Charybdis glaucophylla Bacch., Brullo, D'Emerico, Pontec. & Salmeri, 2012
|
publication ID |
https://doi.org/ 10.11646/phytotaxa.69.1.4 |
|
persistent identifier |
https://treatment.plazi.org/id/03D187B4-0A0E-FFA8-A89B-3A8ED660FD80 |
|
treatment provided by |
Felipe |
|
scientific name |
Charybdis glaucophylla Bacch., Brullo, D'Emerico, Pontec. & Salmeri |
| status |
sp. nov. |
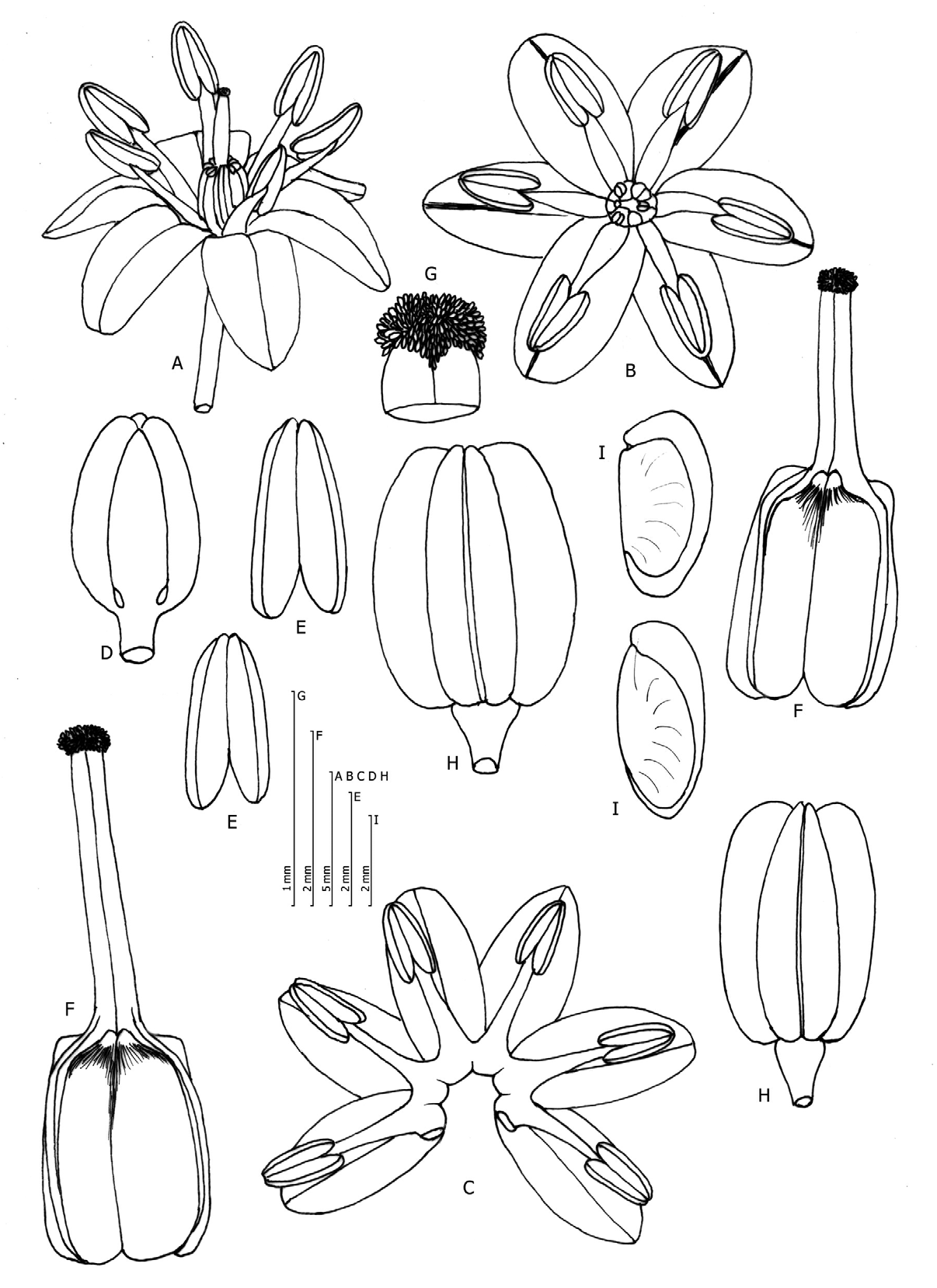
Charybdis glaucophylla Bacch., Brullo, D'Emerico, Pontec. & Salmeri , sp. nov. ( Fig. 1 View FIGURE 1 , 2 View FIGURE 2 )
Affinis Charybde pancratione sed bulbo 5–8 x 6–10 cm, tunicis et radicibus albis, foliis glauco-pruinosis, numero (5–)6–9 varians, oblanceolatis, (16–)22–28(–34) x (3–)4.5–8(–10) cm, inflorescentia (10–)20–40(–57) cm longa ad 150–200 flores composita, perigonii lobis 7–7.5 mm longis, ovario 2.6–2.8 mm longo, stigmate capitati differt.
Type: — ITALY. Sardinia: Isola di San Pietro: Cala Vinagra, Carloforte , 63 m a.s.l., 38° 09’ 47,49’’N, 8° 14’ 37,75’’E, 19 July 2004, G. Bacchetta & C. Pontecorvo s.n. (holotype CAT!; isotypes CAG!, CAT!) GoogleMaps .
Bulb ovoid, 5–8 × 6–10 cm, with outer tunics coriaceous and brown in colour, the inner ones whitish. Leaves (5–) 6–9 in number, glaucous-pruinose, rigid, oblanceolate, (16–)22–28(–34) × (3–)4.5–8(–10) cm, obtuse to acute, cucullate and apiculate at the apex. Stem 28–35 cm long, greenish, tinged with violet in the upper part. Raceme cylindrical, greenish, (10–)20–40(–57) cm long, with 150–200 flowers. Pedicels erect-patent, 12–18 mm long, longer than perigon, extending in fruiting plants. Flower buds white, sometimes tinged with pink, 7–8 mm long. Perigon white, stellate, 15–16 mm in diameter; lobes 7–7.5 × 3.4–3.8 mm, oblong to oblongelliptic, the inner ones rounded, the outer ones obtuse, midrib purplish. Stamens subequal or shorter than the perigon; anthers greenish, 3.0– 3.2 mm long; filaments white, subulate, 3.5–4.2 mm long. Ovary ellipsoid, green, 2.6–2.8 × 1.9–2 mm; style white, 2.2–3.2 mm long; stigma capitate, white, papillose. Fruiting raceme linear-cylindrical. Capsule trigonous, ellipsoid, 8.5–10 × 6–7.5 mm, truncate at the base. Seeds oblong, black, shining, 4.3–5 × 2–2.3 mm.
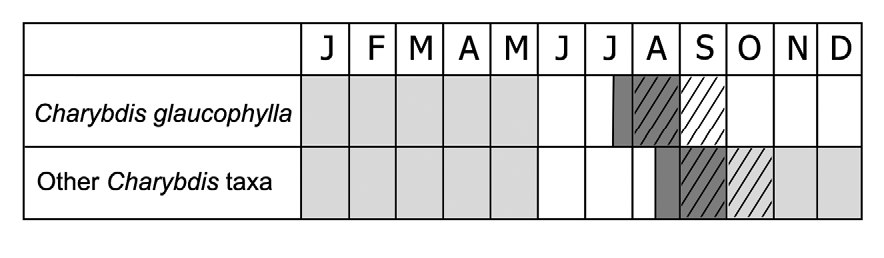
Phenology: —Flowering late July to August, fruiting August to September, foliation January to May.
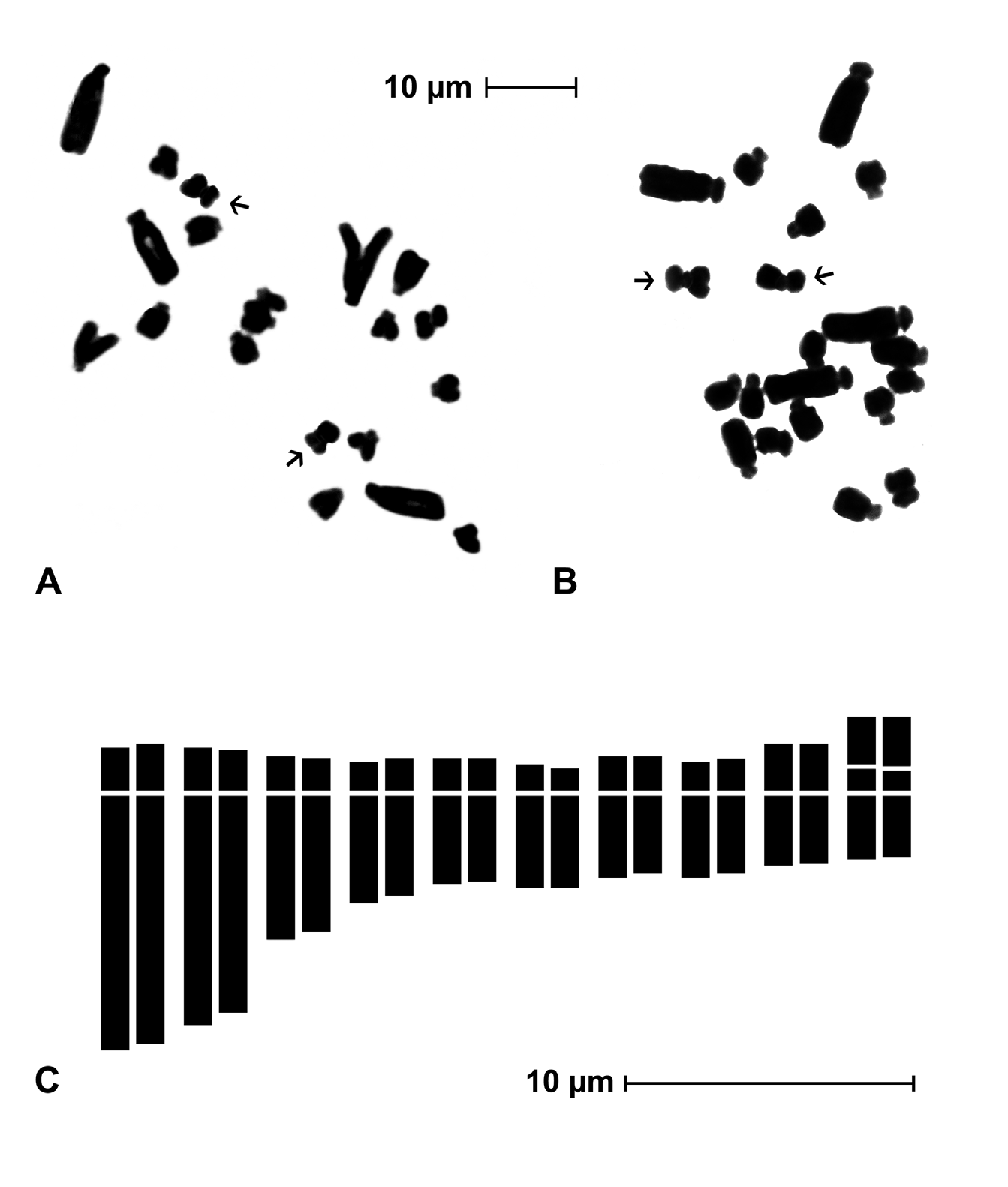
Karyology: —The somatic chromosome number of Charybdis glaucophylla ( Fig. 3A, B View FIGURE 3 ) was found to be 2n = 2x = 20 in all studied samples. The karyotype ( Fig. 3C View FIGURE 3 ) consists of 5 subtelocentric pairs, 2 of which much longer than others, 4 submetacentric pairs, one of which provided with a very long satellite on the short arm, and 1 meta-submetacentric pair (arm ratio 1.5). Chromosome measures and symmetry indices ( Tab. 1) show a relatively high degree of karyotype asymmetry both in chromosome relative size (big pairs vs. small pairs) and in centromere position (many subterminal pairs). Chromosome length varies from 10.37 ± 1 µm of the longest chromosome to 3.77 ± 0.4 µm of the shortest one, while the relative length ranges from 9.54% ± 1 to 3.45% ± 0.2. The karyotype formula can be expressed in 2n = 2x = 20: 10 st + 6 sm + 2 sm sat + 2 msm.
Habitat: —This species is typically linked to rocky and sandy places preferably next to sea and strongly windy (Mistral), where it grows on different substrata, like sand, limestone, metamorphic and volcanic rocks. Usually, it is a member of subhalophilous plant communities characterized by some endemic species linked to rocky coasts, such as Bellium crassifolium Moris (1827: 26) , Hyoseris taurina ( Pampanini 1948: 138) Martinoli (1953: 257) , Limonium sulcitanum Arrigoni (1981: 233) . On sandy dunes this species grows together with various psammophytes [ Ephedra distachya Linnaeus (1753: 1040) , Genista arbusensis Valsecchi (1984: 291) , Scrophularia ramosissima Loiseleur-Delongchamps (1807: 381) ], hence it appears to be well adapted to the marine salt spray. A small population was found in a mountain stand on metamorphic rocks at ca. 1000 m elevation. Here it grows in garigues characterized by Teucrium marum Linnaeus (1753: 564) , Genista sulcitana Valsecchi (1986: 193) and Helichrysum microphyllum ( Willdenow 1803: 1863) Cambessedes (1827: 272) subsp. tyrrhenicum Bacchetta, Brullo & Giusso (in Angiolini et al. 2005: 272).
Distribution: —On the basis of our field investigations, this species is quite rare and scattered just along the south-western part of Sardinia ( Fig. 4 View FIGURE 4 ). Currently, only four populations are known, three of them [S. Pietro (Carloforte), Pranu Sartu (Buggerru and Iglesias) and Monte Linas (Gonnosfanadiga)] occur in rocky habitats and the other one [Scivu (Arbus)] is typical of sandy dunes.
Etymology: —The name refers to the characteristic waxy and greyish-blue leaves (from Greek glaucos = greyish-blue and phyllon = leaf).
Conservation: —Despite the four populations are threatened by grazing, population decline was not observed. However, due to the population small size and the risk that the threat level can quickly increase (i.e. human activity or stochastic events), following the IUCN Red List Categories and Criteria (2001) we suggest that Charybdis glaucophylla should be treated as Vulnerable VU = D2, as the total number of mature plants ranges from 500 to 1000, distributed in less than 20 km 2.
Observations: —Historically, the Mediterranean populations of Urginea Steinheil (1834: 321) characterized by big bulb, large leaves and very long inflorescence were attributed to U. maritima ( Speta 1998) , while the plants with small bulb and leaves, and short inflorescence were referred to U. undulata ( Desfontaines 1798: 300) Steinheil (1834: 330) , if provided with flat and undulate leaves, or to U. fugax ( Moris 1827: 46) Steinheil (1834: 328) , if leaves are filiform. On the basis of genetic analyses, only U. fugax is actually included in the genus Urginea , while the remaining species are included within Charybdis ( Speta 1998, 2001). Karyological studies on these species revealed distinct ploidy levels (from 2x to 6x) within various populations, especially in C. maritima s.l. ( Boscaiu et al. 2003). The occurrence of different cytotypes, supported by genetic and morphological diversity, helped to redefine the taxonomic treatment of many populations, restoring neglected species ( Kreen et al. 2001, Speta 2001, Pfosser & Speta 2004).
The species currently belonging to the C. maritima group are: C. aphylla , C. hesperia , C. maritima , C. maura , C. numidica and C. pancration .
As already pointed out, the new species C. glaucophylla may be easily distinguished from the aforesaid species by its morphological, phenological and ecological features.
Due to the diploid chromosome complement, the whitish bulb tunics and the perigon shape, C. glaucophylla resembles C. pancration , which, however, is significantly distinct in having larger bulb (12–20 × 9–18 cm), red roots, 9–12 lanceolate green leaves, (33–)38–42(–45) × (6–)8–10(–11) cm, inflorescence 175–190 cm long, provided with 400–450 flowers, perigon lobes 7.5–8.2 mm long, ovary 3.7–4.2 mm long, and flattened stigma. These species further differ in their life cycle, since C. glaucophylla shows early flowering (July–August) and four months dormancy between flowering and leaf sprout. Leaves of C. glaucophylla actually develop in winter (January), while both in C. pancration and other species of C. maritima group they usually sprout in early autumn, afterwards flowering ( Fig. 5 View FIGURE 5 , 6 View FIGURE 6 ).
Based on its glaucous leaves, C. glaucophylla is similar to C. aphylla and C. maura , but relevant characters allow these taxa to be distinguished well enough: C. aphylla is a tetraploid species, with red bulb tunics and lanceolate leaves; instead, C. maura , despite the same diploid chromosome count and white bulb tunics as C. glaucophylla , deeply differs in leaf morphology, showing very long leaves, narrowly lanceolate and acute.
As far as chromosome morphology is concerned, the karyotype of C. glaucophylla appears clearly bimodal in having two outstanding groups of different mean sizes (big chromosomes vs. small ones). This morphology agrees fairly well with old reports quoted for some Italian populations of C. maritima s.l., especially with reference to the peculiar feature of the submetacentric chromosomes having an intercalary pseudosatellite ( Giuffrida 1950, Maugini 1953, Battaglia 1957a). The same structure was also pointed out in C. maura populations from Morocco ( Battaglia 1957a). Recent reports for C. maritima s.l. cytotypes (Boscaiu at al. 2003) and C. pancration ( Rosselló et al. 2005) , however, do not evidence this kind of chromosomes, maybe because satellites on small chromosomes are often not clearly visible. Actually, karyotypes in Charybdis taxa and populations are quite similar, indicating the existence of interspecific stability of chromosomes, against a great variability of morphological and anatomical traits and a strong geographic pattern of different cytotypes. This process can be explained by karyotype orthoselection mechanism, where structural chromosome mutation occurs in a certain way resulting in uniformity of basic number and gross morphology of chromosomes ( White 1973, Brendham 1983). Similar results, indeed, have been found in many other monocots, belonging to Asparagaceae , Xanthorrhoeaceae (Alooideae) and Amaryllidaceae ( Brendham & Doherty 1998, Vosa 2005, Cisternas et al. 2010).
As Pfosser & Speta (2004) hypothesized, all diploid Charybdis and Urginea populations most likely displayed a clustering in the western Mediterranean area as far back as Early Miocene, when the main islands Sardinia, Corsica, Sicily and the Balearic Archipelago were much further west and closest to the Iberian and African coasts. This overlapping distribution of diploid taxa and the current geographic pattern of Charybdis cytotypes seems to suggest an early colonization of W Mediterranean by the diploid populations (which are probably the ancestral ones), starting from the Iberian peninsula and NW Africa, with a subsequent eastward migration. Then, geographic isolation and ecological adaptation may have allowed some populations to well differentiate, while autopolyploidy or hybridization processes gave rise to several distinct cytotypes and genetic patterns.
As a matter of fact, C. glaucophylla is a diploid species endemic to the Sulcis-Iglesiente territory, which forms the SW part of Sardinia and is isolated from the rest of the island by the Graben of Campidano. This area, including the oldest geologic elements of Sardinia dates back to the Paleozoic, representing a well distinct biogeographic sector that is very rich in rare and endemic taxa, many of which are paleoendemics ( Bacchetta & Pontecorvo 2005, Bacchetta et al. 2007). In this biogeographic sector C. glaucophylla is sympatric only with tetraploid populations of C. maritima s.l. ( Boscaiu et al. 2003), but in the four locations currently known it is found exclusively, and nearby do not find C. maritima ; for this reason C. glaucophylla can be considered allopatric with respect to the latter.
Based on these considerations, supported by distinctive morphological features, unusual life cycle, diploid arrangement, geographic confinement and scattered distribution, C. glaucophylla can be considered a relictual schizoendemic arisen by gradual diversification as a consequence of a long geographic isolation.
Paratypes: — ITALY. Sardinia: Isola di San Pietro: Canale di Basilio, Carloforte (CI), 39°9'58.29"N, 8°14'52.94"E, 15 August 2002, G. Bacchetta & C. Pontecorvo s.n. (CAG); Cala Vinagra , Carloforte (CI), 39°9'47.66"N, 8°14'30.95"E, 3 September 2005, G. Bacchetta & C. Pontecorvo s.n. (CAG); Canalgrande , Pranu Sartu , Iglesias (CI), 39°21'19.50"N, 8°23'37.86"E, 2 April 2005, G. Bacchetta & C. Pontecorvo s.n. (CAG); Penisola a N di Punta Cubedda , Pranu Sartu , Iglesias (CI), 39°21’11.60’’N, 8°23’11.20’’E, 27 August 2005, G. Bacchetta, C. Pontecorvo & T. Carai s.n. (CAG); Scivu-Is Arenas, Arbus (VS), 29 March 2010, G. Bacchetta s.n. (CAG); Monte Linas, Punta Cammedda, Gonnosfanadiga (VS), 39°26'13.55"N, 8°38'13.45"E, 27 July 2012, G. Bacchetta & C. Pontecorvo s.n. (CAG) GoogleMaps .
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |