Congochromis pugnatus, STIASSNY & SCHLIEWEN, 2007
|
publication ID |
https://doi.org/ 10.1206/0003-0082(2007)3576[1:CANCGT]2.0.CO;2 |
|
publication LSID |
lsid:zoobank.org:pub:9447E641-4CA3-4A29-9F1A-2D71F62411DD |
|
persistent identifier |
https://treatment.plazi.org/id/4B97CD96-DE96-4F27-8EC7-446189BDE643 |
|
taxon LSID |
lsid:zoobank.org:act:4B97CD96-DE96-4F27-8EC7-446189BDE643 |
|
treatment provided by |
Carolina |
|
scientific name |
Congochromis pugnatus |
| status |
sp. nov. |
Congochromis pugnatus View in CoL , new species figures 3–5 View Fig View Fig View Fig
HOLOTYPE: AMNH 6079 View Materials , 48.2 View Materials mm SL, adult male, Democratic Republic of Congo, Kisangani (Stanleyville), H. Lang and J.P. Chapin, May 1915.
PARATYPES: Eight paratypes with same data as holotype : AMNH 237670 View Materials , 2 ex., 1 C&S, 37.0–51.5 mm SL ; FMNH 57121 View Materials , 3 ex., 37.8–49.1 mm SL ; MRAC 2006-45 View Materials -P-1, 50.4 mm SL ; ZSM 34981, 2 ex., 37.0– 49.2 mm SL .
DIAGNOSIS: A Congochromis diagnosed by the possession of a compound urostyle + fused hypural plate ( fig. 2C View Fig ). Further differs from all congeners in possessing a strongly inclined lower jaw and expanded cheek musculature ( fig. 3 View Fig ).
DESCRIPTION: Based on the holotype and eight paratypes. See table 1 for a summary of morphometric and meristic data for the new taxon and for comparative data on type specimens of all congeners. Morphological characteristics and general pigmentation pattern can be observed in figure 4 View Fig , and of congeners in figure 6. A View Fig robust, relatively deep-bodied species (depth 29.3–35.5%, mean 31.7% SL). Greatest body depth at (males), or slightly behind (females), level of pelvic-fin insertion. Head short (length 31.2–33.2%, mean 32.1% SL) and deep (depth 21.2– 25.1%, mean 23.1% HL). Cheek deep (depth 24.4–28.8%, mean 26.2% HL). Snout short and broad, jaws isognathous, with lower jaw strongly inclined and ventral section of adductor mandibulae muscle large and bulbous in anteroventral region of cheek ( fig. 3 View Fig ). Lips well developed and fleshy, lower lip fold discontinuous at symphysis. Dorsal head profile straight to midorbit, bulbous to dorsal fin origin; markedly so in large males. Dorsal body profile curving gently downward along length of dorsal fin base to short, deep caudal peduncle. Ventral body profile more or less straight (males) or strongly convex (females).
Flanks covered with large, regularly imbricating, cycloid scales. A few deeply embedded, cycloid scales scattered over opercle and subopercle. Cheek with small round, cycloid scales restricted to one or two rows at dorsoposterior margin. Occipital region with numerous small, imbricating cycloid scales to level of midorbit. Small cycloid scales over pectoral-fin base, chest naked. Belly scales slightly smaller with a gradual transition in size; scales on ventral portion of belly and anal-genital region of same size as lateral belly scales. Upper lateral line originates behind occipital margin of opercle, ascends gradually to dorsal-fin base reaching highest point at level of 10th to 12th dorsal fin spine, continues with half an intervening scale or no intervening scale between lateral line and dorsal-fin base. Pored scales interspersed with more numerous nonpored scales along length of upper lateral line. Lower lateral line short, usually consisting of only two or three pored scales interspersed among nonpored scales. Upper lateral line separated from lower lateral line by two scales (excluding pored rows). Caudal-fin base with a single large pored scale medially (not included in longitudinal scale count) and numerous small scales over basal eighth of fin.
Dorsal fin with XVI–XVIII (mode XVII) spines and 6–9 (mode 8) rays. Anal fin with III spines and 5–6 (mode 6) soft rays. Dorsal-fin spines gradually increase in length to 14th or 15th spine, remaining spines of equal length. Soft dorsal and anal fins in males with tapering filamentous extensions reaching to
TABLE 1 Morphometric and Meristic Data for the Holotype and Eight Paratypes of Congochromis pugnatus , n.sp., Two Syntypes of C. squamiceps, Two Largest Syntypes of C. dimidiatus , and Two Paratypes of C. sabinae basal third of caudal fin. In females soft dorsal and anal fins are pointed but not produced and do not reach base of caudal fin. Caudal fin rounded with 14 branched rays; appears lance-shaped, subacuminate when adducted. First pelvic fin ray longest in both sexes, reaching anal fin base in males, shorter in females. Pectoral fin rounded, reaching vertical approximately at midpoint of spinous dorsal fin.
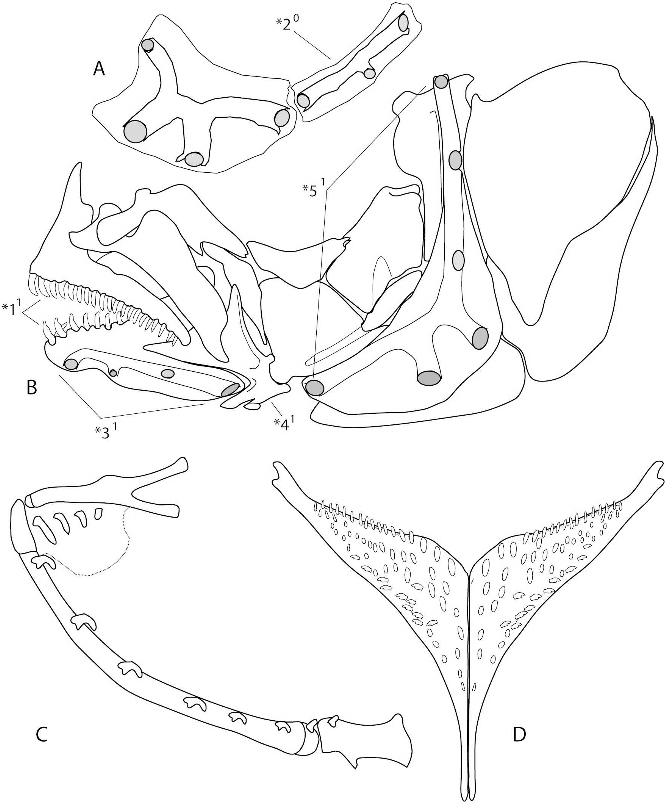
Eight to 10 small gill rakers along outer row of lower limb of first gill arch (including larger more elongate raker in angle of arch) and three to five bulbous epibranchial rakers ( fig. 5C View Fig ). In common with most other chromidotilapiine cichlids, a prominent visorlike, hanging pharyngeal pad is developed on epibranchial 2, and no microbranchiospines are present on outer face of second, third, or fourth gill arches.
Outer row dentition on both premaxilla and dentary composed of relatively robust, recurved, unicuspid teeth ( fig. 5B View Fig ). Teeth are evenly spaced along each jaw, three or four symphysial teeth on dentary somewhat enlarged and procumbently implanted. Anteriorly in both jaws three to four short inner rows of recurved teeth taper to a single row posteriorly.
Lower pharyngeal jaw ( fig. 5D View Fig ) relatively gracile, with narrow horns and a short blunt keel. Dentigerous surface sparsely covered with bicuspid teeth. Posterior row teeth elongate, erect, closely spaced bicuspids with strongly hooked major cusp and smaller minor cusp. Anteriorly lower pharyngeal jaw teeth weakly erect, somewhat shouldered, robust, unicuspids.
Vertebrae column with a total of 26–27 (mode 26) vertebrae.
MISCELLANEOUS OSTEOLOGY AND ANAT- OMY: In common with other Congochromis , the first infraorbital of C. pugnatus has four sensory canal pores and is followed by a single, elongate, dorsoposteriorly oriented infraorbital element ( fig. 5A View Fig ). Four pores perforate the laterosensory canal in the dentary, the anguloarticular lacks a canal, and six pores perforate the preopercular canal ( fig. 5B View Fig ). The pharyngeal apophysis has an extensive exoccipital contribution to the ventral articular surface of the apophysis.
Primitively in the caudal skeleton of chromidotilapiines the hypural plate is comprised of five separate hypural elements, each of which articulates with an autogenous terminal urostyle (e.g., fig. 2A, B View Fig ). In Congochromis various patterns of hypural fusions are evident (e.g., fig. 2C–F View Fig ), but uniquely in C. pugnatus hypurals 1+2 and 3+4 are fused into a single element, and the resultant compound hypural plate is fused with the urostyle ( fig. 2C View Fig ); this is the case even in the smallest specimens examined. By contrast, C. sabinae ( fig. 2E View Fig ), C. squamiceps ( fig. 2D View Fig ), and C. dimidiatus ( fig. 2F View Fig ) have hypurals 3+4 (or 3 and 4 in the case of C. dimidiatus ) fused with the urostyle, but hypurals 1+2 ( C. sabinae ) or hypurals 1 and 2 ( C. squamiceps and C. dimidiatus ) remain autogenous, even in the largest specimens available for study. In C. pugnatus the adductor mandibulae muscle is well developed, and in large individuals the anterioventral portion of the muscle complex is enlarged and voluminous, lending a characteristic bulge to the cheek ( fig. 3 View Fig ).
COLORATION IN PRESERVATIVE ( fig. 4 View Fig ): Ground color is more or less uniformly pale brown. Specimens have been in preservative for more than 90 years and pigmentation is faded; nonetheless, each flank scale has a narrow pigmented bar on its exposed posterior edge. Scale centers retain traces of a silvery iridescence, and this silvery iridescence is most strongly marked midlaterally and over the bloated abdomen of female specimens. All specimens lack a clearly defined dark longitudinal band or series of midlateral blotches extending from the eye to the caudal peduncle (males) or end of the caudal fin (females), a pigmentation that is claimed to be characteristic of other Congochromis (Lamboj 2004) ; however, the absence of this feature may be an artifact of long-term preservation. Both males and females retain a heavily pigmented, scaleless opercular blotch. In males the soft dorsal, anal, and caudal fins are heavily maculate with alternating rows of light and dark maculae creating a striped patterning. In females these fins are hyaline and lack rows of maculae, and a single large black blotch is present in the soft dorsal fin ( fig. 4B View Fig ).
COLORATION IN LIFE: No data.
GEOGRAPHICAL DISTRIBUTION: Currently known only from the Upper Congo from a single collection from the vicinity of Kisangani (Stanleyville) in the Democratic Republic of Congo.
HABITAT: The species is currently known only from historically collected specimens, and no record of habitat preference is provided by Nichols and Griscom, who reported only that the specimens were collected in 1915 during the AMNH Lang-Chapin Congo Expedition, in the vicinity Kisangani. The town of Kisangani lies between the Lindi River and the Congo mainstream, and as a result it is possible that Lang-Chapin specimens from ‘‘Kisangani’’ may refer to collections made in different rivers. Congochromis pugnatus and C. squamiceps may occur syntopically in the vicinity of Kisangani, as indicated by an individual of the latter species collected by Lang and Chapin (AMNH 225399) from the Kisangani locality, but this is unconfirmed at present.
ETYMOLOGY: From the Latin pugno, meaning to contend or fight, in reference to the heavy-jawed, pugnacious aspect of the species.
| ZSM |
Bavarian State Collection of Zoology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |