Macrobiotus kristenseni, Guidetti & Peluffo & Rocha & Cesari & Peluffo, 2013
|
publication ID |
https://doi.org/ 10.1080/00222933.2013.800610 |
|
DOI |
https://doi.org/10.5281/zenodo.5197614 |
|
persistent identifier |
https://treatment.plazi.org/id/F9369705-FFE8-FF9B-FDB0-F9C8EAF6FCDA |
|
treatment provided by |
Felipe |
|
scientific name |
Macrobiotus kristenseni |
| status |
sp. nov. |
Macrobiotus kristenseni View in CoL sp. nov.
( Figures 1–5 View Figure 1 View Figure 2 View Figure 3 View Figure 4 View Figure 5 and Table 2)
Diagnosis
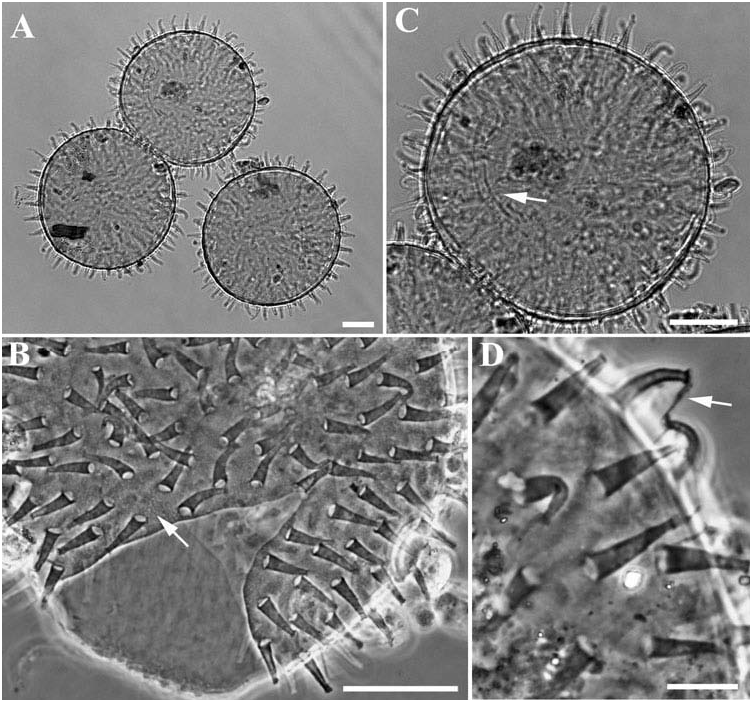
Size medium. Cuticle with pearls (pores). Eye-spots present. Buccal tube of average width. Buccal armature with two bands of teeth and transverse ridges. Bulb with two macroplacoids and small microplacoid. Long Y-shaped claws with two short accessory points on the main branch, lunule smooth or weakly crenate in the hind legs. Free eggs with processes in shape of elongated cones with truncated and enlarged apexes. Limnoterrestrial.
Description
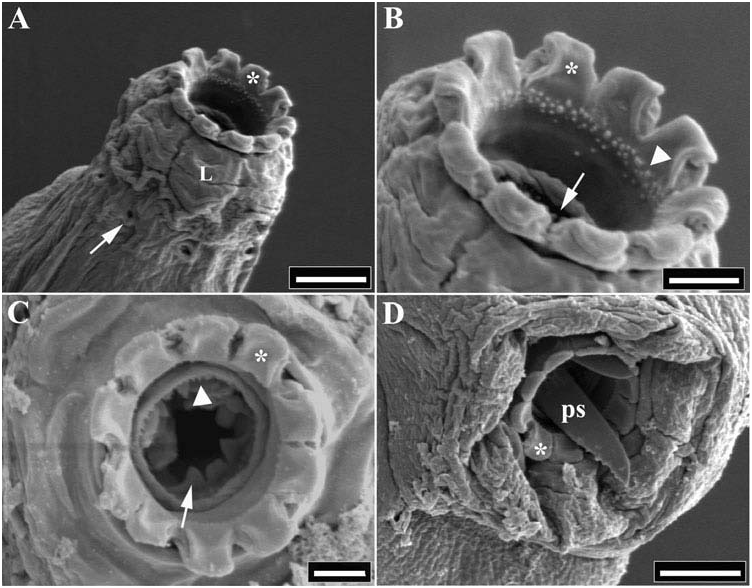
Size range: 247–544 µm long. Colourless, yellowish, greyish or light brown, sometimes with transverse bands with brown pigment. Eye-spots present in all specimens observed ( Figure 1 View Figure 1 ), eye-spot colour not detected. Cuticle smooth under LM, except small granulations on outer part of legs. Cuticular pores rounded or ovate approximately 1 µm in diameter ( Figure 2 View Figure 2 ), generally with thickened edges, irregularly distributed on the body surface (dorsally and ventrally). Near the anterior end, a ring of pores with evident thickened edges and placed in a regular manner. Ten small peribuccal lamellae surround the mouth opening, followed by six buccal sensory lobes ( Figure 3 View Figure 3 ). Buccal armature, poorly distinguishable under LM, formed by an anterior band of small teeth of variable thickness at the base of the buccal lamellae, sometimes reduced to a single row of teeth ( Figures 1 View Figure 1 and 3 View Figure 3 ); a posterior band of larger teeth, sometimes in a single row, followed by three dorsal and three ventral fine transverse ridges ( Figures 1 View Figure 1 and 3 View Figure 3 ). Ventral transversal ridges clearly smaller than the dorsal, lateral ridges larger than the middle ones ( Figure 1 View Figure 1 ). Long ventral strengthening bar, about 65% of buccal tube total length, with an anterior crest on its ventral margin ( Figure 1 View Figure 1 ). Stylet support insertion point posteriorly placed, at about 75% the distance of the buccal tube from the anterior margin of the stylet sheaths. Anterior end of the piercing stylet with external edge straight and serrate, and internal edge curved and smooth ( Figure 3 View Figure 3 ). Pharyngeal bulb oval to circular, with well-developed pharyngeal apophyses. Two elongate macroplacoids and a small elongate microplacoid close to the second macroplacoid. First macroplacoid – the longest – with a small medial constriction, second macroplacoid quadrangular or circular ( Figure 1 View Figure 1 ). Slanting pharyngeal bars present between the apophyses and the first macroplacoids ( Figure 1 View Figure 1 ).
Long double-claws. Main branch long with two fine accessory points, not reaching the distal bend of the main branch, and not clearly visible at LM ( Figure 2 View Figure 2 ). Secondary branch long and evidently bended. Short and large common tract, without distal part, and with an evident stalk connecting the claw to the lunule ( Figure 2 View Figure 2 ). Hind claws larger than the others. Lunules smooth in legs I–III, larger and with weakly crenate edges in the hind legs ( Figure 2 View Figure 2 ).
Measurements of different animal cuticular structures, pt values and a descriptive statistical analysis are given in Table 2.
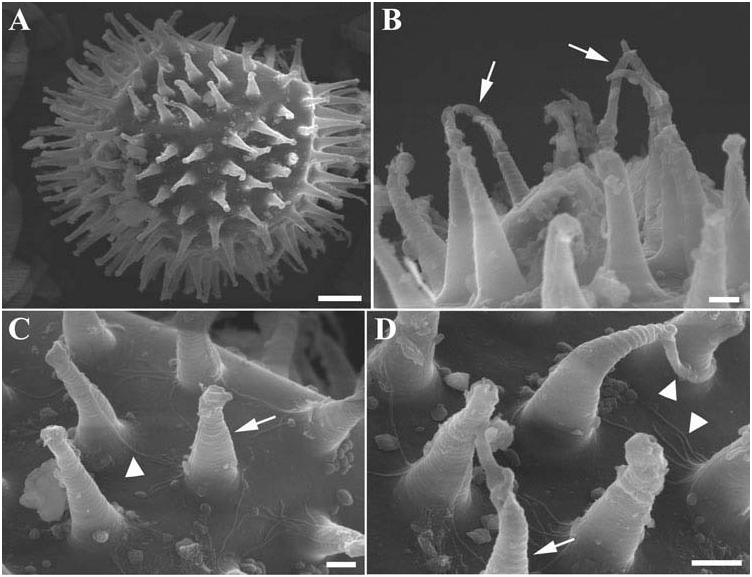
Ornamented round egg laid free ( Figures 4 View Figure 4 and 5 View Figure 5 ). Egg ornamentation formed by processes in the shape of elongated empty cones (height 6.2–12.7 µm, diameter of their base 2.0–3.3 µm) with truncated and enlarged apexes (diameter less than 2 µm) ( Figures 4 View Figure 4 , 5 View Figure 5 ). Several processes with a long thick filament developing from the distal disk ( Figures 4 View Figure 4 , 5 View Figure 5 ). Ornamentations with smooth surface frequently ringed. Diameter of eggs without processes 73.0–96.3 µm, diameter of eggs with processes 94.0–125.0 µm. Number of processes present on a diameter 10–13. Chorion smooth; in some eggs a fine granulation and very small scattered pores in between processes visible by LM ( Figure 4 View Figure 4 ). Three embryonated eggs were found ( Figure 4 View Figure 4 ). In addition to the typical eggs described above, few eggs present slightly higher and more flexible processes internally septated and with a smaller base ( Figures 4 View Figure 4 , 5 View Figure 5 ).
Type locality
South America, Argentina, La Pampa Province, city of Santa Rosa (36 ◦ 39 ′ S, 64 ◦ 17 ′ W) at 177 m above sea level, epiphytic lichens GoogleMaps .
Type material
Holotype (MLP-Oi 3726), four paratypes (MLP-Oi 3727) and three eggs are housed at the Museum of Natural Sciences, National University of La Plata ( Argentina), and 32 paratypes are housed in J. Peluffo’s personal collection in the Department of Natural Sciences at the National University of La Pampa ( Argentina). The voucher specimens (two pictures of the hologenophore animals, 16 cryoconserved paragenophore animals, eight paratypes / paragenophore animals and eggs) and a fragment of the lichen are deposited in the MOD:BRT collection of the Department of Life Sciences ( University of Modena and Reggio Emilia , Italy) registered in the Registry of Biological Repositories (www.biorepositories.org).
Additional material
South America, Argentina, La Pampa Province, city of General Pico at 143 m above sea level (125 km northeast of Santa Rosa) and Natural Reserve Parque Luro (35 km south of Santa Rosa , caldén forest), in all cases on epiphytic lichens .
Etymology
The name is dedicated in honour of Prof. Reinhardt M. Kristensen, of the Invertebrate Department of The Natural History Museum of Denmark of the University of Copenhagen.
Remarks
Under SEM, the general surface of the cuticle appears slightly wrinkled ( Figure 2 View Figure 2 ). It should be verified whether this character is an effect of SEM preparation. An egg observed by SEM had processes with filamentous strips on their surface ( Figure 5 View Figure 5 ). These strips seem to be present on the same side of all processes: it is not clear whether they are artefacts or real morphological structures.
Where M. kristenseni sp. nov. appears in a sample, it is either the only or the dominant tardigrade species. In the latter case, it is accompanied by one or more of the following species: Echiniscus rufoviridis du Bois Raymond-Marcus, 1944 ; Milnesium cf. tardigradum ; Ramazzottius cf. oberhaeuseri. The phorophyte of the lichen communities in which it was recorded more frequently is the autochthonous tree species Prosopis caldenia (Burk.) .
Taxonomic remarks. Macrobiotus kristenseni sp. nov. is similar to species of the M. hufelandi group. Among these species, Macrobiotus sandrae Bertolani and Rebecchi, 1993 is the most similar, sharing a weak buccal armature, reduced ventral transversal crests, and indented lunule only on the fourth leg pair, but it differs from M. kristenseni sp. nov. by longer claw accessory points and larger dorsal transversal crests. Macrobiotus kristenseni sp. nov. differs from all the M. hufelandi group of species in that the shape of the egg processes is different from any other egg of the species group. The most similar eggs are those of Macrobiotus recens Cuénot, 1932 , which share similar thin, long, conical processes with a truncated apex but in M. recens an evident series of small indentations around the base of the egg processes is present ( Pilato and Bertolani 2004).
Molecular results
The molecular phylogeny based on the 18S rRNA gene (897 bp) indicates that M. kristenseni sp. nov. does not share its 18S rRNA sequence with any other Macrobiotus species sequenced so far ( Figure 6 View Figure 6 ), but it is in a sister-group relationship with Macrobiotus sapiens Binda and Pilato, 1984 within a well-supported clade grouping all the M. hufelandi group of species (i.e. M. sapiens , Macrobiotus hufelandi C.A.S. Schultze, 1834 , Macrobiotus polonicus Pilato, Kaczmarek, Michalczyk and Lisi, 2003 , Macrobiorus vladimiri Bertolani, Biserov, Rebecchi and Cesari, 2011 ).
Molecular analysis of the mitochondrial DNA cox1 gene was carried out on sequences of 491–624 bp. Two different sequences (i.e. two haplotypes) are present in the five specimens analysed: three specimens are of one haplotype, two specimens are of the other ( Table 3; Figure 7 View Figure 7 ). The two haplotypes are very similar and the intraspecific genetic distance among M. kristenseni sp. nov. specimens is low (1.4–1.5%; Table 3). Mean K2P distances between specimens pertaining to different species are very high (18.2–33.4%; Table 4). In particular, among the considered species, M. kristenseni sp. nov. has the greatest genetic difference with respect to the other M. hufelandi group of species (23.1–33.4%), while the difference among the considered species excluding M. kristenseni sp. nov. is 18.2–24.4%. The neighbour-joining dendrogram based on cox1 sequences confirms these differences between the two M. kristenseni sp. nov. haplotypes, and the differences among this species and the other species of M. hufelandi group ( Figure 7 View Figure 7 ).
| MOD |
University of Modena and Reggio Emilia, Department of Biology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |