Chelisoficula caussaneli, Nel & Waller & Albouy & Menier & Plöeg, 2003
|
publication ID |
https://doi.org/ 10.5281/zenodo.4665054 |
|
persistent identifier |
https://treatment.plazi.org/id/03838780-F077-FFE8-FE98-13EEC3E153CD |
|
treatment provided by |
Felipe |
|
scientific name |
Chelisoficula caussaneli |
| status |
sp. nov. |
Chelisoficula caussaneli n. sp. ( Figs 1-5 View FIG View FIG View FIG View FIG View FIG )
TYPE MATERIAL. — Female holotype specimen PA 29, male paratype specimen PA 205, both specimens
mounted in Canada balsam, in collection De Ploëg and Indivision Langlois-Meurine, deposited in Muséum national d’Histoire naturelle, Paris . Specimens collected in Le Quesnoy all bear the letters PA for Paris (meaning Paris basin), the following number is the ordinal number in the collection.
ETYMOLOGY. — Named after the late Professor Claude Caussanel, former director of the Laboratoire d’Entomologie du Muséum national d’Histoire naturelle de Paris and a well known specialist of Dermaptera .
TYPE LOCALITY. — Le Quesnoy, Chevrière, region of Creil, Oise department, France.
A
B
GEOLOGICAL AGE. — Lowermost Eocene, Sparnacian, level MP 7 of the mammal fauna of Dormaal. We have demonstrated that the amber is autochthonous and very different from the Baltic amber in age, chemical composition and origin ( Feugueur 1963; De Ploëg et al. 1998; Nel et al. 1999).
STATE OF PRESERVATION. — Both holotype and paratype are complete very well preserved specimens in clear pieces of amber. Numerous small air bubbles surround the cerci of the holotype.
DESCRIPTION
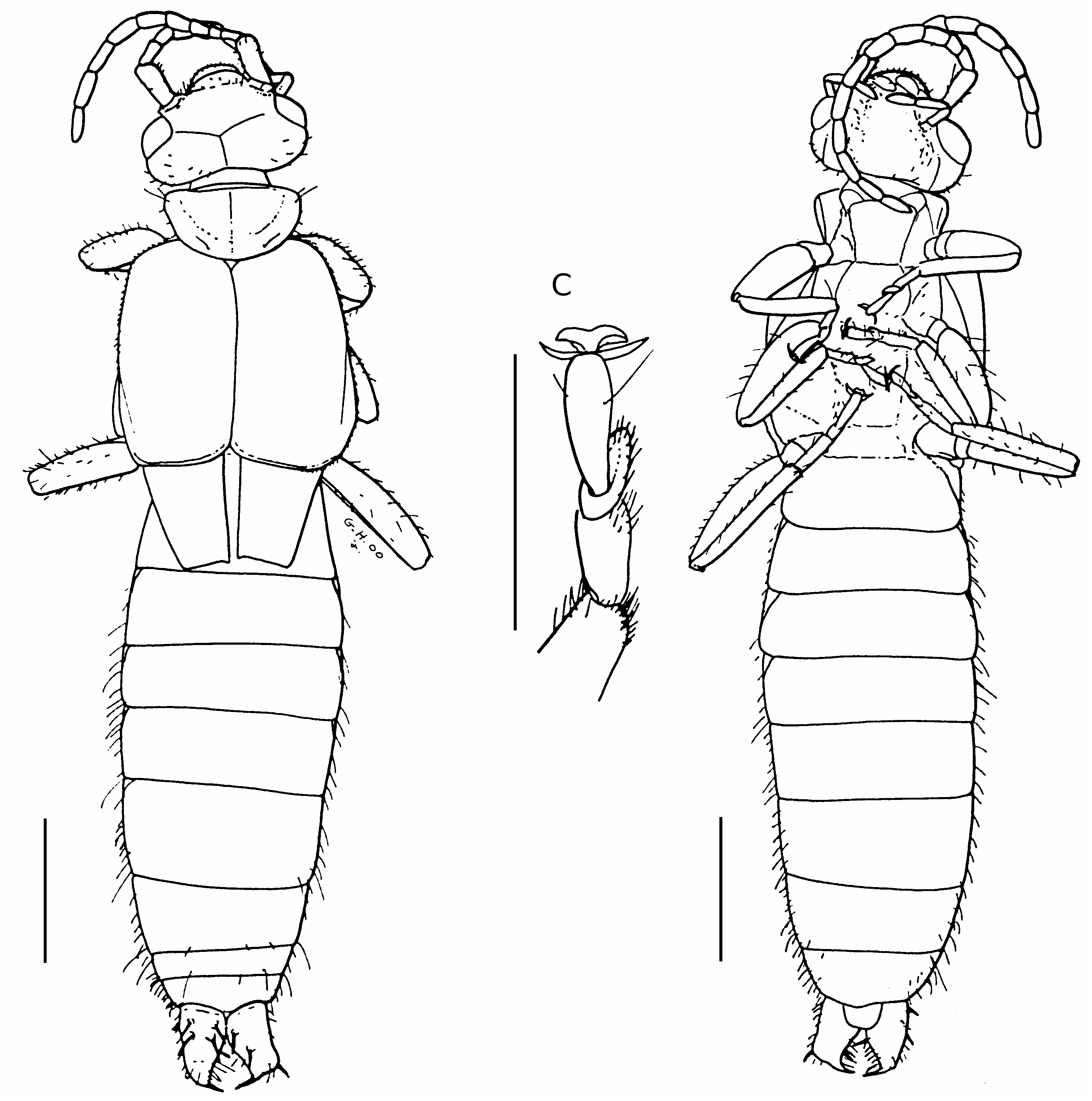
Female holotype specimen PA 29 ( Figs 1-3 View FIG View FIG View FIG )
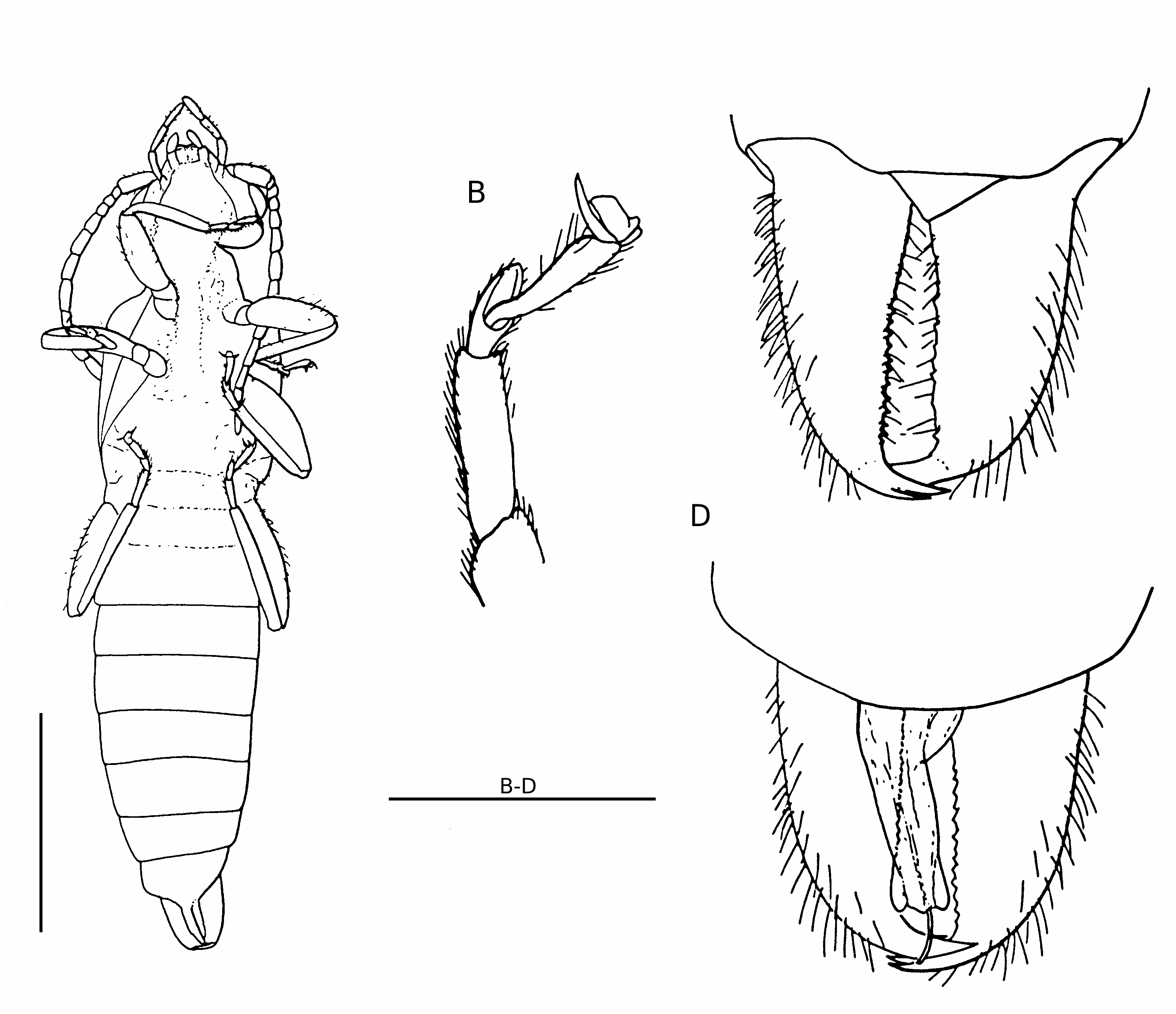
Body about 8.0 mm long, including the cerci, 0.72 mm long; body dark yellow; head prognathous, 1.2 mm wide, 0.86 mm long; eyes 0.3 mm wide, slightly smaller than the distance between them and the back of the head; antenna divided into 12 smooth segments, all of equal
A
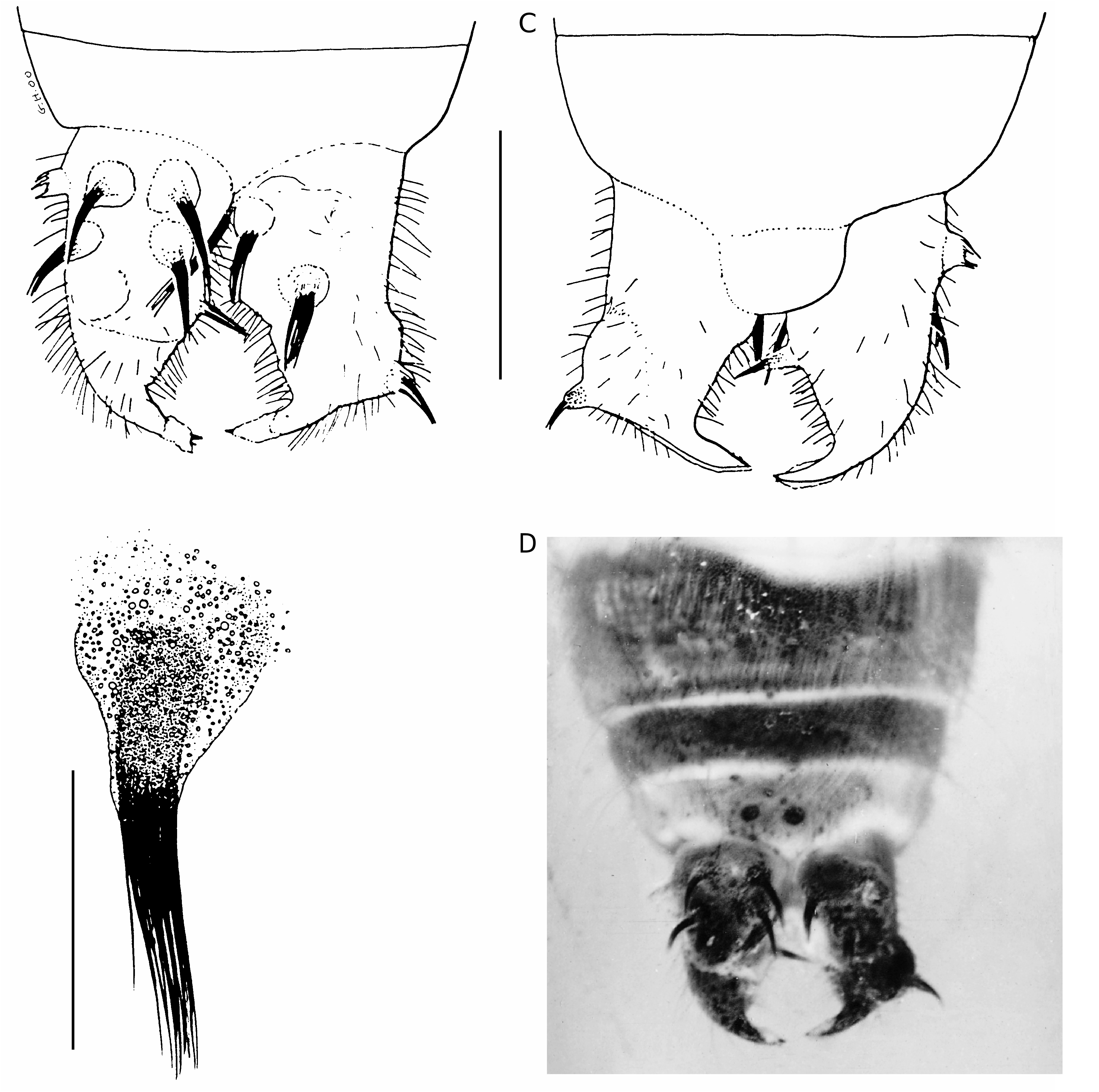
length from the third to the apex; antennal scape three times longer than wide; second antennal segment small, shorter than the third; occiput slightly concave with the angles well rounded; frontal and occipital sutures not visible; labial and maxillary palps visible and similar to those of a modern Dermaptera (see Albouy & Caussanel 1990); mandibles not visible; pronotum transverse, 1 mm wide and 0.6 mm long, slightly broader than long, with anterior part slightly convex and posterior part semi-circular; one strong setae at each anterior angle of the pronotum, other setae on the outer margin; tegmina strongly bulging, with its anterior margin round- ed leaving place to a small equilateral scutellum, posterior margin slightly concave; hindwings clearly visible, covering the second and third abdominal segments; thoracic sternites similar to those of modern Dermaptera : Forficulidae , i.e. prothoracic sternite smaller than the others, with a posterior constriction and a lateral carina anteriorly, mesothoracic sternite nearly quadrangular; metathoracic sternite as long as the pro- and mesothoracic sternites together, not posteriorly concave ( Waller et al. 1999); forelegs slightly smaller than median and hindlegs; femora bearing no dorsal or ventral carina; second tarsal segments not bilobed and strongly extending below the thirds, from which they are deeply separated ( Fig. 1C View FIG ); all tarsal claws stout and separated by a large arolium; abdomen progressively narrowed; eight abdominal segments; last visible segment 0.3 mm long and 1.0 mm wide, wider than long; tegument of abdomen punctuated, with no dorso-lateral tubercle; numerous visible setae on the posterior edges of the segments; pygidium not visible, probably absent; two thirds of total length of inner margin of cerci straight; apex of cerci strongly curved, making an angle of 90° with the inner margin; cerci covered by a tegument-like material from which emerge about 10 tubercles, on their whole surface, except the apical parts ( Fig. 2A, C, D View FIG ); in the centre of each tubercle, presence of a thick and long spine made of agglutinated setae, apically separated ( Fig. 2B View FIG ). We have no argument if it was movable or fixed. At low magnification, these spines appear like “normal” spines, i.e. very strong and stout. When
A B
seen at higher magnification, these spines appear as a set of thinner hair, agglomerated and approximate. The cerci bear also numerous long single setae. The last abdominal sternite bears two dark spots, which may correspond to remnants of spines like those of the cerci.
Male paratype specimen PA 205 ( Figs 4 View FIG ; 5 View FIG )
Body about 8.0 mm long, including the cerci, 0.8 mm long. The characters not visible on the female holotype and differences with it are as follows: the two apical teeth of the mandibles are clearly visible and sharp; neck of forficuloid type, i.e. posterior lateral sclerite enlarged, postero-lateral sclerite completely reduced, posterior ventral sclerite enlarged and joining prosternum ( Steinmann 1986); nine visible abdominal segments; cerci identical to those of the female, but crossing and with numerous small denticles on their inner margin ( Fig. 5C, D View FIG ); cerci covered by no special tegumentlike material; cerci bearing no tuft of long setae or strong spines, except for two small apical spines, that could be homologous to the female spines; male genitalia partly exposed; only one genital lobe visible, from which the virga is clearly extruded; the two parameres seem to be visible at the base of the lobe.
DISCUSSION
All the visible differences between the two specimens are sexual characters. The main difference is the presence of strong spines on the female cerci. This unique character justifies by itself the creation of a new genus and species.
The phylogenetic relationships between the different families of Dermaptera remain very controversial. We discuss the possible phylogenetic relationships of Chelisoficula n. gen. after the different existing classifications.
A C
Steinmann (1986), on a strict systematic point of view, proposed to divide the Dermaptera into two suborders, i.e. Catadermaptera and Eudermaptera, on the basis of the male genitalia: bilobate for the first suborder and with only one lobe for the second. Thus at least one of these suborders (Catadermaptera) can be suspected to be paraphyletic. Chelisoficula n. gen. probably has only one lobe, which is currently considered as the derived state. It would belong to the Eudermaptera (= Labiidae + Chelisochidae + Forficulidae ). Within this group, considering the key of the families proposed by Steinmann (1990), Chelisoficula n. gen. would belong to the Forficulidae because of its reduced number of antennal segments, as well as in the Chelisochidae because of its unilobed tarsal segment.
After Popham (1965, 1985) and Albouy & Caussanel (1990), Chelisoficula caussaneli n. gen., n. sp. can be excluded from the Pygidicranidae because of its neck of forficuloid type. It would belong to the group of families Forficuloidea (= [( Apachyidae + Labiduridae ) + ( Chelisochidae + Forficulidae )] sensu Popham 1985 and sensu Albouy & Caussanel 1990). After Albouy & Caussanel (1990), it would belong more precisely to ( Chelisochidae + Forficulidae ), because of its second tarsal segments strongly prolonged below the third. In the Labiduridae Allosthetus Verhoeff, 1903 , the second segments are prolonged below the third, but in Allosthetus, the third segments are much longer than the seconds, unlike in Chelisoficula n. gen. and ( Chelisochidae + Forficulidae ). Also, the second segments of Allosthetus are not as long as in Chelisochidae Burr, 1907 and Chelisoficula n. gen.
Also, Chelisoficula n. gen. would be related to the Forficulidae because it has less than 13 antennal segments. The reduced number of antennal segments (between 10 and 16) is supposed to be an apomorphy of the Forficulidae , after Popham (1985). Nevertheless, the Chelisochidae Chelisochella superba (Dorhn, 1865) may have 17 antennal segments (pers. obs.). Steinmann (1983, 1993) indicated that the number of antennal segments greatly varies in Chelisochidae , i.e. 11 for Hamaxas singhi Kapoor, 1966 , 14 for H. bidentatus Ramamurthi, 1965 , 15 for Schizoproreus delicatulus (Burr, 1911) , 16 for Adiathetus glaucopterus (Bormans, 1888) , and 36 segments for Genitalata mahajani Kapoor, 1974 . Steimann (1983) added that the number of segments varies between 15 to 20 in Chelisoches Scudder, 1876 . Thus, the value of this character remains dubious. The second tarsal segment deeply separated from the third is a character supposed to be only present in the Forficulidae . Hovewer it occurs in the chelisochid Proreus simulans (Stål, 1860) (pers. obs.). Thus the value of this character is also dubious. The second tarsal segment bilobate is only present in Forficulidae (+ the Pygicranidae Tagalina ) ( Steinmann 1986). Thus it is probably a synapomorphy of the modern Forficulidae , even if its presence in Tagalina suggests that it can be subject to convergency. Consequently, if attributed to the Forficulidae , Chelisoficula caussaneli n. gen., n. sp. could be in a basal position within this group. Furthermore the second tarsal segment being very long is a character only present in the Chelisochidae , and is probably apomorphic.
Chelisoficula caussaneli n. gen., n. sp. has very well developed arolia, which could be a plesiomorphic state, as it is present in several genera of the Pygidicranidae Verhoeff, 1902 View in CoL ( Echinosoma Audinet-Serville, 1839 View in CoL , Bormansia Verhoeff, 1902 View in CoL , Diplatys Audinet-Serville, 1831 View in CoL , Haplodiplatys Hincks, 1955 , Lobodiplatys Kirby, 1891 View in CoL ; see Giles 1963; Waller et al. 1999), which is supposed to be the most basal family after Popham (1985). Within the Forficuloidea sensu Popham (1985), the arolia are absent in ( Chelisochidae View in CoL + Forficulidae View in CoL ), but present in Apachyidae Verhoeff, 1902 View in CoL (at least in Apachyus Audinet-Serville, 1831 View in CoL ) ( Waller et al. 1999). The arolia are also absent in some Labiidae Burr, 1909 View in CoL (at least in Spongovostox cornutus Brindle, 1973 View in CoL ), but present in others (at least in the Geracinae Nesolabia longicollis Hincks, 1957 View in CoL ). It is absent in some Labiduridae View in CoL ( Forcipula (Decolyi) decolyi Bormans, 1900 View in CoL ) but present in Allosthetus lombokianum Verhoeff, 1904. Thus, the character “presence versus absence of arolia” is clearly homoplastic within the whole order.
If we admit the phylogenetic hypotheses of Popham and of Albouy & Caussanel, the three solutions: 1) Chelisoficula n. gen. as sister group of ( Chelisochidae View in CoL + Forficulidae View in CoL ); 2) Chelisoficula n. gen. as sister group of Chelisochidae View in CoL ; and 3) Chelisoficula n. gen. as sister group of Forficulidae View in CoL , all imply two convergences. But these hypotheses are based on weakly polarized and/or homoplastic characters.
Sakai (1987, 1988) and Haas (1995) also considered the Forficulidae and Chelisochidae as sister groups. These authors differentiated these families on the basis of the second tarsal segment. More precisely, Haas (1995) considered that the “forficuloid-type lobed” or “chelisochoid-type lobed” are both derived from a primitive, “normal” type. Chelisoficula n. gen. would belong to the Chelisochidae after this hypothesis. This does not solve the problem related to the presence of arolia in Chelisoficula n. gen.
We consider that there is an unresolved trichotomy between the three taxa Chelisoficula n. gen., the Forficulidae and Chelisochidae . The discovery of Chelisoficula n. gen. suggests that the characters that are currently used in the classification and phylogenetic analyses of the Dermaptera are probably more homoplasic than previously thought.
| MP |
Mohonk Preserve, Inc. |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Genus |
Chelisoficula caussaneli
| Nel, André, Waller, Alain, Albouy, Vincent, Menier, Jean-Jacques & Plöeg, Gaël De 2003 |
Chelisoficula caussaneli
| Nel & Waller & Albouy & Menier & Plöeg 2003 |
Chelisoficula
| Nel & Waller & Albouy & Menier & Plöeg 2003 |
Chelisoficula
| Nel & Waller & Albouy & Menier & Plöeg 2003 |
Chelisoficula
| Nel & Waller & Albouy & Menier & Plöeg 2003 |
Spongovostox cornutus
| Brindle 1973 |
Nesolabia longicollis
| Hincks 1957 |
Haplodiplatys
| Hincks 1955 |
Labiidae
| Burr 1909 |
Chelisochidae
| Burr 1907 |
Chelisochidae
| Burr 1907 |
Chelisochidae
| Burr 1907 |
Labiduridae
| Allosthetus Verhoeff 1903 |
Pygidicranidae
| Verhoeff 1902 |
Bormansia
| Verhoeff 1902 |
Apachyidae
| Verhoeff 1902 |
Forcipula (Decolyi) decolyi
| Bormans 1900 |
Lobodiplatys
| Kirby 1891 |
Echinosoma
| Audinet-Serville 1839 |
Diplatys
| Audinet-Serville 1831 |
Apachyus
| Audinet-Serville 1831 |
Forficulidae
| Stephens 1829 |
Forficulidae
| Stephens 1829 |
Forficulidae
| Stephens 1829 |