5*; holotype with 4 on right side); median supratemporal: 3(21*); anterior infraorbital: 3(21*); posterior infraorbital: 3(1), 4(20*); supraorbital: 2(1), 3(7), 4(13*); frontal: 0(8), 1(3), 2(10*), 3(4); commissural: 1(8), 2(13*); anterofrontal: 1(1), 2(20*); nasal: 1(21*).
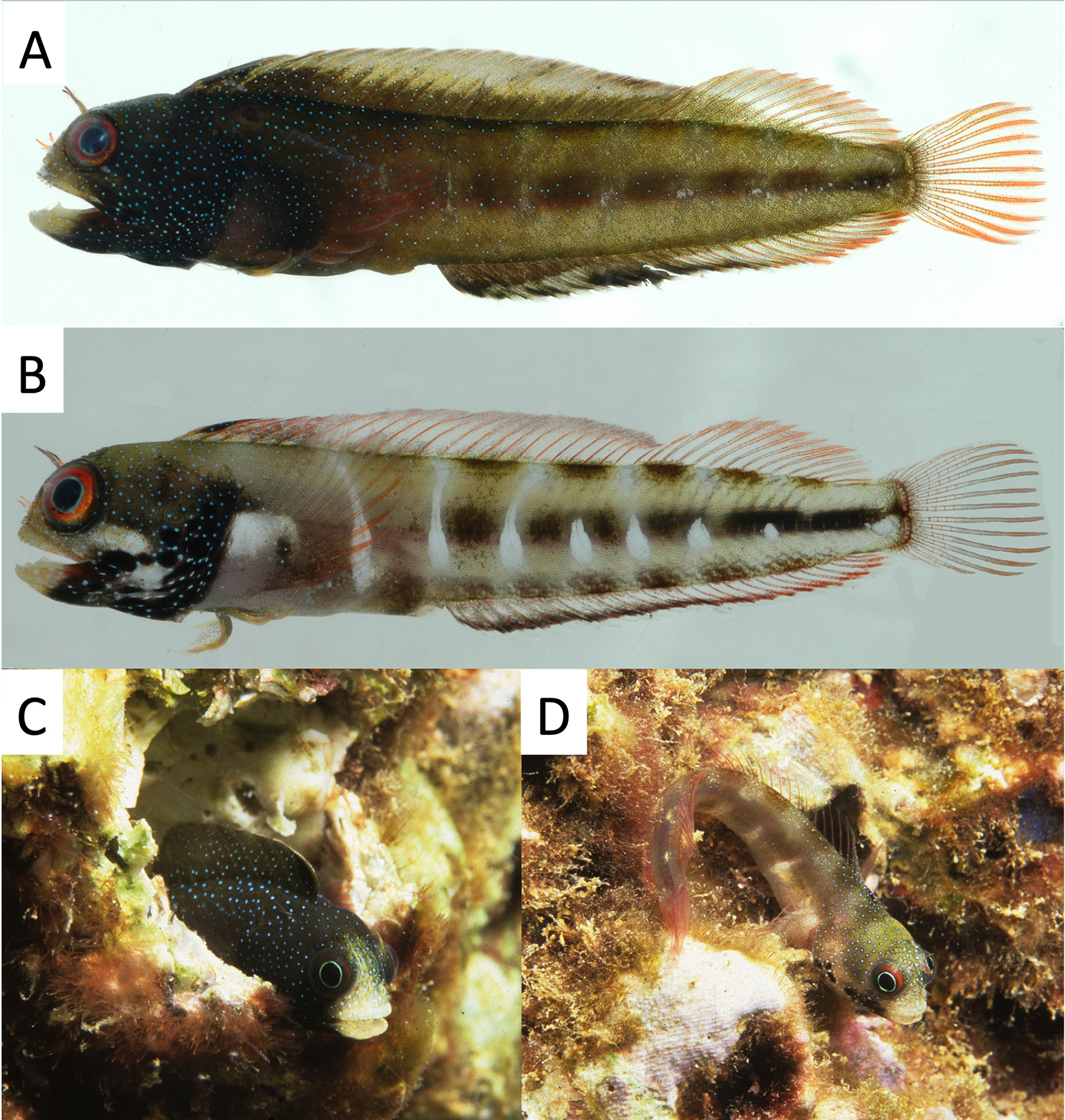
Coloration of male holotype in preservative ( Fig. 1A View FIGURE 1 ): snout, upper jaw and lower jaw lightly pigmented with numerous small melanophores. Head from anterior orbit to posterior margin of operculum, dark with densely spaced and expanded melanophores. Black spots or blotches present at posterior tip of upper jaw, operculum and branchiostegal membranes: left side with one spot above posterior tip of jaw, three black blotches on lower operculum and four on branchiostegal membranes; right side with one black spot at posterior tip of lower jaw, one on lower preopercle, two on operculum and three on branchiostegal membranes. Body and abdomen densely covered with melanophores, those along lateral aspect of body concentrated in broad bars extending to dorsal midline: two over abdomen, five on body between anal-fin origin and insertion, becoming less distinct posteriorly; areas between bars less pigmented. Dorsal fin covered with dense melanophores, numerous anteriorly decreasing in density posteriorly. Membrane between first and second dorsal-fin spines with a prominent black spot covering most of membrane. Proximal anal fin covered with dense melanophores; distal tips of rays white. Caudal fin with melanophores along rays (none on membranes). Pectoral-fin base covered with dense melanophores extending outward onto fin, including rays and membrane. Pelvic fin with dense melanophores along rays and membranes, less dense along anterior margin of first ray. Very fine light spots evident on head and anterior dorsal fin (these correspond to blue dots seen in living specimens; Fig. 3 View FIGURE 3 ).
Coloration of other specimens in preservative variable, especially in extent and concentration of melanophores. Prominent black spots on lower posterior portion of head variable in number, size and location. One to three spots near posterior tip of jaw; some specimens with spots both above and below jaw, and some with large and variously fused spots. Operculum with one to five spots, preopercle area with none to three spots, and branchiostegal membranes with three to twelve spots. Some specimens with branchiostegal membranes densely covered with melanophores and no evident spots. Smaller males and females less dark on head and body but bars present on body.
In life, sexually mature males ( Fig. 3A,C View FIGURE 3 ) melanistic with black obscuring underlying coloration except for upper and lower jaws and interorbit that lack pigment, and faint whitish bars along body. Numerous blue dots cover body, head, and anterior dorsal fin. Iris dark with narrow yellowish internal ring around pupil ( Fig. 3C View FIGURE 3 ). Anterior dorsal fin with a narrow reddish margin; posterior dorsal fin with red along fin rays. Anal fin with a dark distal margin; proximal anal fin dark anteriorly, with red along rays posteriorly. Caudal fin with red along rays. Non-melanistic individuals ( Fig. 3D View FIGURE 3 ) light brown with evident lateral bars, distinct black spots on posterior jaw and across branchiostegal membranes. Iris orange with an inner blue ring. Fine blue spots cover head and anterior body.
The underlying pigment pattern of freshly caught males and females variable. Female photographed shortly after capture ( Fig. 3B View FIGURE 3 ) with melanophores on head concentrated in a narrow ring around posterior margin of orbit, on operculum and ventrally across branchiostegal membranes and posterior half of lower jaw. Dark spots present along posterior jaw and on branchiostegal membranes. Distinct white areas on head and body located in a crescent from lower orbit to branchiostegal membranes, anterior of pectoral fin, and laterally along body in seven bars separating dark bars and extending to dorsal midline anteriorly, restricted to midline posteriorly. Dark bars interrupted posteriorly, appearing as dark saddles dorsally, dark blotches along midline (elongate along caudal peduncle) and dark blotches above anal fin. Head and anterior body covered with fine blue dots. Iris distinctly orange with inner blue ring. Supraorbital cirrus dark, nasal cirrus reddish. Anterior dorsal fin with a black spot between first and second spines, few faint melanophores anteriorly and distally on spinous portion, with red along spines and rays. Similar red along rays of pectoral, caudal, and anal fins. Anal fin with a dark distal margin, grading to reddish posteriorly. A dark spot at base of pectoral fin. Pelvic fin tinged with yellowish orange.
Distribution. Acanthemblemaria aceroi occurs along the northern coast of South America including Colombia and Venezuela. It appears to be restricted to the southern Caribbean upwelling system that extends from Trinidad (61.1° W) to Barranquilla, Colombia (75.51° W; Rueda-Roa & Muller-Karger 2013). A significant biogeographic break in this region has been identified for other groups including catfishes of the genus Cathrops (Betancur et al. 2010)
Etymology. Acanthemblemaria aceroi is named for Arturo Acero Pizarro, who first documented meristic differences between populations of “ A. rivasi ” from Central and South America, in recognition of his contributions to ichthyology, including the systematics of chaenopsid blennies. The common name “Blue-spotted Barnacle Blenny” refers to the profusion of fine blue spots on the head, anterior body and dorsal fin.
Comparisons. Acanthemblemaria aceroi can be distinguished from other Atlantic members of the genus (excluding its closest relative, A. rivasi ) by its stout body, relatively short, rounded head spines, simple typically unbranched supraorbital cirrus, and distinctive red to orange iris with a narrow, bright blue inner ring. It differs from the similar A. rivasi in having: 1) a shorter posterior extent of the infraorbitals ending well short of the posterior tip of the maxilla (extending to near the tip in A. rivasi ; Fig. 2A,B View FIGURE 2 ); 2) a smooth supraorbital margin (spines or serrations present in A. rivasi ; Fig. 2C,D View FIGURE 2 ); 3) a smaller frontal wedge of spines that intersects the orbital margin at the anteriormost supraorbital sensory pore well anterior to the junction of the upper infraorbital with the frontal (frontal wedge intersects the orbital margin at the posteriormost supraorbital pore and at the junction with second infraorbital with the frontal in A. rivasi ; Fig 2 View FIGURE 2 A-D); 4) fewer, larger teeth on the palatine ( Fig. 2E,F View FIGURE 2 ); and 5) modally higher meristic counts, especially of the total dorsal-fin elements ( Table 1). In addition, the COI sequence of the new species differs from that of A. rivasi by 4.4-4.7 percent (Eytan, pers. observ.). Based on data from several genes, Eytan et al. (2012) estimated the divergence time of the two species as 0.97 million years (range = 0.38-1.71).
Remarks. Acero (1984) indicated that specimens of “ A. rivasi ” from South America are larger than those from Central America. The largest known specimen of A. aceroi from Venezuela is the 40.8 mm SL holotype (SIO 02- 106) and the largest reported from Colombian waters is 40.4 mm SL ( Acero 1984), while the largest specimen of A. rivasi from Panama is 32.0 mm SL (SIO 07-69). The larger body size of the new species may be related to its occurrence in colder, more productive waters compared to the closely related species from Central America. Acero (1984) noted similar meristic and body size differences in Central and northern South American populations of Acanthemblemaria betinensis . The genetics of this form have not been examined but may reveal significant differences that warrant recognition of two separate species in the southern Caribbean.
Comparative material examined: A. rivasi . Panama: SIO 67-45 View Materials (1, 16 mm SL; paratype) Toro Point ; SIO 71- 272 View Materials (3, 23–28) San Blas ; SIO 71-286 View Materials (3, 20.5–28.5) San Blas ; SIO 01-09 View Materials (1, 24), San Blas ; SIO 03-141 View Materials (7, 22–28) Bahia Azul , Bocas del Toro ; SIO 07-69 View Materials (3, 26–32), Portobello .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.