Sphaeridops amoenus ( Lepeletier & Serville, 1825 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5020.3.5 |
|
publication LSID |
lsid:zoobank.org:pub:CB9F5F75-AAF9-40AB-9275-355E324E3F75 |
|
persistent identifier |
https://treatment.plazi.org/id/EF4C87CE-5F55-6A11-FF7E-FBE339490BFD |
|
treatment provided by |
Plazi |
|
scientific name |
Sphaeridops amoenus ( Lepeletier & Serville, 1825 ) |
| status |
|
Sphaeridops amoenus ( Lepeletier & Serville, 1825) View in CoL
( Figs. 1–49 View FIGURES 1–3 View FIGURES 4–7 View FIGURES 8–13 View FIGURES 14–19 View FIGURES 20–23 View FIGURES 24–30 View FIGURES 31–36 View FIGURES 37–43 View FIGURES 44–49 )
Reduvius amoenus Lepeletier & Serville, 1825: 275 . Syntype (s): Brasil [= Brazil]; NHMW.
Sphaeridops eulus Maldonado & Santiago-Blay, 1992: 509 View in CoL . Holotype: ♂, Paraguay: Horqueta ; USNM. New subjective synonym.
Sphaeridops amoenus: Amyot & Serville (1843: 382–383) View in CoL (redescription, figures), Stål (1872: 113) (checklist), Walker (1873b: 10) (catalog), Wygodzinsky (1949: 64) (catalog), Putshkov & Putshkov (1985: 99) (catalog), Maldonado (1990: 490) (catalog), Maldonado & Santiago-Blay (1992: 509) (figures, comparison with S. eulus View in CoL ), Gil-Santana et al. (1999: 1, 2) (citations), Gil-Santana et al. (2000: 1, 5) (citations, comparison with S. aurantius View in CoL , in key), Sehnal (2000: 15) (type material), Gil-Santana & Alencar (2001: 96) (citation), Forero (2004: 164) (citation), Weirauch (2008: 231, 236, 246, 247, 261) (listed, photos, drawings, morphology, genitalia, included in cladistic analysis), Weirauch et al. (2014: 102) (photo), Gordon & Weirauch (2016: 68, 70, 71) (photo, bionomics, included in cladistics analysis), Gil-Santana & Oliveira (2019: 101, 116) (citation, genitalia).
Sphaeridops amaenus [sic]: Lethierry & Severin (1896: 115) (catalog), Pinto (1927: 43, 44) (citations, figures), Costa Lima (1940: 207, 209) (citation, reproduction of figures of Pinto 1927).
Sphaeridops eulus: Gil-Santana et al. (1999: 1 View in CoL , 2) (citations), Gil-Santana et al. (2000: 1, 5) (citations, comparison with S. aurantius View in CoL , in key), Gil-Santana & Alencar (2001: 96) (citation), Forero (2004: 164) (citation), Gil-Santana & Oliveira (2019: 102, 116) (comments on some of its characteristics). New subjective synonym.
Distribution. Paraguay and Brazil.
Note. Additional comparative morphological studies by Weirauch (e.g. 2004, 2005a,b, 2006, 2007) in which specimens of S. amoenus were studied together with numerous other species of Reduviidae were not included in the literature review, being not primarily taxonomic.
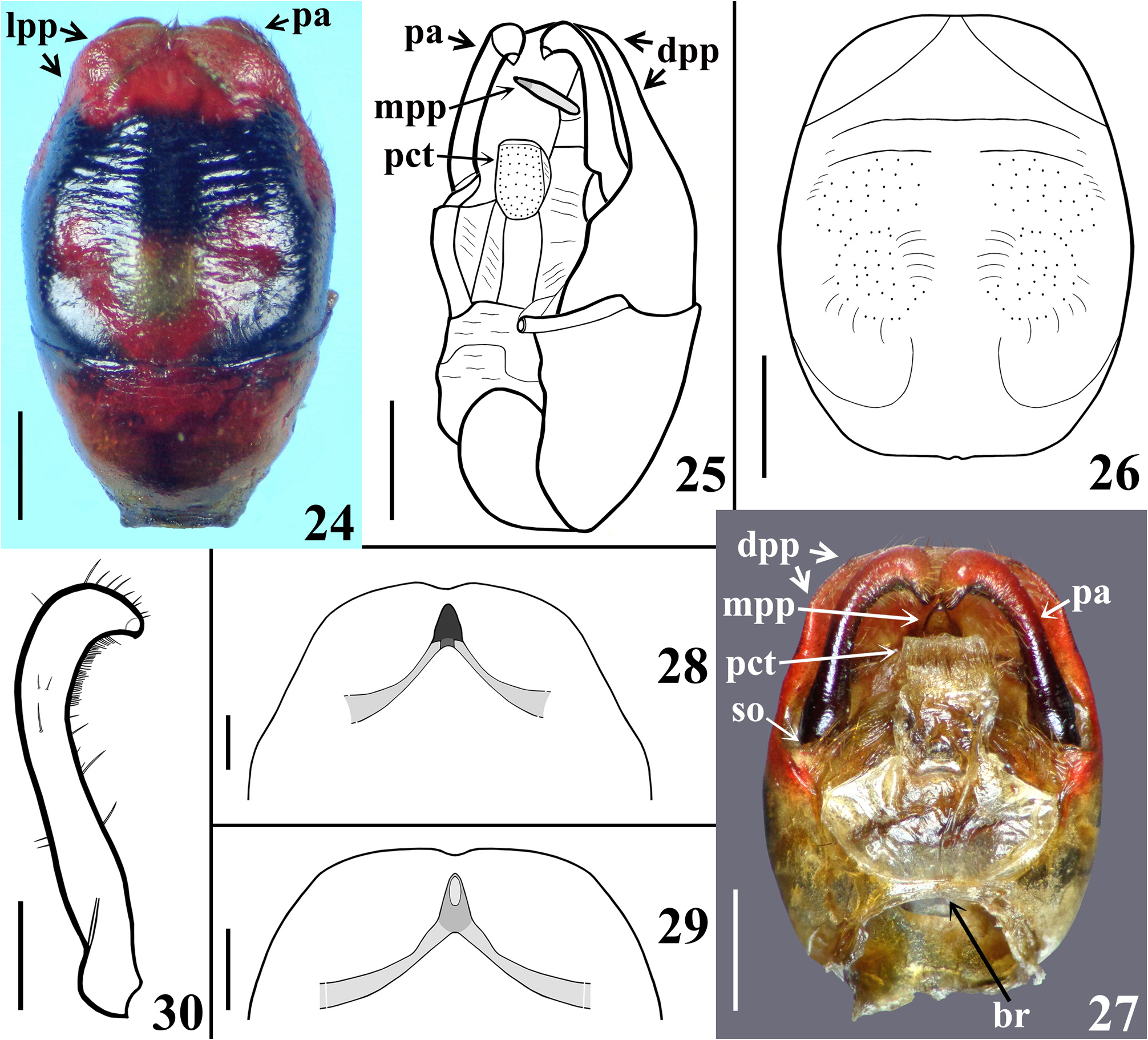
Diagnosis. Sphaeridops amoenus can be separated from other species of Sphaeridops mainly by its general coloration: reddish to pale reddish or orange reddish with blackish markings or portions ( Figs. 1–2 View FIGURES 1–3 , 4–6 View FIGURES 4–7 , 8–24 View FIGURES 8–13 View FIGURES 14–19 View FIGURES 20–23 View FIGURES 24–30 , 44–49 View FIGURES 44–49 ), while in the other species the general coloration is pale to pale orange, pale yellowish or pale whitish with blackish markings or portions. The male genitalia of S. amoenus differ from those of other congeners by the medial process of pygophore (mpp), which, in anterior view, is not elongated or slightly enlarged, in its basal portion ( Figs. 27–29 View FIGURES 24–30 ) as in S. aurantius and S. pallescens , respectively; the basal processes (bp) of the endosoma are moderately large with margins faintly or not undulated ( Fig. 41 View FIGURES 37–43 ), while in S. aurantius and S. pallescens they are somewhat smaller, narrower ( S. aurantius ), with almost all margins ( S. aurantius ) or only the basal margins ( S. pallescens ) undulated; pair of groupings of sclerotized longitudinal linear shallow ridges of the subbasal processes (sbp) slightly curved at distal half in S. amoenus ( Fig. 42 View FIGURES 37–43 , dh), slightly divergent towards their apices in S. pallescens , and subparallel in S. aurantius ; in the latter species, the ridges are more numerous at approximately the distal third of the groupings, while in the other species, at approximately the distal two-thirds of them.
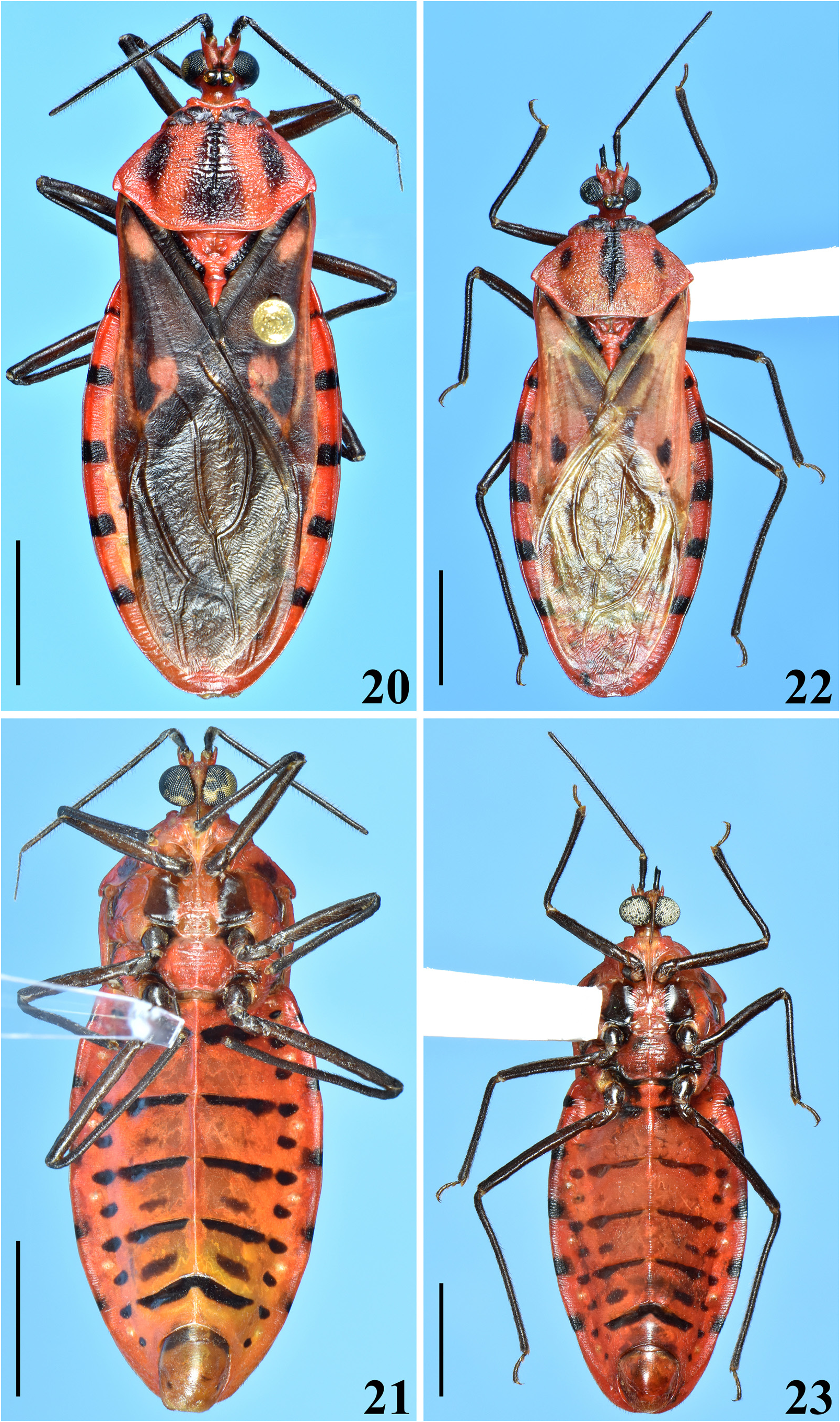
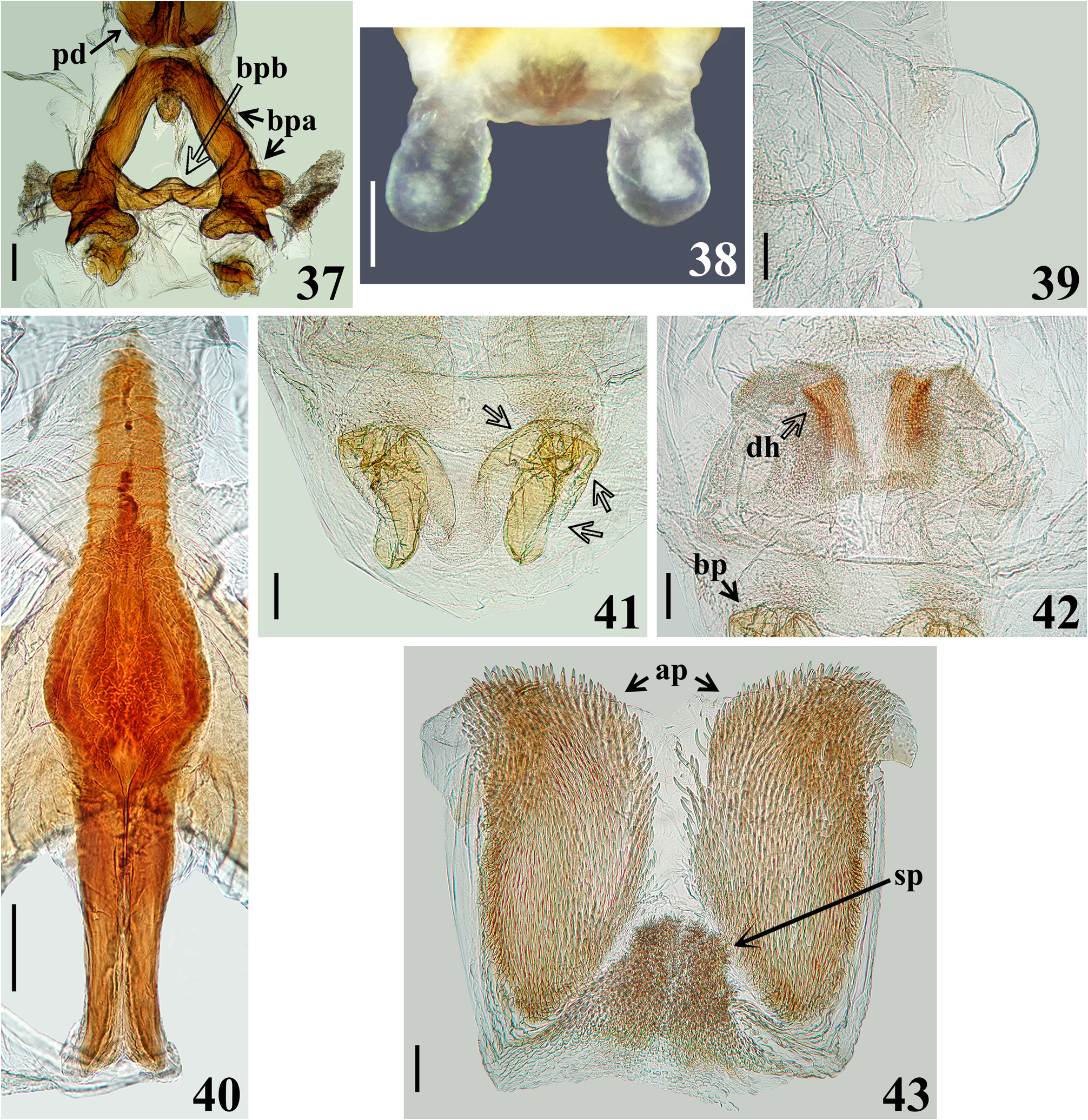
Redescription. Body length: males: 20.0–25.5 mm; females: 19.0–23.5 mm; maximum width of abdomen: males: 7.0–9.7 mm; females: 7.5–10.0 mm. Coloration and variability ( Figs. 1–2 View FIGURES 1–3 , 4–6 View FIGURES 4–7 , 8–24 View FIGURES 8–13 View FIGURES 14–19 View FIGURES 20–23 View FIGURES 24–30 , 44–49 View FIGURES 44–49 ): General coloration of body reddish, pale reddish or orange reddish with blackish markings or portions. Antennae and legs generally dark brownish to blackish; coxae and trochanters sometimes with pale markings or portions, variable in extension on different legs; tibial pads pale. Head with blackish portions on ocellar tubercle, antennifers, area behind eyes and neck with blackish portions of variable extent, in some specimens head mostly dark; labium paler to brownish, sometimes segments differently colored. Thorax. Anterior collar and anterolateral angles generally reddish or pale reddish, sometimes with variable dark markings; remaining of fore lobe of pronotum mostly to partially blackish ( Figs. 8, 10 View FIGURES 8–13 , 14, 16, 18 View FIGURES 14–19 , 20 View FIGURES 20–23 , 44, 46, 48 View FIGURES 44–49 ), sometimes with alternating blackish and reddish areas ( Figs. 12 View FIGURES 8–13 , 22 View FIGURES 20–23 ); hind lobe of pronotum with blackish longitudinal median stripe and a pair of blackish lateral markings; median longitudinal stripe runs over median longitudinal sulcus, extending approximately as far as the latter or extending towards posterior margin for a variable distance, varying in width, being narrow ( Figs. 16, 18 View FIGURES 14–19 ), somewhat larger at midportion ( Fig. 8 View FIGURES 8–13 ), subdistally ( Figs. 10 View FIGURES 8–13 , 22 View FIGURES 20–23 , 44 View FIGURES 44–49 ), even more enlarged towards apex ( Figs. 14 View FIGURES 14–19 , 20 View FIGURES 20–23 ) or absent with some portions with dark markings along midline, but without forming a complete stripe ( Fig. 12 View FIGURES 8–13 ); lateral dark markings also largely variable in shape and size ( Figs. 4 View FIGURES 4–7 , 8, 10, 12 View FIGURES 8–13 , 14, 16, 18 View FIGURES 14–19 , 20, 22 View FIGURES 20–23 , 44, 46, 48 View FIGURES 44–49 ). Lateral margins of scutellum dark. Pleurae with variable dark markings, more commonly present only on their upper portion or sometimes more extensively downwards. Mesosternum largely dark at lateral portion ( Figs. 21, 23 View FIGURES 20–23 ); metasternum sometimes darkened on lateral margin, just above hind coxae, or variably more extensively to almost completely dark. Hemelytra with following set of variations ( Figs. 1 View FIGURES 1–3 , 4 View FIGURES 4–7 , 8, 10, 12 View FIGURES 8–13 , 14, 16, 18 View FIGURES 14–19 , 20 View FIGURES 20–23 , 44, 46, 48 View FIGURES 44–49 ): clavus entirely darkened or with a basal or subbasal pale spot variable in size; corium mostly darkened with the basal portion (humeral angle) reddish to orange reddish or darkened with a pale spot and a subapical large pale spot, with a blackish spot, variable in size, within it or the subapical pale spot smaller and curved and/or without a blackish spot within it; lateral portion of corium between humeral angle and the subapical spot sometimes with the same pale coloration continuous to them, or corium pale reddish with dark portions, including small dark subapical markings ( Fig. 22 View FIGURES 20–23 ); membrane darkened in most individuals, sometimes slightly paler at median portion, anteriorly ( Fig. 10 View FIGURES 8–13 ), almost completely pale yellowish in the specimens with pale reddish coria ( Fig. 22 View FIGURES 20–23 ). Abdomen. Connexivum with dark markings at approximately distal third to distal fifth of segments II–VI, respectively ( Figs. 1 View FIGURES 1–3 , 6 View FIGURES 4–7 , 8–23 View FIGURES 8–13 View FIGURES 14–19 View FIGURES 20–23 , 44–49 View FIGURES 44–49 ). Sternites with transverse dark stripes on basal portions of segments, interrupted at median portion (except on sternite VII) and small rounded lateral dark spots at basal and mid portion of the segments ( Figs. 11, 13 View FIGURES 8–13 , 23 View FIGURES 20–23 ); in other individuals, besides these markings, a pair of submedian, lateral transverse dark stripes may be present ( Figs. 2 View FIGURES 1–3 , 6 View FIGURES 4–7 , 9 View FIGURES 8–13 , 15 View FIGURES 14–19 , 45, 49 View FIGURES 44–49 ); all those markings varying in size and extension and their presence/absence in all or only some segments; in other individuals, sternites almost completely dark, except on lateral margin, close to connexivum and sometimes on midline too ( Figs. 17, 19 View FIGURES 14–19 , 47 View FIGURES 44–49 ); similarly, the extension of the darkened portions showing variation among segments and individuals. Genitalia with variable dark markings on exposed portion (e.g. Figs. 11, 13 View FIGURES 8–13 , 19 View FIGURES 14–19 , 21 View FIGURES 20–23 , 24 View FIGURES 24–30 , 47, 49 View FIGURES 44–49 ). Structure generally as described for Sphaeridops . Integument of hind lobe of pronotum variably more or less transversely wrinkled; median sulcus reaching approximately distal third, its deepness and elevation of the ridges at its margins variable, in few individuals almost completely indistinct (e.g. Fig. 46 View FIGURES 44–49 ); lateroposterior and midposterior margins varying from straight to slightly curved ( Figs. 1 View FIGURES 1–3 , 4 View FIGURES 4–7 , 8, 10, 12 View FIGURES 8–13 , 14, 16, 18 View FIGURES 14–19 , 20, 22 View FIGURES 20–23 , 44, 46, 48 View FIGURES 44–49 ). Process of scutellum very short or variably elongated ( Figs. 4 View FIGURES 4–7 , 8, 10, 12 View FIGURES 8–13 , 14, 16, 18 View FIGURES 14–19 , 20, 22 View FIGURES 20–23 , 44, 46, 48 View FIGURES 44–49 ). Median keel on sternites limited to sternite II and basal portion of sternite III in females ( Figs. 45, 49 View FIGURES 44–49 ); in males extending on sternites II–VI ( Fig. 9, 11 View FIGURES 8–13 , 15, 17, 19 View FIGURES 14–19 , 23 View FIGURES 20–23 ), sometimes also to sternite VII on which it can be present only on its basal portion or run entirely or partially between the anterior margin and the margin above sternite VIII (e.g. Fig. 21 View FIGURES 20–23 ); in most individuals, it is more pronounced on segments II, II–III or II–IV and absent on distal portion of sternite VI. Male terminalia. Abdominal segment VIII ( Figs. 24–25 View FIGURES 24–30 ): anterior margin of ventral face straight or slightly curved; posteroventral margin straight ( Fig. 24 View FIGURES 24–30 ), with or without a small median small notch. Male genitalia ( Figs. 24–43 View FIGURES 24–30 View FIGURES 31–36 View FIGURES 37–43 ). Parameres not visible or only with their posterior margins barely visible in ventral view of pygophore ( Figs. 24, 26 View FIGURES 24–30 ); ventral face of pygophore with a pair of submedian basolateral depressions, variable in deepness among individuals, lateroposterior portions (lpp) slightly elevated and prominent ( Figs. 24, 26 View FIGURES 24–30 ); distal portion of pygophore (dpp) large, forming a horizontal extension of pygophore wall ( Figs. 25, 27 View FIGURES 24–30 ), with lateral shallow excavations in internal surface, where apical portions of parameres rest, corresponding to lateroposterior elevated portions (lpp) of ventral surface ( Figs. 24, 26 View FIGURES 24–30 ). Medial process of pygophore (mpp) only visible in dorsal and lateral views, situated at some distance from posterior margin; subtriangular in anterior view; somewhat variable in dimensions of apical half among individuals ( Figs. 25, 27–29 View FIGURES 24–30 ). Phallus ( Figs. 31–40 View FIGURES 31–36 View FIGURES 37–43 ): basal plate bridge (bpb) bent ventrally ( Figs. 31–32 View FIGURES 31–36 , 37 View FIGURES 37–43 ); each membranous lobe on endosoma (mle) rounded to shortly digitiform ( Figs. 31–32, 35 View FIGURES 31–36 , 38–39 View FIGURES 37–43 ). Processes of endosoma ( Figs. 41–43 View FIGURES 37–43 ): basal processes (bp) large with margins faintly or not undulated ( Fig. 41 View FIGURES 37–43 ); pair of groupings of sclerotized linear longitudinal shallow ridges of the subbasal processes (sbp) slightly curved at distal half (dh); ridges numerous or present only at approximately distal two-thirds ( Fig. 42 View FIGURES 37–43 ).
Type material examined.
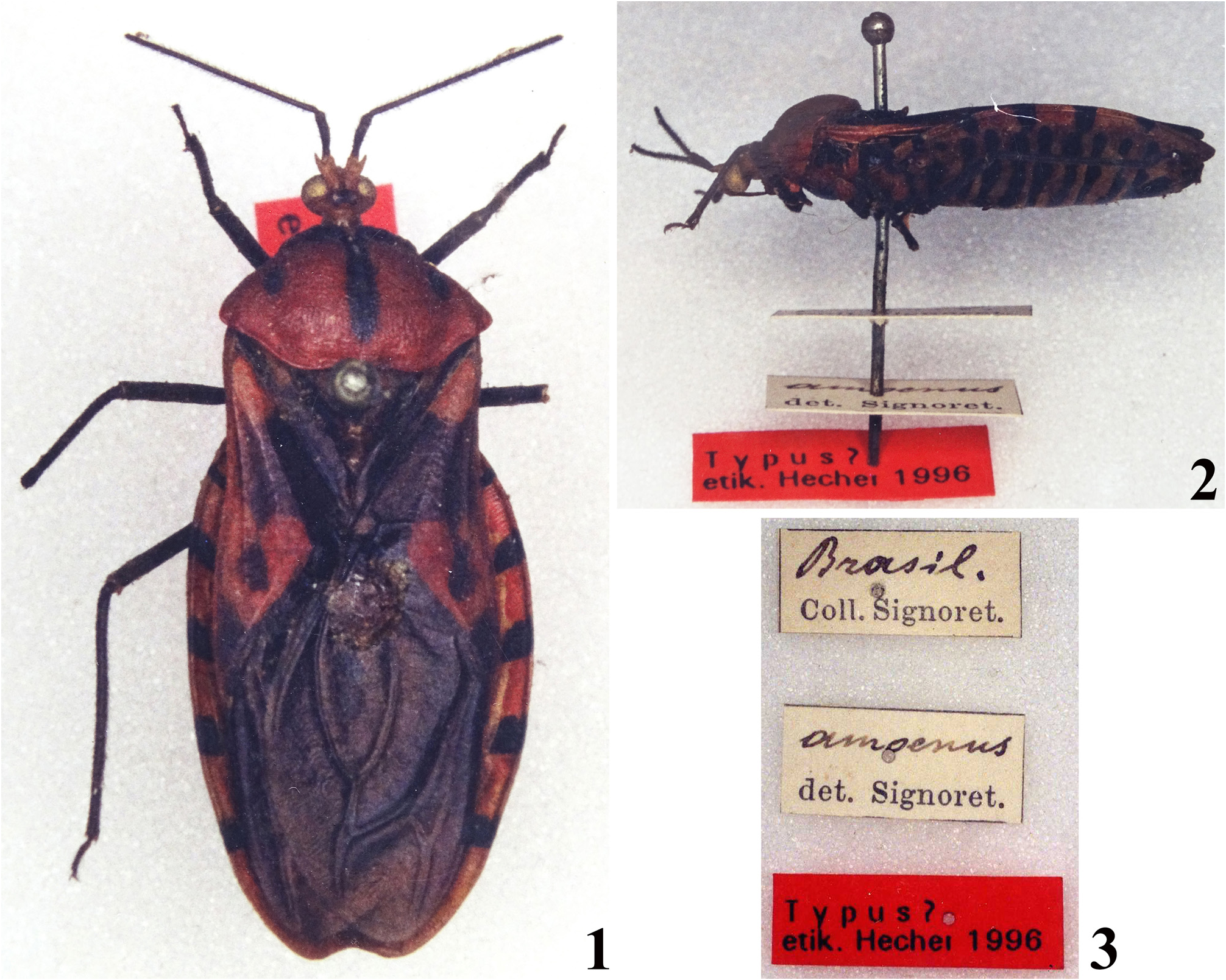
Reduvius amoenus Lepeletier & Serville, 1825 . Syntype: ♀, [ BRAZIL]: [handwritten:] Brasil. / [printed:] Coll. Signoret. // [handwritten:] amoenus / [printed:] det. Signoret. // [printed red label:] Typus? / etik. Hecher 1996 ( NHMW) .
Sphaeridops eulus Maldonado & Santiago-Blay, 1992 . Holotype: ♂, [ PARAGUAY]: [printed labels:] Horqueta / Paraguay / 57–10, W. 23–24, N. // [red label:] Holotype // USNM _ENT QR code / UCR_ENT 00008061 // [handwritten:] Sphaeridops / eulus / holotype / [printed:] det. J Maldonado C. 19 [handwritten:] 92. [Pygophore pinned separately] [printed labels:] [red label:] Holotype // [handwritten:] Sphaeridops / eulus / holotype / [printed:] det. J Maldonado C. 19 [handwritten:] 2 ( USNM).
Additional specimens examined. BRAZIL: Bahia, Campo Formoso, xi. 2004, 1 male; Encruzilhada, 10.xii.1997, leg. N. Tangerini, 1 male; Maranhão, Balsas, 08º48’41”S 46º21’49”W, x.1996, leg. M. Eklein, 2 males; Feira Nova do Maranhão, Retiro, 07º00’31”S 46º26’41”W, 29–30.xi.1995, leg. M. Eklein, 1 female, 3 males; Mato Grosso, [handwritten:] Chavantina / XI-1946 / Sick // Sphaeridops / amoenus / (L.& S.) / [printed:] Wygodzinsky det. [handwritten:] ’47, 1 female; Diamantino, Alto Rio Arinos, 14º25’S 56º29’W, 30.iv.2002, leg. E. Furtado, 1 male; Reserva Vale da Solidão, 14º22’S 56º07’W, 02.x.2002, leg. E. Furtado, 1 male; Minas Gerais, Águas Vermelhas, 12–14.xii.1992, light trap, leg. N. Tangerini, 2 males; Carangola, 18.xi. 1997, 760 m. a.s.l., leg. M. Koike, 1 male; Datas, Marques, 19º20’S 44º39’W, 21–22.xi.1989, 1.236 m.a.s.l., leg. M. Eklein & S. Ekroejoim, 2 males; [printed label bordered with black lines:], [written vertically:] J.F. Zikán / [printed horizontal line] / Mar de Hespanha [currently, Espanha] / E, Minas-Brazil / [handwritten in red:] 28.-X.-1908 // [printed label bordered with black lines:] No / [handwritten:] 48 / J.F. Zikán // Sphaeridops / amaenus [sic] / (Lep. et S.) / [printed:] Wygodzinsky det., 1 female; Ouro Preto, xi.1990, leg. Narciso, 1 male; São João del-Rei, xii.1951, 1 male (all specimens in MNRJ).
Discussion. The potential female syntype of S. amoenus was not available for examination during my visit to the NHMW in 2018, because it was on loan by another researcher (and it still is; H. Zettel, pers. comm.), but photographs taken by C. Hecher (formerly, C. Sehnal) almost two decades ago were seen ( Figs. 1–2 View FIGURES 1–3 ). The coloration of this specimen agrees well with the original description of the species ( Lepeletier & Serville 1825), reinforcing the assumption that it represents a syntype of S. amoenus .
The dimensions, vestiture, overall coloration and structural features of the examined non-type males and females were very similar, with the exception of the median keel on sternites which was limited to sternite II and basal portion of sternite III in females ( Figs. 45, 49 View FIGURES 44–49 ), while in males it extended on sternites II–VI ( Fig. 9, 11 View FIGURES 8–13 , 15, 17, 19 View FIGURES 14–19 , 23 View FIGURES 20–23 ) and sometimes also to sternite VII ( Fig. 21 View FIGURES 20–23 ). Therefore, the sexual dimorphism seems to be restricted to the extension of the shallow median keel along the sternites, but examination of more specimens, first of all more females, would be necessary to confirm this. The medial process of pygophore showed some variation in the dimensions of the apical half among males examined (e.g. Figs. 28–29 View FIGURES 24–30 ); these differences occurred randomly among individuals with different color forms, making plausible that it represents an intraspecific variation. Intraspecific variation in shape of the medial process of pygophore has been recorded in other reduviids as well, e.g. in Pothea jaguaris (Carpintero, 1980) (Ectrichodiinae) ( Gil-Santana 2020). Therefore, it is possible that this structure may be subject to intraspecific variation in some species of Reduviidae , reinforcing the aforementioned assumption in relation to the recorded variation of this structure in S. amoenus .
Maldonado & Santiago-Blay (1992) proposed the following set of characters for separating S. amoenus from S. eulus : (1) median longitudinal black stripe on the hind lobe of pronotum, narrow in S. amoenus , broad in S. eulus , also with differences in sculpture (cf. their figs. 4 and 12); (2) each abdominal sternites almost completely blackish in S. amoenus , with two transverse blackish stripes in S. eulus (cf. their figs. 1 and 10); (3) humeral (basal) angle of hemelytra pale in S. amoenus , dark with a pale spot in S. eulus (cf. their figures 4 and 12); (4) subapical pale spot of corium of hemelytra small, simple and curved in S. amoenus , large, suboval, with a blackish spot within it in S. eulus (cf. their figs. 6 and 8), and (5) “many details of the dorsal genitalia” (cf. their figs. 3, 5, 9, 11). The lateral margin of the hind lobe of pronotum of S. eulus was recorded as slightly concave on the middle third (paratype) or uniformly convex (holotype).
The examined specimens identified as S. amoenus included all color patterns previously considered as belonging to S. eulus and S. amoenus ( Figs. 8–9 View FIGURES 8–13 , 14–15; 18–19 View FIGURES 14–19 ) and also several intermediate forms ( Figs. 10–13 View FIGURES 8–13 , 16–17 View FIGURES 14–19 , 20–23 View FIGURES 20–23 ). All diagnostic characters proposed by Maldonado & Santiago-Blay (1992) for separating S. amoenus from S. eulus were considered in this relatively broad sample. Specimens exhibiting various mosaic-like combinations of characters thought to be diagnostic for S. amoenus and S. eulus were common, e.g. the degree or elevation of the ridges at margins of the median sulcus, and the corrugation of the integument of the hind lobe varied randomly with no regard to the width of the median dark stripe. Based on the examined sample all of the features listed by the above authors are judged to be independently variable among individuals and, therefore, do not have specific significance. The above author´s recognition of diagnostic color features used for defining S. amoenus is in conflict with the original description of the species ( Lepeletier & Serville 1825), mentioning a large subapical reddish large spot on the corium enclosing a dark spot, and sternites decorated with two transverse dark lines in each segment, most of them being interrupted at mid-portion; accordingly, some of the color characteristics stated by them as diagnostic for S. eulus are actually diagnostic for S. amoenus , what poses further doubt on the validity of S. eulus . Furthermore, the “many details of the dorsal genitalia” of the two putative species were not specified by these authors, and their schematic drawings (their figs. 3, 5, 9, 11) of the pygophore only show subtle differences. Differences in the medial process of pygophore similar to their drawings (broader in S. amoenus , narrower in S. eulus ) were indeed observed among the examined specimens, but they occurred randomly among individuals of different color, suggesting that they are probably subject of intraspecific variation of the same species. Other differences seen in the drawings are subtle and they were inconsistent in the examined sample, therefore they are not judged to be of specific importance. Finally, the phallus, which has a significant diagnostic value at species level, was not mentioned by Maldonado & Santiago-Blay (1992), but found to be highly similar among all males studied. As a conclusion it can be stated that individuals of different color patterns all exhibited highly similar male genitalia ( Figs. 24–43 View FIGURES 24–30 View FIGURES 31–36 View FIGURES 37–43 ), supporting the hypothesis that the two taxa are conspecific, resulting in the following synonymy propose here: Sphaeridops amoenus ( Lepeletier & Serville, 1825) = Sphaeridops eulus Maldonado & Santiago-Blay, 1992 , syn. nov.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Sphaeridops amoenus ( Lepeletier & Serville, 1825 )
| Gil-Santana, Hélcio R. 2021 |
Sphaeridops eulus:
| Gil-Santana, H. R. & Oliveira, J. 2019: 102 |
| Forero, D. 2004: 164 |
| Gil-Santana, H. R. & Alencar, J. 2001: 96 |
| Gil-Santana, H. R. & Costa, L. A. A. & Zeraik, S. O. 2000: 1 |
| Gil-Santana, H. R. & Zeraik, S. O. & Costa, L. A. A. 1999: 1 |
Sphaeridops eulus
| Maldonado, J. C. & Santiago-Blay, J. A. 1992: 509 |
Sphaeridops amaenus
| Costa Lima, A. 1940: 207 |
| Pinto, C. 1927: 43 |
| Lethierry, L. & Severin, G. 1896: 115 |
Sphaeridops amoenus: Amyot & Serville (1843: 382–383)
| Gil-Santana, H. R. & Oliveira, J. 2019: 101 |
| Gordon, E. R. L. & Weirauch, C. 2016: 68 |
| Weirauch, C. & Berenger, J. - M. & Berniker, L. & Forero, D. & Forthman, M. & Frankenberg, S. & Freedman, A. & Gordon, E. & Chamberlain, R. & Hwang, W. S. & Michael, A. & Udah, O. & Watson, C. & Zhang, G. & Zhang, J. 2014: 102 |
| Weirauch, C. 2008: 231 |
| Forero, D. 2004: 164 |
| Gil-Santana, H. R. & Alencar, J. 2001: 96 |
| Gil-Santana, H. R. & Costa, L. A. A. & Zeraik, S. O. 2000: 1 |
| Sehnal, C. 2000: 15 |
| Gil-Santana, H. R. & Zeraik, S. O. & Costa, L. A. A. 1999: 1 |
| Maldonado, J. C. & Santiago-Blay, J. A. 1992: 509 |
| Maldonado, J. C. 1990: 490 |
| Putshkov, V. G. & Putshkov, P. V. 1985: 99 |
| Wygodzinsky, P. 1949: 64 |
| Walker, F. 1873: 10 |
| Stal, C. 1872: 113 |
| Amyot, C. J. - B. & Serville, J. - G. A. 1843: ) |
Reduvius amoenus
| Lepeletier, A. L. M. & Serville, J. - G. A. 1825: 275 |