Phagocata armeniaca ( Komárek, 1916 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4969.2.4 |
|
publication LSID |
lsid:zoobank.org:pub:3B93D7A3-5624-4EA0-8EBE-9814386FF44E |
|
DOI |
https://doi.org/10.5281/zenodo.4784190 |
|
persistent identifier |
https://treatment.plazi.org/id/03E95D02-FFF7-FFA7-C8F4-FA791A01FA84 |
|
treatment provided by |
Plazi |
|
scientific name |
Phagocata armeniaca ( Komárek, 1916 ) |
| status |
|
Phagocata armeniaca ( Komárek, 1916)
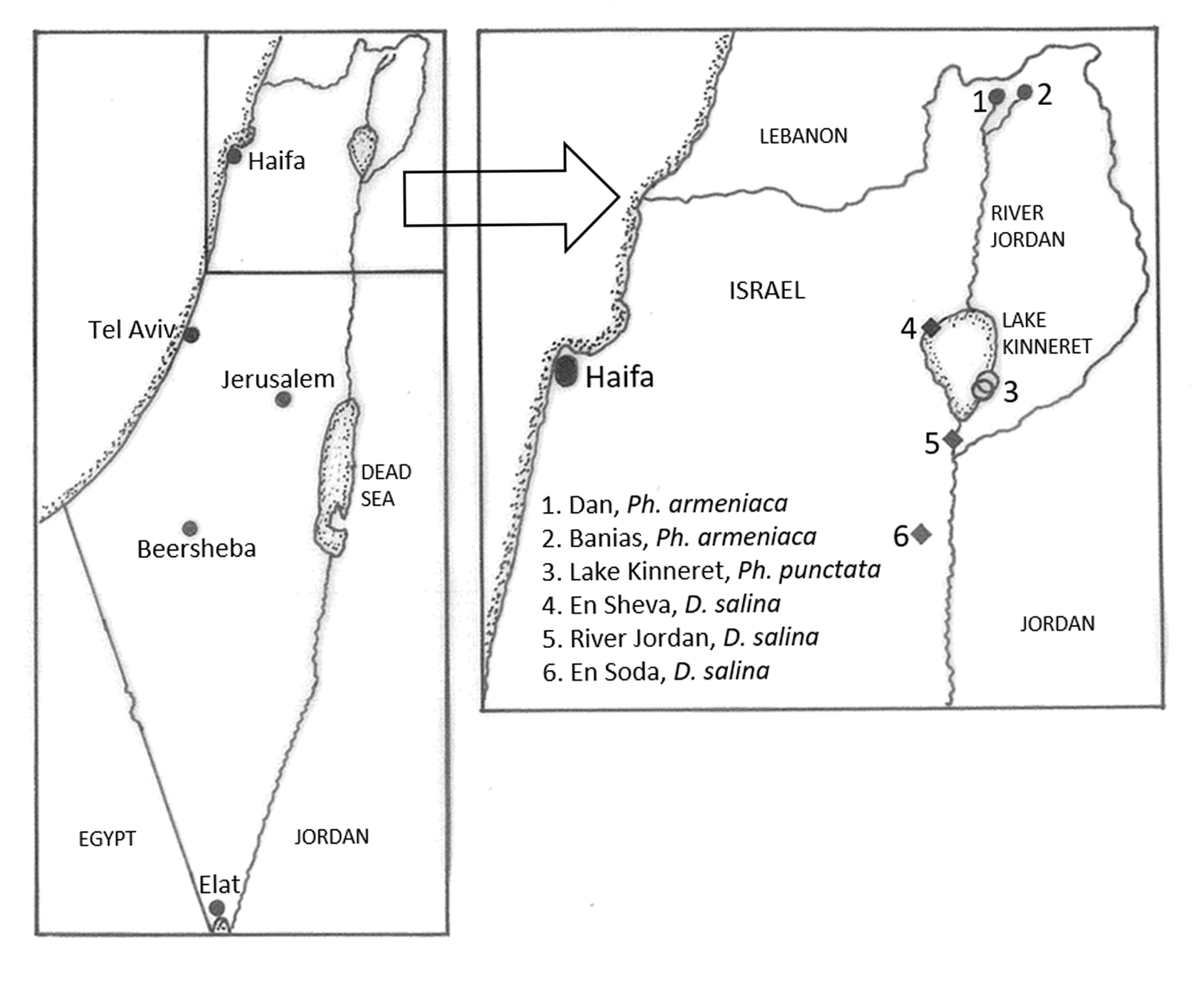
( Figs 1 View FIGURE 1 , 2B View FIGURE 2 , 6 – 8 View FIGURE 6 View FIGURE 7 View FIGURE 8 ; Tables 1 – 5 View TABLE 1 View TABLE 2 )
Material examined. Material was collected from the spring sites of Dan, N 33.248824 E35.652379 and Banias, N33.248663048 E 35.695301297 in northern Israel GoogleMaps . Slide preparations examined were from Banias as follows: HUJ. Ban.3, sagittal sections on 4 slides; HUJ.Ban.4, transverse sections on 7 slides; HUJ.Ban.5, transverse sections on 15 slides; HUJ.Ban.6, sagittal sections on 5 slides. From laboratory culture, collected in the field, June 1972. Material deposited in the Zoological Collections of the Department of Ecology , Systematics and Evolution, Hebrew University, Jerusalem, Israel .
Habitat. The spring habitats of both Dan and Banias in northern Israel, where Ph. armeniaca was found, have water of an almost constant temperature of around 15–16 oC and the average salinity was 8–12 mg /l Cl´ at Dan and 12–16 mg /l Cl´ at Banias. The water is mainly fast flowing over a stony substrate, but some stones were partly submerged in sand or silt. The water depth where the triclads were found was usually in the range of 10 to 30 cm. Dan is a large complex of springs and streams and there are areas of slower flowing water adjacent to the main river. Other Tricladida found at these sites were Dendrocoelum dani Bromley, 1982 , Dugesia golanica Bromley & Benazzi, 1991 and Dugesia biblica Benazzi & Banchetti, 1973 , now considered to be a junior synonym of Dugesia sicula Lepori, 1948 ( Solà et al. 2015).
Description. Comprehensive descriptions of Ph. armeniaca have been made by both Komárek (1916) and by De Beauchamp (1958). The description following here is intended to point out several differences in the reproductive apparatus between the two Phagocata species found in northern Israel. Table 1 View TABLE 1 shows a comparison of the external morphology of the two species. Many of the internal features are shared by both species, and in order to avoid unnecessary repetition, the description will be compared with that of Ph. punctata in some cases.
The pigmentation of Ph. armeniaca is seen dorsally as numerous granules scattered throughout the mesenchymal cells underlying the dorsal muscle layer, and fewer granules in the ventral mesenchyme.
The ovaries are situated ventrally, just behind the brain, at about 1/8 th of the distance between the brain and the root of the pharynx. The pharynx is composed of two muscle layers in the outer muscle sheet. The outer, longitudinal layer is about 2.5 µm thick and the inner, circular layer is about 7.5 µm thick. The mouth is located just posterior to the hind end of the pharyngeal pouch.
Cyanophilous glands are abundant, especially in the anterior quarter of the body, dorsally and ventrally, between the gut branches and the mesenchymal cells internal to the body wall musculature. They are also very prominent anterior to the eyes, in a median region between the eyes and the brain, as well as in the central region between and behind the ovaries, extending back as far as the first and second testes. They were also seen in the central body region immediately anterior to the pharynx insertion.
The female reproductive system is essentially similar to that described above for Ph. punctata .
The position and extent of the testes are similar in both species of Phagocata . However, there are significant differences in the details of the male reproductive systems between the two species.
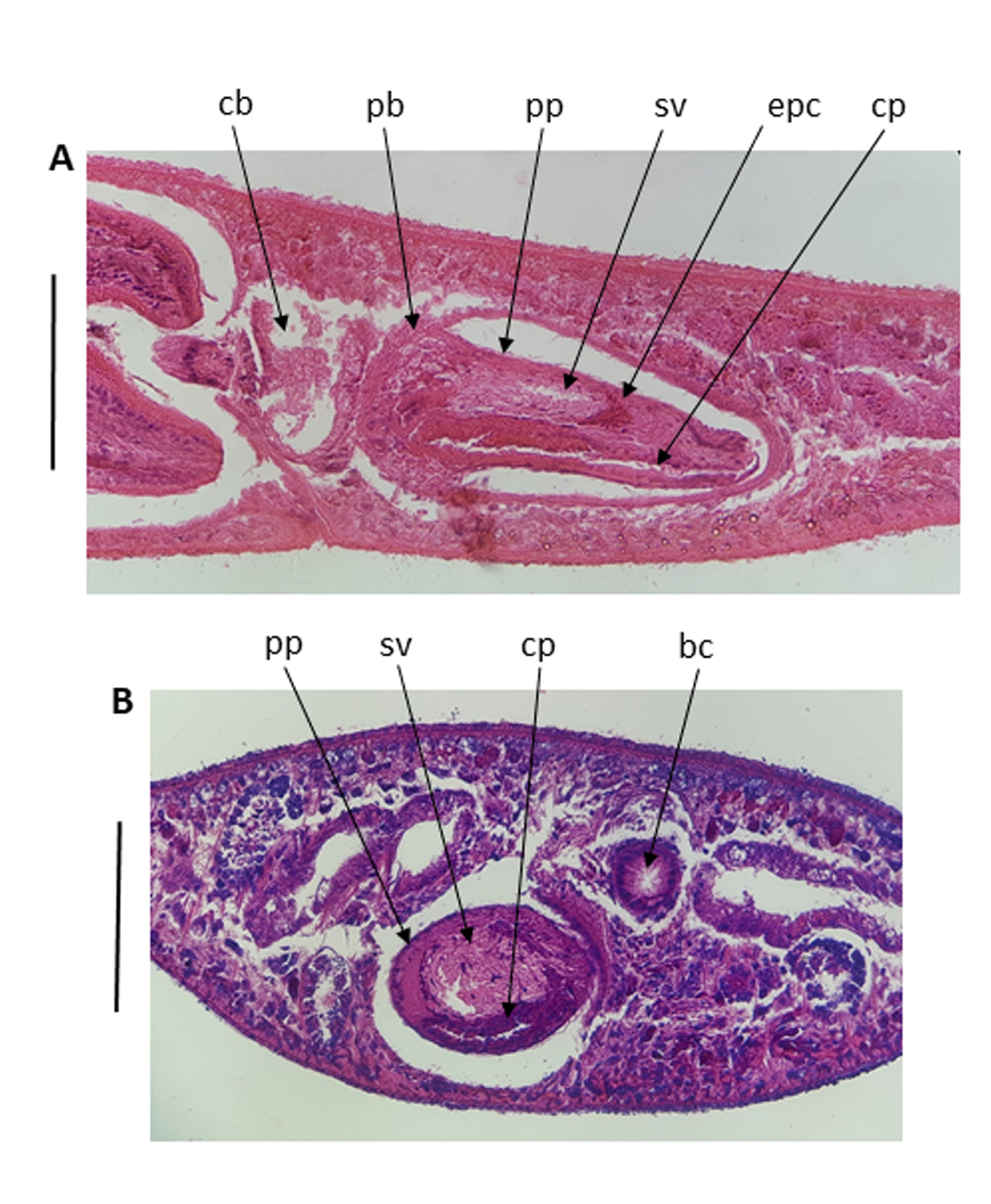
Ph. armeniaca has a small penis bulb, while the papilla is large, cylindrical and pointed, with a ring of circular muscle almost at the tip. The vasa deferentia enter the lateral walls of the penis bulb. The seminal vesicle is represented by a wide tube, lined by very tall cells, which almost fill the lumen of the penis bulb and anterior part of the papilla where the two vasa deferentia enter. The seminal vesicle is constricted about 2/3 along its length by an eosinophilous plug of cells which apparently separate it from the ejaculatory duct, which opens terminally ( Figs 6 View FIGURE 6 , 7 View FIGURE 7 ).
Similar to that described above for Ph. punctata , the penis of Ph. armeniaca has a blind-ending, kidney-shaped caecum lying ventrally to the ejaculatory duct in the penis papilla. The caecum extends back the whole length of the penis papilla and slightly into the penis bulb, remaining narrow for its whole length. The lumen of the caecum and of the ejaculatory duct become continuous at the tip of the penis papilla in both species.
Karyology of Ph. armeniaca . Mitotic metaphase plates were studied in five specimens, and 17 pairs of chromosomes were clearly counted in 25 metaphase plates. Figure 8 View FIGURE 8 shows three sets of chromosomes. The first pair of chromosomes is significantly larger than all the others, and is about twice the length of pair 2. Number 3 is noticeably smaller than number 2 and pairs 4 to 17 decrease gradually in size, although several pairs are indistinguishable. Pair 17 is about 1/5 th the size of pair one.
Chromosome 1 is large and metacentric with the highest centromeric index value. Chromosomes 2, 3, 4, 7 and 11 are all submetacentric. All other chromosomes are metacentric or borderline metacentric-submetacentric. The standard deviations for the centromeric indices are rather high in many cases.
Tables 2 View TABLE 2 and 3 show a comparison of mean values and standard deviations of the relative lengths and of centromeric indices of both species of Phagocata .
Reproductive behaviour of the two Phagocata species. Collections of Ph. armeniaca at all seasons from both
Dan and Banias included young, immature as well as larger, sexually mature animals. At Banias, where the population was larger and easier to monitor, cocoons were found on stones bearing mature animals during both summer and winter. The cocoons were round to oval in shape, unstalked and attached to the substrate such that the long axis of an oval cocoon was always parallel to the substrate. No evidence of fission was ever seen and presumably sexual reproduction occurs throughout the year.
Ph. punctata was first discovered in Lake Kinneret during the spring and summer of 1972 (April to September) when only asexual animals were found with no evidence of fission having occurred. The specimens were up to 7 mm in length, a size normally associated with mature specimens of Ph. armeniaca . However, during the winter months (November to March), both immature and sexually mature animals were found, and on a few occasions, cocoons were found on stones also bearing mature planarians. It thus appears that conditions suitable for sexual reproduction of Ph. punctata occur only when the water temperature is 20 oC or lower.
In the laboratory, both species of Phagocata were very easy to maintain and breeding was established at 18 oC. Asexual reproduction was never observed although the regenerative powers of both species, when cut, was good. Comparative data on cocoons and emergence of young at 18 oC is given in Table 4 View TABLE 4 .
The cumulative rate of cocoon production of 10 animals of each species, kept separately, was followed for 100 days at temperatures of 15 oC, 18 oC and 20 oC rising gradually to 25 oC. In both species, cocoon production was greatest at 18 oC, lowest at 15 oC (the temperature at Banias) and an intermediate rate at 21–23 oC, which was the first phase of the high temperature period ( Table 5 View TABLE 5 ). Cocoon production slowed down greatly or stopped and cocoons already deposited did not hatch at temperatures above 23 oC ( Ph. armeniaca ) and 25 oC ( Ph. punctata ) at which temperature some animals of both species began to break up. By day 100, the cumulative egg production of the Ph. punctata animals was approximately twice that of the Ph. armeniaca animals, and that of the F 1 cross was intermediate ( Table 5 View TABLE 5 ).
The following observation indicates that the induction of sexual maturity in asexual animals of Ph. punctata is temperature dependant. Asexual animals were collected from the field in May, 1973, when the water temperature was 25 oC. Half the animals were cultured at 18 oC and the others at room temperature of 20–28 oC. After 3 months, the 18 oC animals were all sexually mature and depositing cocoons, while none of the room temperature animals had matured. When the latter were transferred to 18 oC, they matured within a few weeks.
Cross-breeding experiments. Since the morphology and karyology of both species of Phagocata were very similar, an attempt was made to cross-breed them. Immature animals of each species were raised in isolation to maturity at 18 oC and then one specimen of each, per jar, was cultured together. Both parental types deposited fertile cocoons. The cocoons were often larger than either parental type (ranging from 1.2 x 0.9 to 1.5 x 0.9 mm), and the number of young emerging was generally higher (5–17 per cocoon, usually 7–10). The hatching time was approximately the same (16–21 days) as was the range in size of emerging young (0.5–2.75 mm).
Most of the F 1 animals were normal and healthy and the examination of 100 animals showed a range of gut branching patterns, i.e., fairly branched like Ph. armeniaca , fairly sparse like Ph. punctata , or intermediate (in a ratio of 2:2:1). Likewise, after a few days when pigmentation patterns became clear, the range of patterns of the F 1 animals was noted, with some unspotted, some definitely spotted and the majority very slightly spotted, (in a ratio of about 1:1:3). However, later examination showed that very few animals acquired the full spotted pigmentation patterns typical of adult Ph. punctata , thus the ratio changed to 4:1:7 (unspotted like Ph. armeniaca , definitely spotted like Ph. punctata and intermediate).A small percentage of the F 1 animals (about 0.5%)were joined at the head or anterior body region like “Siamese twins”. Such abnormalities were never observed among the pure-bred offspring of either parental type.
Some of the F 1 animals, with a random assortment of pigmentation patterns were left to grow to maturity, and at 7–8 weeks old, the first cocoons were deposited. Cumulative cocoon production of 10 mature F 1 animals was also determined at various temperatures over a period of 100 days as with the pure-bred animals described above. The results are shown in Table 5 View TABLE 5 , and indicate a cocoon production at all temperatures intermediate between those of the two parent types. The F 2 animals showed a range of pigmentation from brown, to brown speckled, pale grey and clearly grey spotted. From the 117 cocoons that hatched during the experimental period, 1065 young were produced, an average of 9.1 per cocoon. Most were normal, but there were 5 “Siamese twins” amongst them.
A separate set of F 1 animals, with a range of pigmentation patterns, was kept for observation and breeding at 18 oC. All animals were mature and measured 8–11 mm in length. 219 cocoons were deposited producing 1279 young (average 5.8 young per cocoon). Again, most of the F 2 offspring (89%) were normal, while the remaining 11% included 15 “twins” representing 1% of the total, as well as a new type of abnormality, small round or oval, pale brown “monsters”, 0.5–1.5 mm in length. There were 128 of these monsters (10% of the total F 2 offspring); they were mainly 2-eyed, with no pigment-free area around the eyes; one had a single eye, one had five eyes; they apparently had a degenerate pharynx and could not eat or grow, and after 15 to 30 days of gliding around the culture jars, they died.
Discussion. While the external features and the habitats of the two species of Phagocata from Israel are very different and completely isolated from each other in nature, it is clear from the above morphological considerations that there is a very close relationship between Ph. armeniaca and Ph. punctata . However, although they share the presence of a penial caecum, they can easily be distinguished by the significant differences in the shape of the both the penis bulb and the penis papilla: large bulb and a short conical papilla in Ph. punctata and small bulb and a large cylindrical papilla in Ph. armeniaca .
Tables 2 View TABLE 2 and 3 show the close karyological similarity between the two species. The relative lengths of the chromosomes in the two species are very similar. The main differences are in the centromeric indices of chromosomes 3 and 10. Chromosome 3 in Ph. armeniaca is clearly submetacentric while in Ph. punctata it is submetacentric bordering on subtelocentric; chromosome 10 in Ph. armeniaca is metacentric, while in Ph. punctata it is submetacentric. In addition, two of the metaphase plates examined in detail for Ph. punctata showed the presence of a small supernumerary chromosome, but there is not enough data to know if this is a constant feature.
The taxonomic position of Ph. armeniaca with regard to other European representatives of the genus was discussed in detail by De Beauchamp (1958). It is noteworthy that De Beauchamp pointed out that in the original description of the penis of Ph. armeniaca by Komárek (1916), he mistakenly believed that the caecum was in fact one of the vasa deferentia, and that both ran independently through the length of the penis. He also notes that Komárek’s material was collected from stenothermic mountainous springs and streams in Armenia, perhaps not too dissimilar to the cold streams of Dan and Banias in Israel. In contrast, De Beauchamp’s material was collected from lakes in eastern Turkey and presented a quite different type of habitat. He suggested that the planarians living in the lakes may become sexually mature either in colder springs nearby or only in the winter. This is the situation with Ph. punctata , which becomes mature in Lake Kinneret only at winter temperatures lower than about 20 oC.
The systematics of the genus with reference to five new pigmented species from Spain have been discussed more recently by Sluys et al. (1995) and Vila-Farré et al. (2011). Most animals now assigned to the genus Phagocata are small white animals with a truncated head and include the former junior synonyms of Albiplanaria Komárek, 1926 , Fonticola Komárek, 1926 , and Penecurva Livanov & Zabusova, 1940 ( Sluys et al. 1995). However, apart from Ph. armeniaca , there are several other pigmented species of Phagocata in Europe, including the five from Spain ( Ph. ullala Sluys, 1995 , Ph. flamenca Vila-Farré & Sluys, 2011 , Ph. asymmetrica Vila-Farré & Sluys, 2011 , Ph. gallaeciae Vila-Farré & Sluys, 2011 , Ph. pyrenaica Vila-Farré & Sluys, 2011 ) as well as the endemic species of Lake Ohrid, Ph. maculata (Stanković, 1938) . These six pigmented species are all quite distinct from Ph. armeniaca and Ph. punctata regarding the penis papilla, which in both these latter species have a blind-ending caecum opening into the distal part of the ejaculatory duct. The only other Phagocata species known to have such a diverticulum is the pigmented North American Ph. velata (Stringer, 1909) , which differs from the Israeli species in other morphological features and especially in its karyological pattern of 60 to 96 chromosomes, corresponding to different degrees of polyploidy ( Whitehead 1965).
The number of chromosomes for several species of Phagocata have been described (see Sluys et al. 1995). Among these, Ph. vitta (Dugès, 1830) and Ph. paravitta (Reisinger, 1923) , both unpigmented species, may have a diploid complement of 34, like Ph. armeniaca and Ph. punctata , although Ph. vitta may also be polyploid. The pigmented species, Ph. ullala and Ph. pyrenaica also have a diploid complement of 34 chromosomes, and like Ph. vitta , as well as Ph. armeniaca and Ph. punctata , the first pair of chromosomes are about twice the length of the second pair, acting as marker chromosomes ( Sluys et al. 1995). However, both Ph. ullala and Ph. pyrenaica have chromosome pairs 2 and 3 of about the same length. In contrast, with both Ph. armeniaca and Ph. punctata , chromosome pair 2 is clearly longer than pair 3 (see Table 2 View TABLE 2 ).
The differences in reproductive behaviour of the two Phagocata species is probably a reflection of the different field conditions. At each temperature, the cocoon production of Ph. punctata was approximately twice as high as that of Ph. armeniaca , which may indicate a higher fecundity of the animals in Lake Kinneret under field conditions, possibly as an adaptation to the shorter breeding period in nature during the winter months.
The cross-breeding experiments showed that despite the morphological differences of the two types of Phagocata , they can, under laboratory conditions, produce mostly fertile offspring. However, the relatively high proportion (up to 11%) of abnormalities amongst the F 2 animals indicates an incomplete reproductive compatibility, favouring the separation at species level. Dahm (1958, page 117) has noted that “The abundance of evidently near-related but still isolated forms (of triclads) is a feature characterizing those animals which are said to represent “young species”, still being in a rapid stage of evolution”. It seems probable that this is the situation with the separation and speciation of the Lake Kinneret animals from the more widely distributed Ph. armeniaca stock and which has taken place in relatively recent geological times.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Continenticola |
|
Family |
|
|
Genus |