Acanthaspis cincticrus Stål, 1859
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3892.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:30C7AE6D-D6AB-4777-B6A3-9760BBB95741 |
|
DOI |
https://doi.org/10.5281/zenodo.4953401 |
|
persistent identifier |
https://treatment.plazi.org/id/6C5587D2-D97F-2E57-FF24-FF15FAC4FD81 |
|
treatment provided by |
Felipe |
|
scientific name |
Acanthaspis cincticrus Stål, 1859 |
| status |
|
Acanthaspis cincticrus Stål, 1859 View in CoL
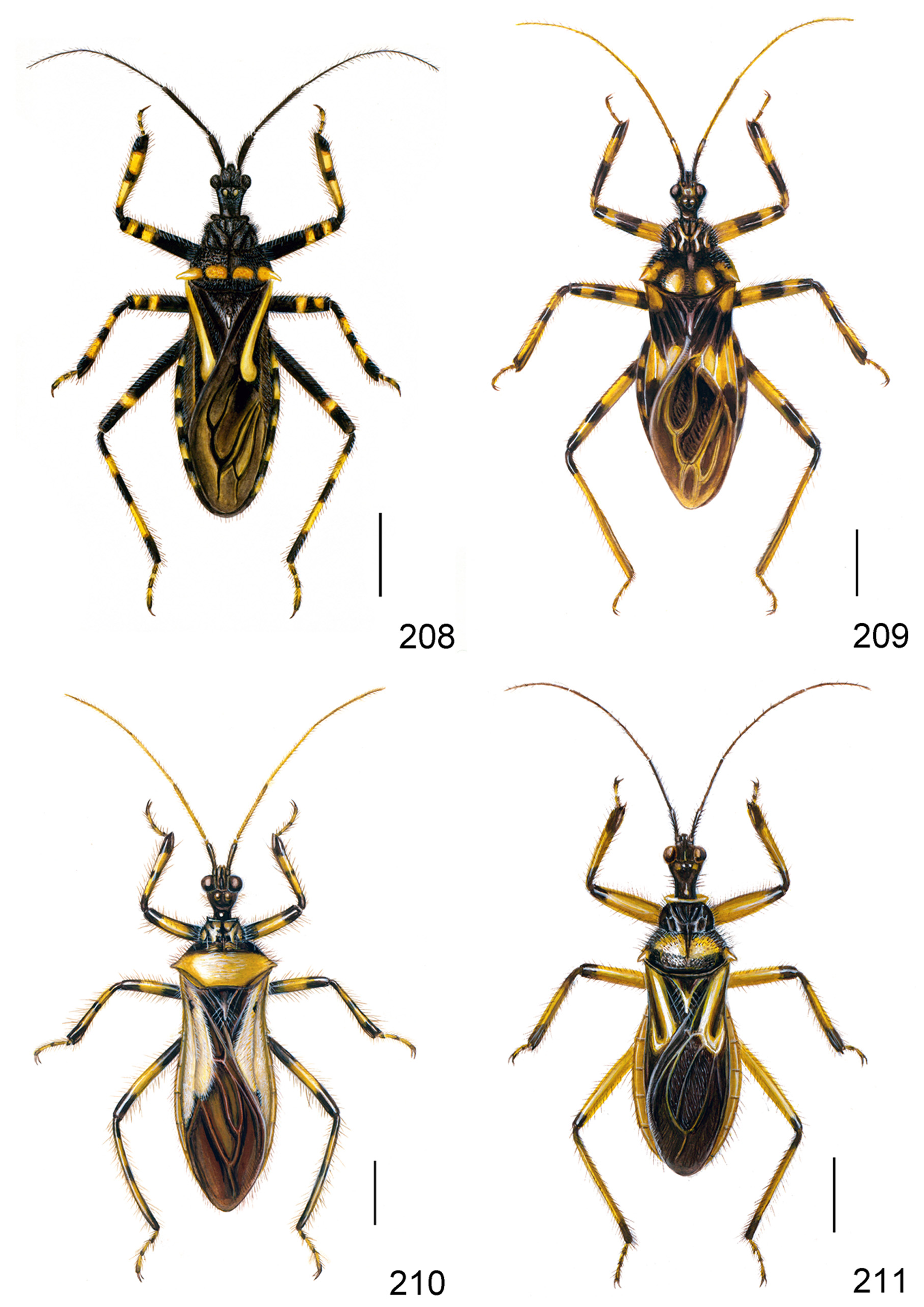
( Figs 1–40, 208 View FIGURES 208–211 )
Acanthaspis cincticrus Stål 1859: 188 View in CoL ; Stål 1866: 243; Stål 1874: 74; Reuter 1887: 157; Horváth 1889: 37; Lethierry & Severin 1896: 104; Distant 1904: 270; Oshanin 1908: 523; Oshanin 1912: 50; Matsumura 1931: 1206; Doi 1933: 90; Wu 1935: 17; Yamada 1936: 21; Ishihara 1937: 728; China 1940: 252; Hoffmann 1944: 17; Hsiao 1976: 80; Hsiao & Ren 1981: 457; Maldonado-Capriles 1990: 384; Lee & Kwon 1991: 20; Putshkov & Putshkov 1996: 186. Tomokuni & Cai 2002: 106.
Acanthaspis humeralis View in CoL (non Scott 1874): Matsumura 1905:27, misidentification.
Acanthaspis albovittata Matsumura 1907: 141 . Synonymized by Fukui 1927: 3.
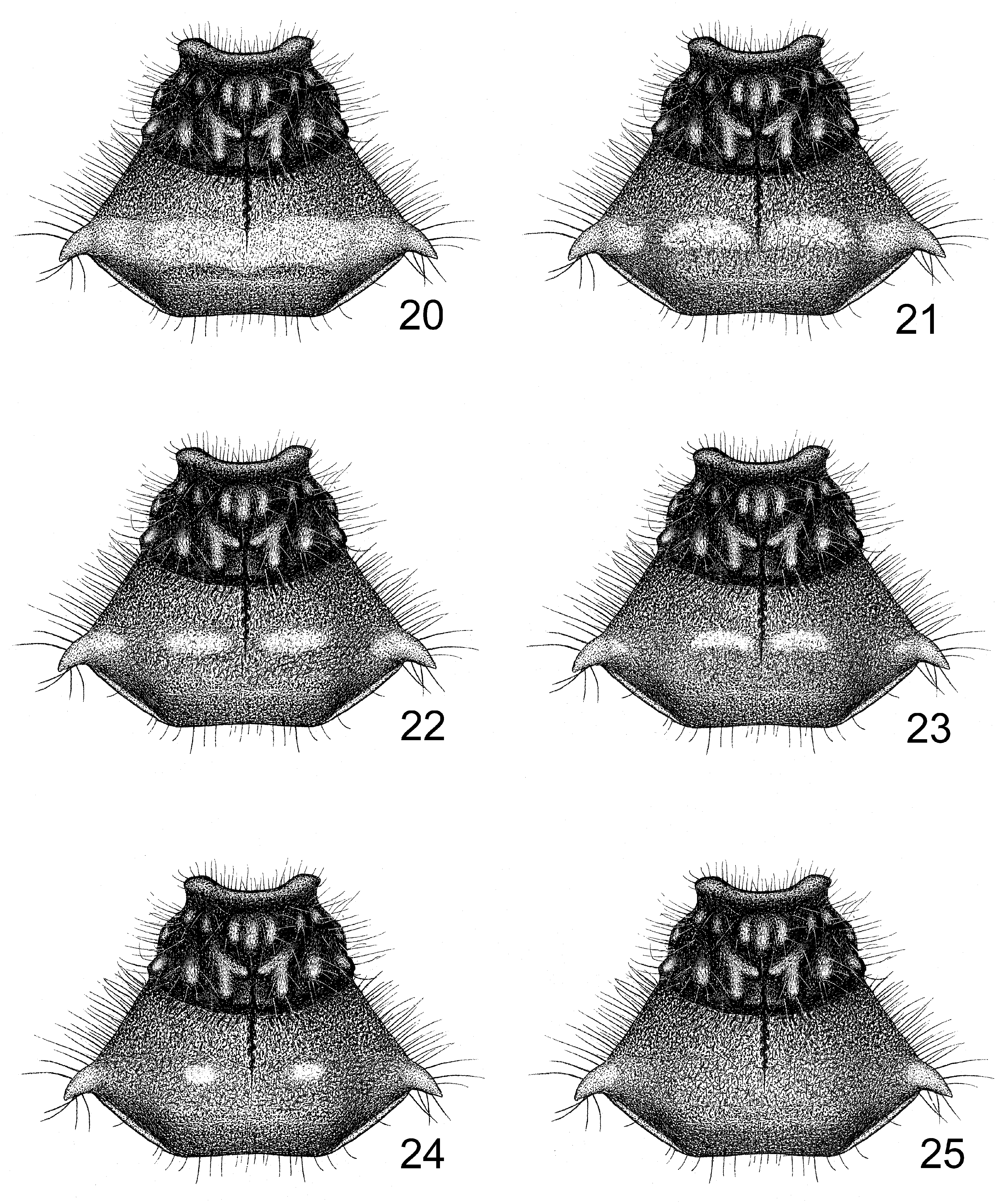
Redescription. Colouration. Black. Ocelli, spots at external side of ocelli (sometimes absent), apical half of gena, rostrum, pronotal humeral angles, spots on discal portion of posterior pronotal lobe, posterior half of each segment of connexivum, two annulations on each tibia, spots on apical portions of pro- and mesofemora, a discontiguous annulation on metafemur, basal half of third tarsomere pale yellow to yellow; eyes dark brown; distal margin of corium, membrane brown to grayish black; veins of membrane black; a long longitudinal fascia on corium white to pale yellow, with outer margin slightly pink ( Figs 1, 208 View FIGURES 208–211 ). Spots on posterior pronotal lobe varied in shape and size ( Figs 20–25 View FIGURES 20–25 ).
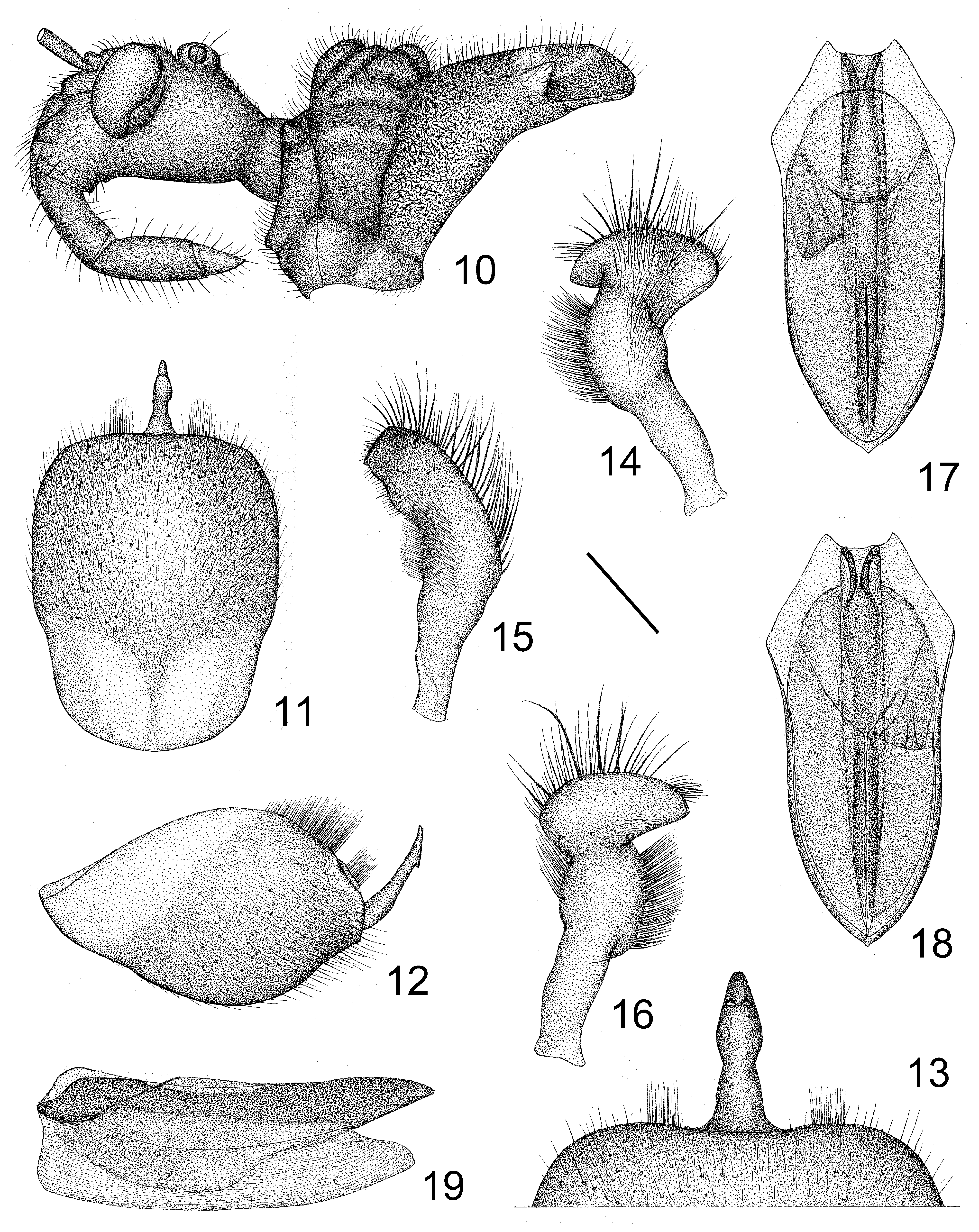
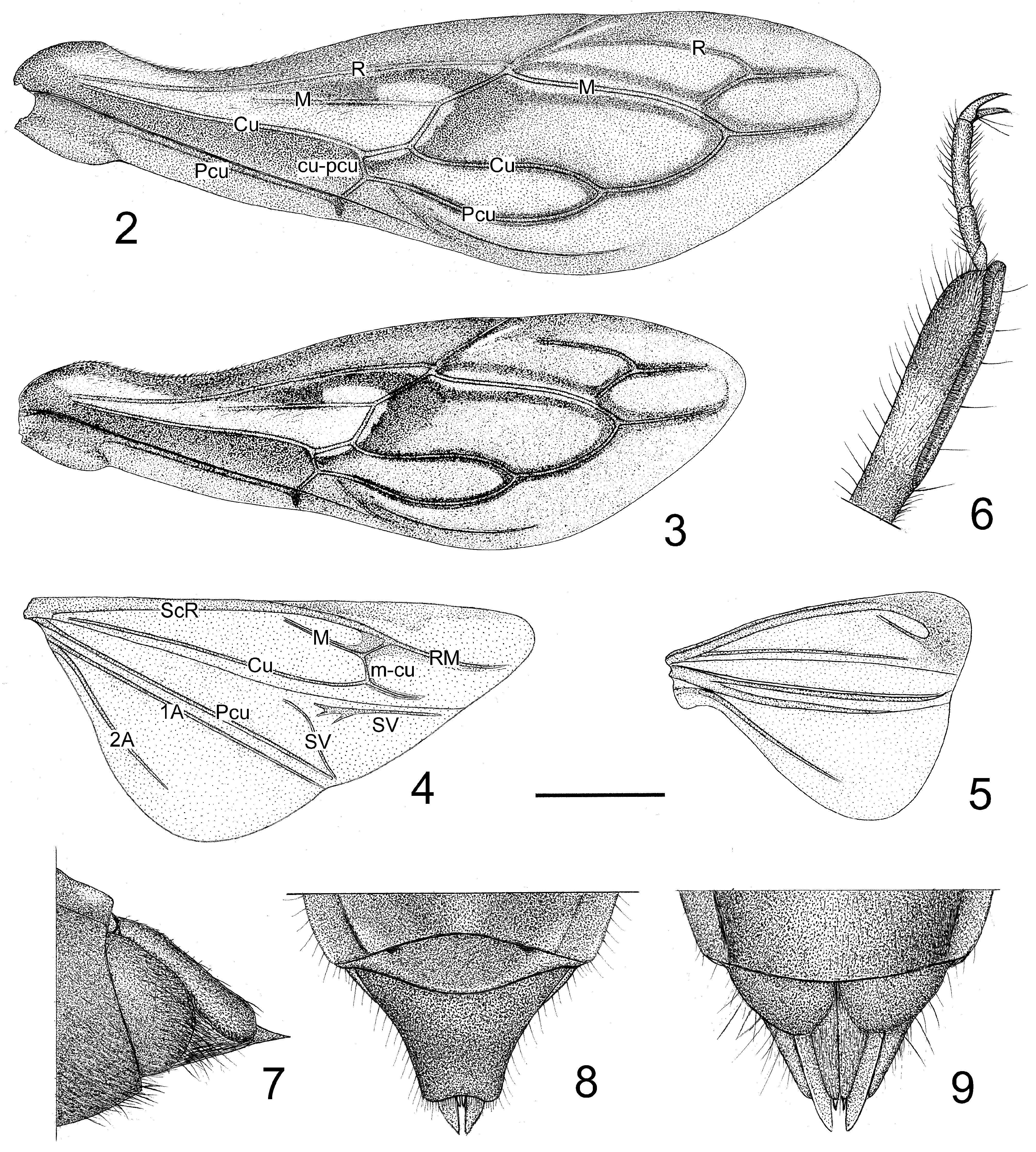
Structure. Medium sized, oblong, pilose. Thochanter and base of pro- and mesofemora ventrally clothed with dense and long setae; lateral surfaces of head, first antennal segment, pronotum, legs, and abdominal venter clothed with short and long brown setae; antennal segments 2–4 clothed with short setae; head ventrally without setae; apical spine of scutellum with several long setae; corium and connexivum above with short setae. Head elongate oblong, smooth ventrally, vertex broad; eyes large; anteocular portion shorter than postocular; deep longitudinal depression between eyes connected with transverse sulcus ( Figs 1, 10 View FIGURES 10–19 ); mandibular plate anteriad to antennifer not higher than clypeus; maxillary plate slightly round, not very prominent; gena flat, with a sculpture between it and maxillary plate. Collar processes produced, tuberculiform; anterior pronotal lobe slightly shorter than posterior, with sculpture and inflations, posterior pronotal lobe wrinkled; pronotal humeral angles spinously produced and recurved; discal area unarmed; middle of posterior margin nearly straight ( Figs 1, 10 View FIGURES 10–19 ); scutellum concave in middle, apical process short and thick; macropterous or brachypterous, hemelytron reaching abdominal tip in macropterous males or reaching fifth or sixth abdominal tergites in brachypterous forms (more commonly in females); fore wing venation as in Figs 2 and 3 View FIGURES 2–9 . Connexivum mottled, posterior angles of second segment prominent. Median process of pygophore long, apex sharp, subapical portion with a swollen ridge downward ( Figs 11–13 View FIGURES 10–19 ); paramere basal and subapical portions attenuated, median and apical portions swollen ( Figs 14–16 View FIGURES 10–19 ); basal plate of phallus thin and short, basal plate bridge short and slender; pedicel long and thick, firm, bilaterally flattened; phallosoma oblong-ovate; dorsal phallothecal sclerite strongly sclerotized, basal part nearly V-shaped concaved, apex sharp; struts fused, reaching nearly to apex of phallosoma; asymmetrically in rest condition, endosome translucent, without any tuber, thorn or spur ( Figs 17–19 View FIGURES 10–19 ). Female with eighth abdominal tergite large, posterior margin convex; ninth and tenth tergites fused, posterior margin concave ( Fig. 8 View FIGURES 2–9 ); posterior angle of first valvifer round; apex of first valvula blunt; styloid sharp, protruding ( Figs 7, 9 View FIGURES 2–9 ).
Measurements [in mm, ♂ (n= 12) / ♀ (n=7)]. Body length 13.0–16.1/13.2–17.3; maximum width of abdomen 3.5–4.6/3.8–5.6; head length 2.1–2.4/2.3–2.9; length of anteocular part 0.8–1.0/0.9–1.3; length of postocular part 0.8–0.95/0.91–1.25; length of synthlipsis 0.7–0.8/0.8–0.95; interocellar space 0.13–0.2/0.15–0.2; length of antennal segments I–IV= 1.1–1.4/1.3–1.5, 2.2–2.5/2.4–2.6, 3.6–4.0/3.6–4.2, 2.3–2.8/2.4–2.8; lengths of visible rostral segments I–III= 0.7–0.8/0.75–0.85, 0.7–0.75/0.7–0.85, 0.4–0.45/0.5–0.6; length of anterior lobe of pronotum 1.2–1.5/1.4–1.7; length of posterior lobe of pronotum 1.5–1.8/1.5–1.9; maximum width of thorax 3.2–3.9/3.4–4.2; length of scutellum 1.2–1.4/1.1–1.3; length of hemelytron 8.3–9.8/5.3–7.1.
Type material. Syntype (s), from “ India Orientalis” [= East India], preserved in the Zoologisches Museum , Humboldt-Universität, Berlin, not examined .
Other specimens examined. 1 ♂, “ China, Beijing, Miyun, Xiaoshijian ; 22.VII.2006; Chen Jin” ( CAU) . 1 ♂, “ China, Beijing, Baiwangshan Park ; 10.IX.2006; Lv Yuan” ( CAU) . 1 ♂, “ China, Beijing, Baiwangshan Park ; 4.VIII.2006; Wang Yan” ( CAU) . 1 ♂, “ China, Shannxi, Yangling ; 3.VIII.1993; Cai Wanzhi” ( CAU) . 1 ♂, “ China, Yunnan, Xishuangbanna, Mengyang , Yexianggu; 27.V.2006; Wang Hesheng” ( CAU) . 1 ♀, “ China, Beijing, Yanqing, Songshan ; 4.VIII.2005; Li Tingjing” ( CAU) . 1 ♀, “ China, Beijing, Changping, Heishanzhai ; 4.IX.2007; Li Yuanyuan” ( CAU) . 2 ♂, “ China, Shanxi, Linfen ; 15.VIII.1987; Zhang Xiaochun” ( CAU) . 1 ♂, “ China, Beijing, Yangtaishan ; 3.IX.2003; Duan Shengnan” ( CAU) . 1 ♂, “ China, Beijing, Jinshan ; 16.VII.1992 ” ( CAU) . 1 ♂, “ China, Beijing, Haidian, Jiufeng ; 8.VIII.2006; Li Hu” ( CAU) . 1 ♂, “ China, Beijing, Haidian, Yaozhiyuan ; 11.VIII.2006; Cui Jianxin” ( CAU) . 1 ♀, “ China, Beijing, 1998-VII-6 ”; “ Acanthaspis cincticrus Stål, Det. W. Cai, 1997 ” ( BMNH) . 1 ♀, “ Pekin [= Beijing, China]”; “Fallou”; “Atinson Coll. 92-6” ( BMNH) . 1♂, “ Pekin [= Beijing, China]”; “25”; “Fallou”; “Atinson Coll. 92-6”; “ Acanthaspis cincticrus Stål , Nevis (?).” ( BMNH) . 2 ♀, “N. China, Shantung [= Shandong]”; “Distant Coll. 1911-383” ( BMNH) . 1 ♀, “45”; “ Acanthaspis cincticrus Stål, det. R. J. Izzard, 1935 ” ( BMNH) . 1 ♂, “ Japan ”; “Distant Coll. 1911-383” ( BMNH) . 1 ♂, “ Japan ”; “14”; “ Acanthaspis cincticrus Stål ” ( BMNH) . 1 ♂, “ India: Behar, Pusa ; 30.vi.1916; The Gov. Entomologist”; “Pres. by Imp. Bur. Ent. Brit. Mus. 1912-191”; “ Acanthaspis cincticrus Stål ” ( BMNH) . 1 ♀, “N. India / 48, 13L”; “ cincticrus Stål ”; “31. Acanthaspis cincticrus Stål ” ( BMNH) . 1 ♀, “ India, Kumaon , Haldwani Dist., H. G. C.” ( BMNH) . 1 ♀, “ E. Garo [in Meghalaya, India], 16.v.(19)02(?), 500, Ch???”; “Distant Coll. 1911-383”; “ cincticrus Stål ” ( BMNH) . 1♀, “ Pusa , Bihar, at light; 17.VI.(19)18, C. S. Misra ” ( BMNH) .
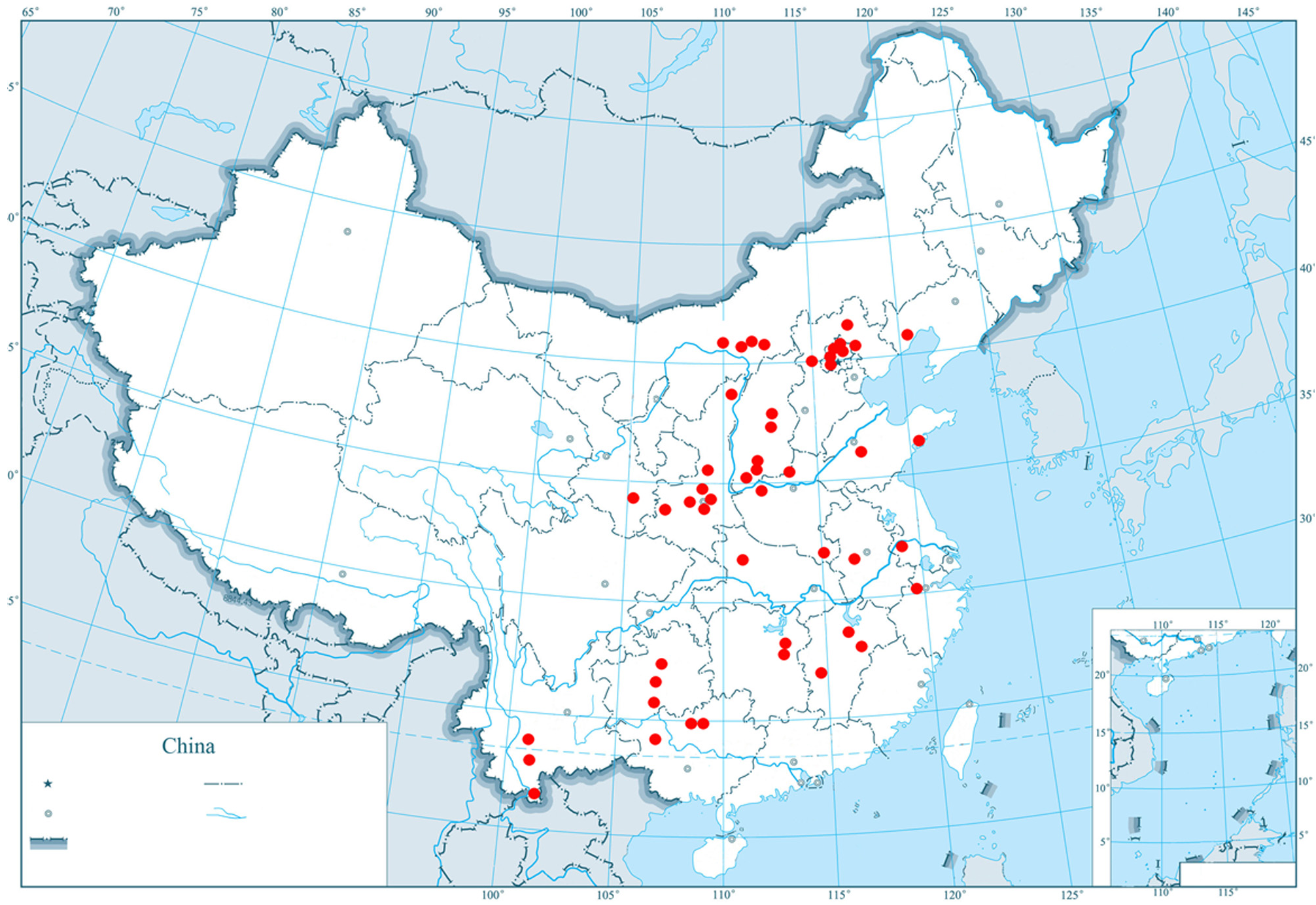
Distribution in China. Liaoning (Jianchang), Inner Mongolia (Hohhot, Baotou, Qahar Youyi Qianqi, Tumd Zuoqi), Beijing (Miaofengshan, Dazhaoshan, Xishan, Juyongguan, Sanbao, Badaling, Wofusi, Qinglongxia), Hebei (Chengde, Xiaowutaishan, Wulinshan, Yuxian), Shanxi (Linfen, Taiyuan, Yuncheng, Taigu, Yuanqu), Shaanxi (Fugu, Yanan, Tongchuan, Fengxian, Wugong, Lintong, Changan), Shandong (Qingdao, Taishan, Laoshan), Henan (Huixian, Xinan, Jigongshan), Jiangsu (Nanjing, Zhenze), Gansu, Anhui (Meishan), Zhengjiang (Tianmushan), Jiangxi (jingangshan, Nanchang, Guixi), Hubei (Shennongjia), Hunan (Changsha, Zhuzhou), Guizhou (Guiyang, Meitan, Wangmo), Guangxi (Guilin, Baise, Huanjiang), Yunnan (Jinggu, Xishangbanna, Xinping) ( Fig. 26 View FIGURE 26 ).
Distribution outside China. India, Japan, Korea ( Maldonado-Capriles 1990), Myanmar.
Biology. The species is univoltine, living under boulders near ant nests. This species oviposits eggs in October to November and overwinters as eggs in the surface layer of soil ( Fig. 27 View FIGURES 27–29 ). The shell of the newly laid egg is white, and turns brown about 20 hours later ( Fig. 29 View FIGURES 27–29 ), its operculum is white with meshy protuberance ( Fig. 28 View FIGURES 27–29 ). The eggs will hatch in later May, and then nymphs develop from first to fifth instars from June to late August ( Figs 30–33 View FIGURES 30–34 ). The newly moulted nymph is whitish, and turns dark a few hours later ( Fig. 34 View FIGURES 30–34 ). The peak of emergence of adults occurs in early and mid-August, in early September the population is composed mostly of adults. The newly hatched adult is reddish and always stays near the exuvium until it become melanized ( Figs 35–36 View FIGURES 35–36 ). In the field, copulating adults ( Fig. 37 View FIGURES 37–38 ) were observed in September, oviposition in late September and early October. Under the laboratory condition, the eggs can hatch from late of October to early January of next year.
Nymphs and adults of this assassin bug apparently exclusively feed on ants. They usually can be found in the vicinity of ant nests, waiting for their preys. Young nymphs always feed on smaller ants, older nymphs and adults can easily overcome larger ants ( Figs 38 View FIGURES 37–38 , 39 View FIGURES 39–40 ). According to our observations in Beijing, we found that they prey mostly on four species of ants, Camponotus japonicus Mayr , Odontomachus monticola Emery , Monomorium chinense Santsch , and Formica cunicularia (Latreille) .
Nymphs of Acanthaspis cincticrus exhibit remarkable camouflaging behavior. They camouflage themselves with a range of materials found in their environment. The camouflaging cover often consists of two layers. The first layer originates from the habitat they live in, consists of small soil and substrate particles; this layer is referred as “natural camouflaging” ( Ambrose 1999) or “dust coat” (Brand & Mahsberg 2002) in the literature. The second layer consists of corpses of ants the nymph has fed on and other materials found in their environment ( Fig. 34 View FIGURES 30–34 ).
Remarks. The species is relatively widely distributed in southern and southeastern Asia, from India to China, extending to Japan and Korea. It is easy to distinguish from other species of Acanthaspis by its black ground colour with an oblique long and white longitudinal fascia on the corium. The Indian individuals are slightly different from those from China, Japan and Korea in body size, colour patterns on hemelytron, structures of pronotum and male genitalia. Both male and female adults from India are distinctly smaller than those from China, Japan and Korea; the oblique longitudinal stripe on Indian individuals is wider, nearly straight, with apex rounded (vs. the stripe thinner, bent apically in Chinese, Japanese and Korean individuals); the inflations on anterior pronotal lobe are less developed than those on Chinese, Japanese and Korean individuals; the phallus is slightly wider than those in Chinese, Japanese and Korean individuals. But these differences are not enough to separate them into two species in our opinion. The measurements are based on Chinese, Indian, Japanese and Korean individuals. The population genetic study is needed to judge their diversification.
| CAU |
China Agricultural University |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Acanthaspis cincticrus Stål, 1859
| Cao, Liangming, Rédei, Dávid, Li, Hu & Cai, Wanzhi 2014 |
Acanthaspis albovittata
| Fukui, T. 1927: 3 |
| Matsumura, S. 1907: 141 |
Acanthaspis humeralis
| Matsumura, S. 1905: 27 |
Acanthaspis cincticrus Stål 1859: 188
| Tomokuni, M. & Cai, W. 2002: 106 |
| Putshkov, P. V. & Putshkov, V. G. 1996: 186 |
| Lee, C. E. & Kwon, Y. J. 1991: 20 |
| Maldonado-Capriles, J. 1990: 384 |
| Hsiao, T. Y. & Ren, S. Z. 1981: 457 |
| Hsiao, T. Y. 1976: 80 |
| Hoffmann, W. E. 1944: 17 |
| China, W. E. 1940: 252 |
| Ishihara, T. 1937: 728 |
| Yamada, M. 1936: 21 |
| Wu, C. F. 1935: 17 |
| Doi, H. 1933: 90 |
| Oshanin, B. 1912: 50 |
| Lethierry, L. & Severin, G. 1896: 104 |
| Horvath, G. 1889: 37 |
| Reuter, O. M. 1887: 157 |
| Stal, C. 1874: 74 |
| Stal, C. 1866: 243 |
| Stal, C. 1859: 188 |