Phagocata punctata, Bromley-Schnur, 2021
|
publication ID |
https://doi.org/10.11646/zootaxa.4969.2.4 |
|
publication LSID |
lsid:zoobank.org:pub:3B93D7A3-5624-4EA0-8EBE-9814386FF44E |
|
DOI |
https://doi.org/10.5281/zenodo.4749041 |
|
persistent identifier |
https://treatment.plazi.org/id/03E95D02-FFF3-FFAE-C8F4-FF021A90FEEC |
|
treatment provided by |
Plazi |
|
scientific name |
Phagocata punctata |
| status |
sp. nov. |
Phagocata punctata sp. nov.
( Figs 1 View FIGURE 1 , 2A View FIGURE 2 , 3 – 5 View FIGURE 3 View FIGURE 4 View FIGURE 5 ; Tables 1 – 5 View TABLE 1 View TABLE 2 )
Material examined. Holotype. HUJ.Kin.1, sagittal sections on 3 slides; Paratypes: HUJ.Kin.2, sagittal sections on 3 slides; HUJ.Kin.3, transverse sections on 7 slides. From laboratory culture, collected in the field, June 1972. Material deposited in the Zoological Collections of the Department of Ecology , Systematics and Evolution, Hebrew University, Jerusalem, Israel .
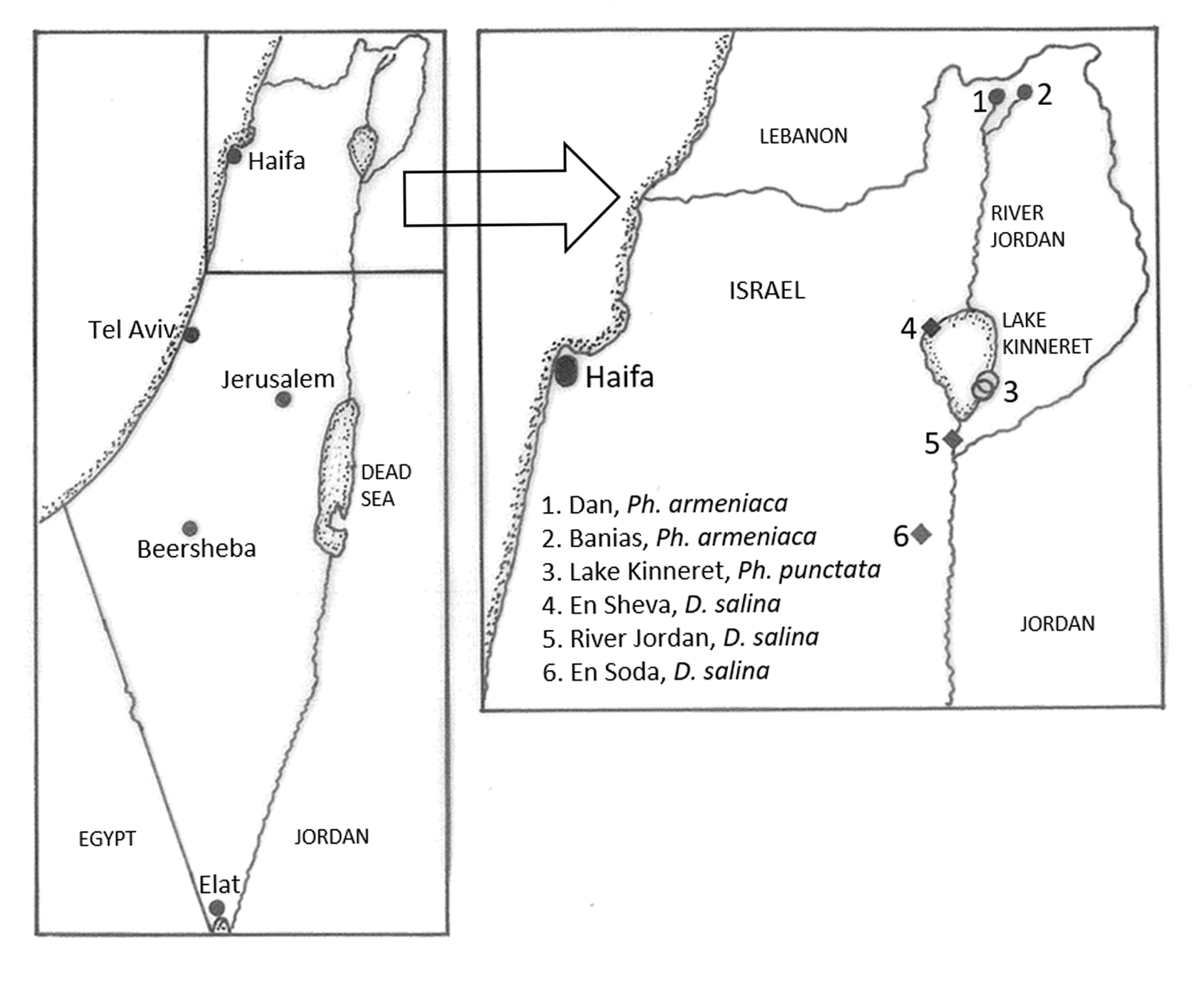
Type locality. Two adjacent sites in the littoral water off the south-eastern shore of Lake Kinneret: N32.764688 E35.638202 GoogleMaps .
Habitat. The type locality of Lake Kinneret where Ph. punctata was found lies in the River Jordan Rift Valley and is presently about 209 m below sea level. Ph. punctata was usually found in clusters in small crevices on the undersides of small pitted rocks and boulders at a depth of 50 to 150 cm. These rocks typically also bore patches of a sponge, Cortispongilla barroisi (Topsent, 1892) and a bryozoan, Fredericella sultana Blumenbach, 1779 . No other triclads were found at these littoral sites.
The lake water is subject to considerable seasonal temperature fluctuations of between 14 and 29 oC and the average salinity measured in 1972–3 was around 230–240 mg /l Cl´. However, in the past, both the water level and the salinity have fluctuated greatly, the water level ranging from a maximum of about 209 m to about 215 m below sea level ( Markel 2014) and the salinity ranging from about 200 mg /l Cl´ to a peak of 390 mg /l Cl´ in the early 1960s. In the mid-1960s, considerable amounts of saline water were diverted into a salt-water channel from western saline springs at the edge of the lake to the lower Jordan River, and subsequently, the salinity levels of the lake decreased by about 30% ( Rimmer & Nishri 2014). In 2018, after several years of drought as well as increased irrigation diversion for agriculture, historically low levels of water in the lake, to - 213 m, resulted, and the salinity again rose to almost 300 mg /l Cl´, one of its highest levels in 50 years. By spring 2020, after an exceptionally rainy winter, the lake level again rose by just over 3 m to almost - 209 m.
Diagnosis. Phagocata punctata sp. nov. is characterized by grey or grey-brown spots on a light grey background dorsally, very light or white ventrally. It has a large distinct penis bulb with a short blunt, conical papilla. There is a blind-ending caecum in the penis, lying ventrally to the ejaculatory duct, extending back into the penis bulb where it has an enlarged lumen. The caecum opens into the distal part of the ejaculatory duct. Chromosome portrait: diploid (2n = 34), with the first chromosome pair about twice as long as the second pair.
Etymology. The specific epithet refers to the spotted or speckled appearance of the new species.
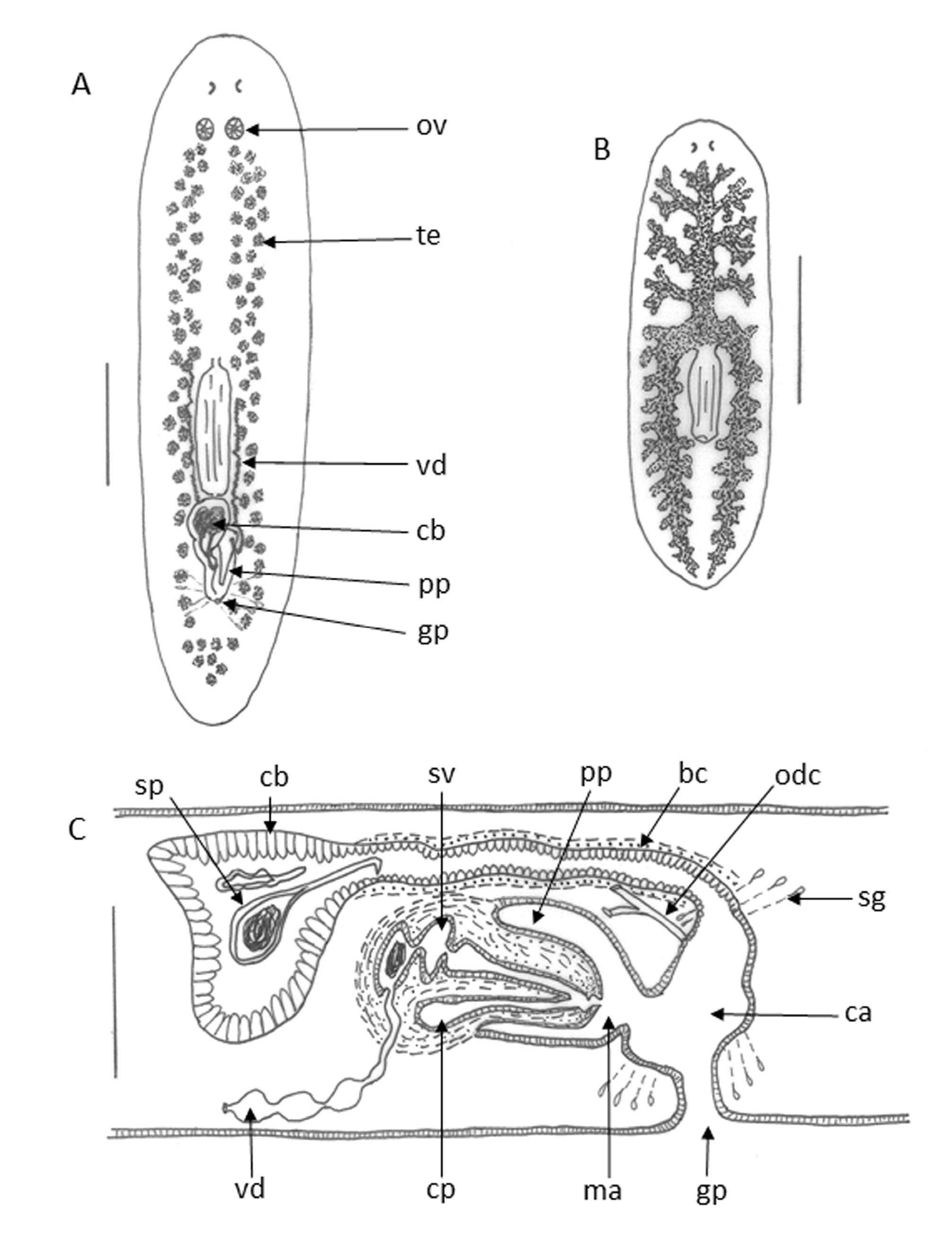
Description. The main external features of Ph. punctata are shown in Table 1 View TABLE 1 , where they are compared with those of Ph. armeniaca , a description of which follows that of Ph. punctata .
There are numerous small pigmentation granules beneath the basement membrane of the dorsal epithelium and large intermittent clusters of pigment underlying the dorsal musculature. The pharynx is composed of two muscle layers in the outer muscle sheet. Both the outer, longitudinal layer and the inner circular layer are about 2.5 µm thick. Cyanophilous glands are seen in the head region under the dorsal musculature, extending posteriorly about halfway to the pharynx insertion and anteriorly to the tip of the head. They are well developed ventrally in the median body region and also around the ovaries where they extend to the dorsal body wall. There are also many cyanophilous glands in the region of the pharynx insertion. The mouth opens just beyond the posterior end of the pharyngeal pouch.
The ovaries are situated ventrally, just behind the brain, at about 1/6 th of the distance between the brain and the root of pharynx, after the first main lateral digestive branch. They are round to oval in shape, measuring 100–175 µm dorso-ventrally and 100–120 µm antero-posteriorly. The oviducts leave the ovaries from a mid-lateral position and lie above the ventral nerve cords. The right oviduct passes on the right side of the penis while the left one lies between the penis and the bursal canal. The common oviduct enters the bursal canal dorsally, near its junction with the male atrium.
The testes have a ventral position, but may extend into the dorsal part of the body as well. The testes extend from the posterior level of the ovaries almost to the tip of the tail. They are round to pear-shaped, 150–200 µm in diameter, lying in two rows, one on either side of the body.
The copulatory bursa is large, sacciform and thin-walled, measuring 200–300 µm dorso-ventrally and 200–400 µm antero-posteriorly. It often contains one or more spermatophores. The spermatophore bulbs measured around 150 µm and the stalks up to 400 µm. The bursal canal exits from the dorsal posterior part of the bursa and lies dorsally, to the left of the penis. The muscle sheath of the canal is of uniform thickness and consists of a thin layer of circular muscle surrounded by an outer longitudinal muscle layer. It is lined with an epithelium of tall, columnar ciliated cells with basal nuclei. The bursal canal dilates near the atrium. The female, male and common atria are distinct. The male atrium has a dorsal epithelium of cuboid cells and a ventral epithelium of flattened cells. It is surrounded by a layer of subepithelial circular and then longitudinal muscle fibres. The female atrium lies to the left of the common atrium and as a diverticulum of it. A group of eosinophilic cells, presumed to be shell glands, enter the common atrium. There is a short gonoduct, about 100 µm in length ( Figs 3 View FIGURE 3 , 4 View FIGURE 4 ).
There is a large distinct penis bulb with a small and blunt, conical papilla. There is a ring of outer longitudinal and inner circular muscle near the papilla tip. The vasa deferentia enter the penis antero-laterally and open into a distinct round or oval seminal vesicle lined with smooth flat epithelium and surrounded by circular muscle. There is a muscular constriction in the globular seminal vesicle which partially divides the seminal vesicle into two parts. The ejaculatory duct exits from the distal part, forming a narrow tube opening terminally ( Figs 3 View FIGURE 3 , 4 View FIGURE 4 ).
The most outstanding characteristic of the reproductive system of Ph. punctata is the caecum, a blind-ending, kidney-shaped tube lying ventrally to the ejaculatory duct in the penis papilla and opening into the distal portion of the ejaculatory duct. The caecum extends back the whole length of the penis papilla and into the penis bulb where it has an enlarged lumen at its blind end. The lumen of the caecum and of the ejaculatory duct become continuous at the tip of the penis papilla.
Karyology of Ph. punctata . Thirty mitotic metaphase plates from five specimens studied revealed 17 pairs of chromosomes. Figure 5 View FIGURE 5 shows three sets of chromosomes, two of which show the presence of a small supernumerary chromosome, about ½ the size of chromosome 17. The first pair of chromosomes are significantly larger than all the others, with number 2 being approximately half the size of number 1. Number 3 is noticeably smaller than number 2 and pairs 4 to 17 decrease gradually in size, although several pairs are indistinguishable. Pair 17 is about 1/5 th the size of pair one.
Chromosome 1 is large and metacentric with the highest centromeric index value. Chromosomes 2, 3, 4, 7, 10 & 11 are all submetacentric, but number 3 is borderline submetacentric-subtelocentric. All other chromosomes are metacentric or borderline metacentric-submetacentric. The standard deviations for the centromeric indices are rather high in many cases.
Tables 2 View TABLE 2 and 3 show a comparison of mean values and standard deviations of the relative lengths and centromeric indices of both species of Phagocata .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Continenticola |
|
Family |
|
|
Genus |