GEOPLANINAE Stimpson, 1857
|
publication ID |
https://doi.org/ 10.1080/00222933.2015.1084057 |
|
DOI |
https://doi.org/10.5281/zenodo.4324237 |
|
persistent identifier |
https://treatment.plazi.org/id/03F7B26E-FFD2-FFB5-B1FB-1E4FAEB9FAED |
|
treatment provided by |
Carolina |
|
scientific name |
GEOPLANINAE Stimpson, 1857 |
| status |
|
Subfamily GEOPLANINAE Stimpson, 1857 View in CoL
Genus Cratera Carbayo et al., 2013 View in CoL
Cratera ochra View in CoL sp. nov.
( Figures 2 – 7 View Figure 2 View Figure 3 View Figure 4 View Figure 5 View Figure 6 View Figure 7 )
Geoplanidae 4: Leal-Zanchet & Carbayo, 2000
Geoplanidae 2: Carbayo et al., 2002
Geoplana sp. 4: Leal-Zanchet & Baptista, 2009
Geoplana sp. 4: Leal-Zanchet et al., 2011
Etymology
The specific name refers to the dorsal pigmentation, which is yellow-ochre.
Type-material
Holotype. MZUSP PL. 1564: leg. P. K. Boll, 1 August 2013, São Francisco de Paula (National Forest of São Francisco de Paula), RS, Brazil – anterior tip: transverse sections on 21 slides; anterior region at the level of the ovaries: sagittal sections on 52 slides; prepharyngeal region: transverse sections on nine slides; pharynx: sagittal sections on 34 slides; copulatory apparatus: sagittal sections on 26 slides.
Paratypes. São Francisco de Paula , RS, Brazil – MZU PL .00187: leg. R. Murowaniecki , 4 May 1998, National Forest of São Francisco de Paula – copulatory apparatus: sagittal sections on 26 slides; MZU PL .00188: leg . F . Carbayo , 8 June 1998, National Forest of São Francisco de Paula – anterior tip: transverse sections on 22 slides; region at the level of the ovaries: sagittal sections on 67 slides; pre-pharyngeal region: transverse sections on eight slides; pharynx: sagittal sections on 24 slides; copulatory apparatus: sagittal sections on 22 slides; MZU PL .00189: leg . F . Carbayo , 25 September 1998, National Forest of São Francisco de Paula – copulatory apparatus: horizontal sections on 22 slides; MZU PL .00190: leg . C . Palácios 17 October 2004, Research and Conservation Center Pró- Mata – pre-pharyngeal region: transverse sections on seven slides; pharynx: sagittal sections on 15 slides; copulatory apparatus: sagittal sections on 12 slides .
Diagnosis
Specimens of Cratera with yellow-ochre dorsal colour mainly with dispersed greyish or greyish-brown pigmentation constituting two broad dorsal bands; eyes dorsal; glandular margin with at least four types of glands; mc: h, 11 – 12%; pharynx cylindrical; prostatic vesicle extrabulbar with proximal portion ample and laterally expanded and distal portion tubular; ovovitelline ducts emerging dorsally from the median third of ovaries and ascending anterior to the gonopore.
Molecular diagnosis. This species includes all populations that cluster with specimens KT 250622 View Materials to KT 250624 View Materials (see Supplementary material, Table S1), with significant support in phylogenetic analyses.
Type-locality
São Francisco de Paula (National Forest of São Francisco de Paula), state of Rio Grande do Sul (RS), Brazil.
Distribution
Rio Grande do Sul (São Francisco de Paula), Brazil.
Description
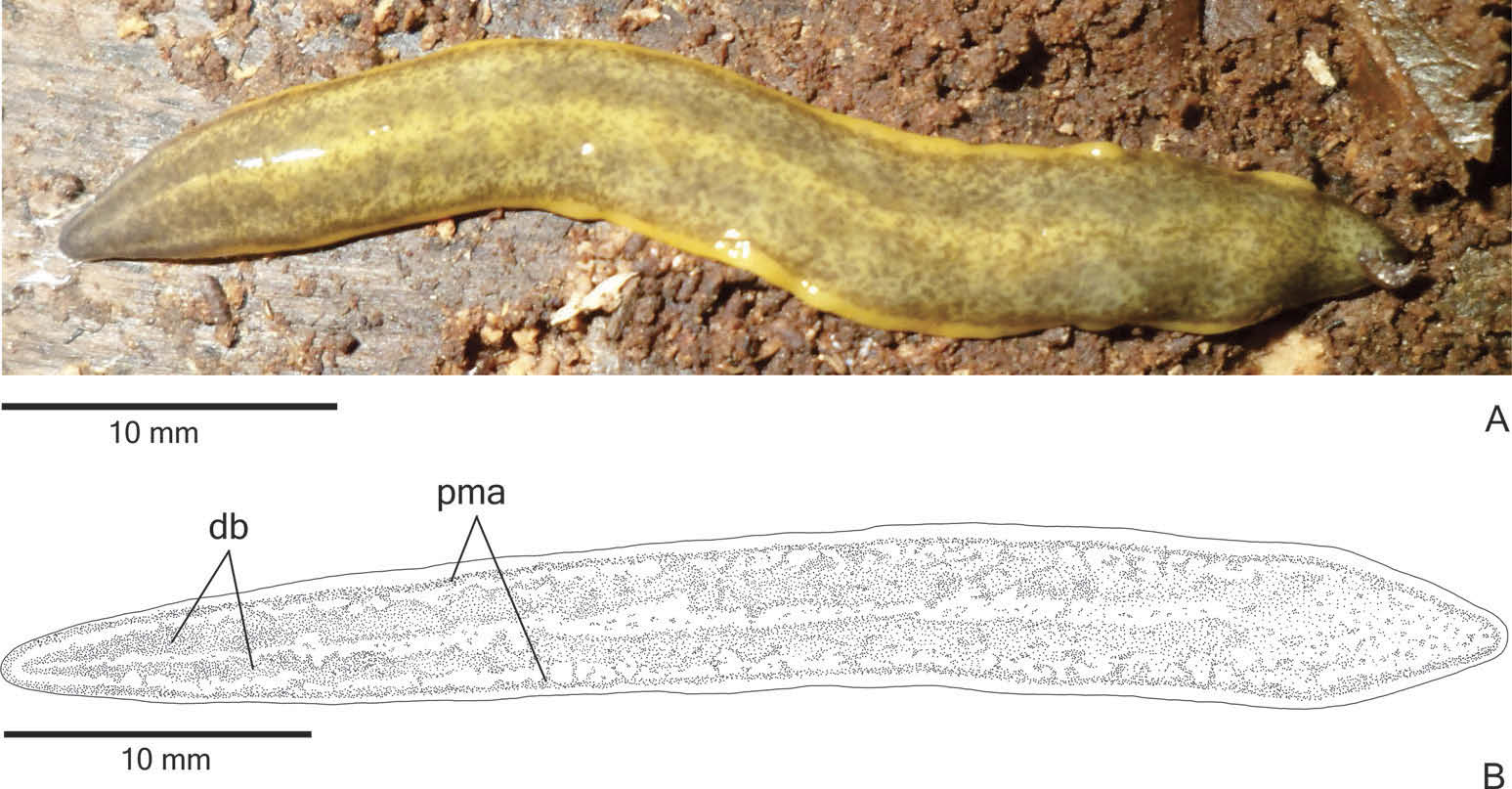
External features. Body broad with parallel margins and dorsal surface convex; anterior tip rounded and posterior tip pointed ( Figure 2A View Figure 2 ). When creeping, maximum length reaches 85 mm. After fixation, maximum length was 52 mm ( Table 1). Mouth and gonopore located at the posterior third of the body ( Table 1).
In living animals, dorsal surface with dispersed greyish or greyish-brown pigmentation constituting two broad dorsal bands; the dorsal yellow-ochre ground colour is mainly visible between the dorsal bands and on the body margins. The ground colour is darker on the body margins than on the rest of the dorsal surface. The dorsal pigmentation concentrates at the anterior extremity ( Figure 2A View Figure 2 ). Under the stereomicroscope, a greyish pigmentation forms two densely pigmented dorsal bands, as well as contours the anterior tip ( Figures 2B View Figure 2 , 3A View Figure 3 ). Posterior to the anterior tip, this pigmentation becomes more irregular and loosely arranged in flecks over the dorsum, making the yellow-ochre ground colour visible ( Figures 2B View Figure 2 , 3B View Figure 3 ). These flecks form the dorsal bands as well as paramarginal stripes. The dorsal bands become inconspicuous towards the posterior tip, where the pigmentation is more irregularly distributed ( Figure 3B View Figure 3 ). Ventral surface pale yellow. After fixation, the dorsal pigmentation becomes greyish and mainly greyish-brown next to the margins; dorsal ground colour becomes pale yellow, as well as the ventral surface of the body.
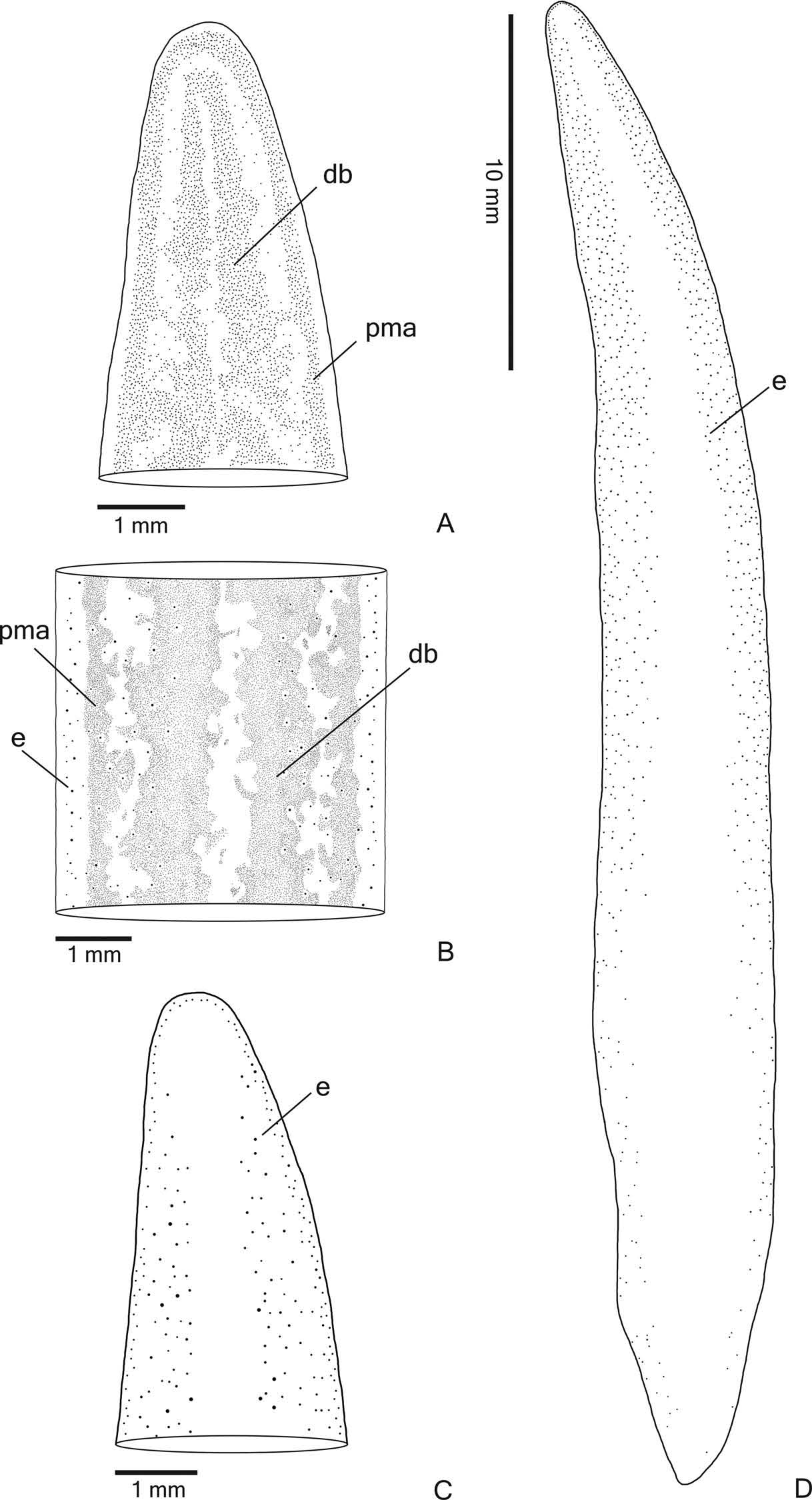
Eyes monolobated. They are initially uniserial, with pigment cups of about 15 μm in diameter, surrounding anterior tip ( Figure 3C View Figure 3 ). After the second millimetre, the monolobated eyes become larger (with pigment cups of about 30 µm in diameter) and spread onto the dorsal surface, occupying the maximum width of about 40% of the body width on each side of the body. Eyes remain dorsal, but become less numerous towards posterior tip ( Figure 3D View Figure 3 ). Inconspicuous clear halos may occur around dorsal eyes ( Figure 3B View Figure 3 ).
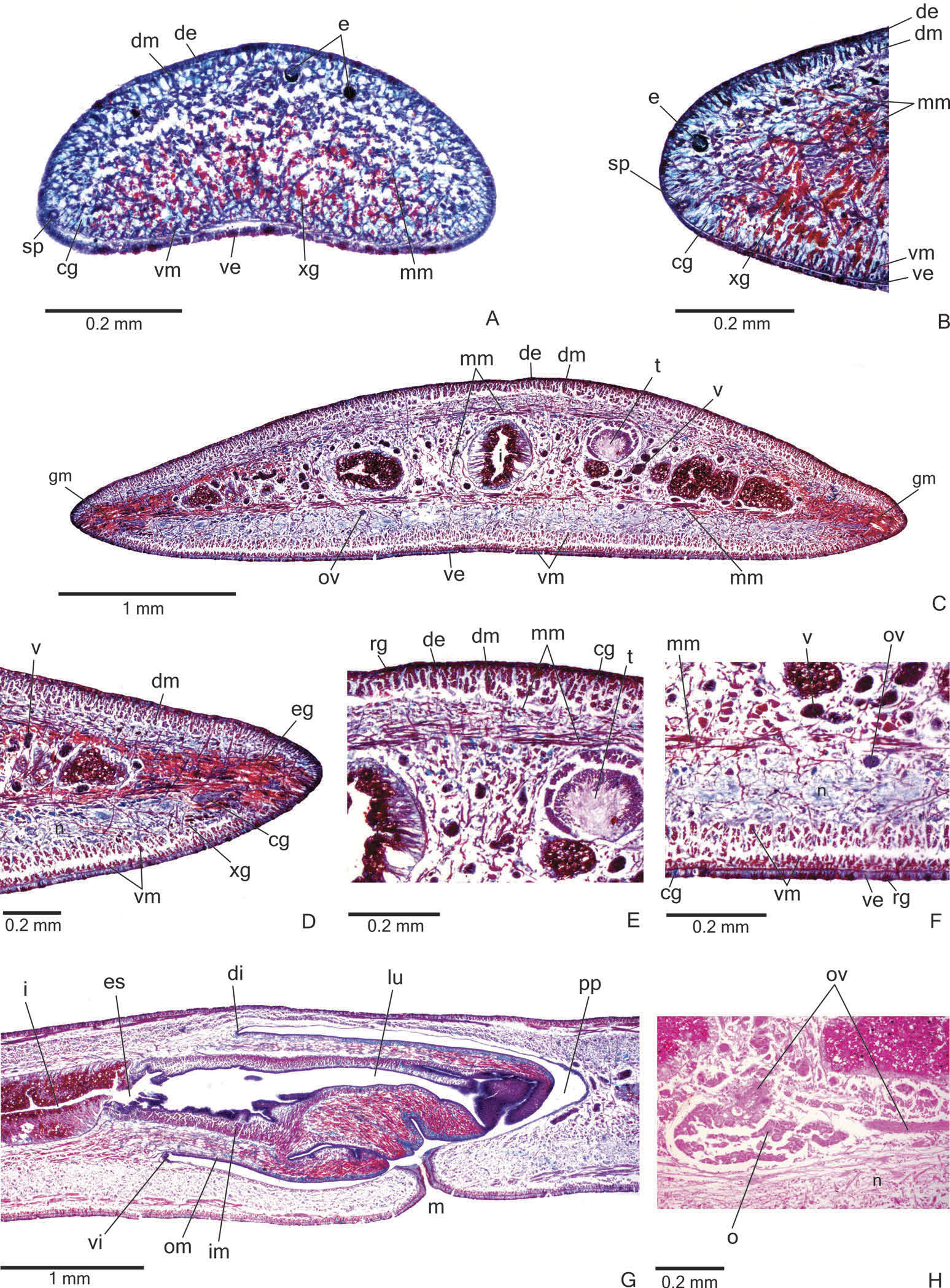
Sensory organs, epidermis and body musculature. Sensory pits ( Figure 4A, B View Figure 4 ), as simple invaginations, contour anterior tip and occur ventromarginally in an irregular, single row in the anterior eighth of the body. Initially they occur at intervals of approximately 18 µm, becoming gradually sparser. Their depth is about 25 – 50 µm.
Creeping sole occupying the whole body width. Two types of glands discharge through dorsal epidermis and body margins of the pre-pharyngeal region: numerous rhabditogen cells with xanthophil secretion (rhammites) and cells with amorphous, cyanophil secretion ( Figure 4E View Figure 4 ). Creeping sole receives the secretion from amorphous, cyanophil glands, as well as few rhabditogen cells with small, xanthophil rhabdites and cells with coarse granular, xanthophil secretion ( Figure 4F View Figure 4 ). The glandular margin ( Figure 4C, D View Figure 4 ), which is visible after the second millimetre of the body, receives openings of at least four types of glands. Two of them are more numerous: glands with erythrophil, coarse granules, and glands with xanthophil, coarse granules. Other two types are scarcer: cyanophil glands with amorphous secretion and erythrophil glands with fine granules. On anterior tip, glands with cyanophil, amorphous secretion and rhabditogen glands with xanthophil secretion open through the whole surface of the body and glands with coarse granular, xanthophil secretion have numerous openings through the ventral epidermis ( Figure 4A View Figure 4 ).
Cutaneous musculature with the usual three layers (circular, oblique and longitudinal layers), longitudinal layer with thick bundles ( Figure 4C – F View Figure 4 , Table 2). Cutaneous musculature thinner in the pre-pharyngeal region than in the anterior region of the body, gradually diminishing its thickness towards anterior tip ( Figure 4A View Figure 4 ). Musculature becoming progressively lower towards body margins. Ventral musculature slightly higher than dorsal at the sagittal plane. Ratio of mc: h 11 – 12% ( Table 2).
Mesenchymal musculature ( Figure 4C, E, F View Figure 4 ) well developed, mainly composed of three layers: (1) dorsal subcutaneous, located mainly close to the cutaneous musculature, with oblique fibres variously oriented (about 5 – 14 fibres thick); (2) supra-intestinal transverse (about 7 – 14 fibres thick); (3) subintestinal transverse (about 14 – 22 fibres thick). In addition, there are scattered transverse subneural fibres and ventral subcutaneous oblique fibres, as well as numerous dorso-ventral fibres. On the anterior region of the body ( Figure 4A, B View Figure 4 ), the mesenchymal musculature is less developed than in the prepharyngeal region.
Pharynx. Pharynx cylindrical, about 5 – 6% of body length, with dorsal insertion posteriorly shifted, but located at the anterior third of pharyngeal pouch. Mouth posterior to the dorsal insertion, in the median third of pharyngeal pouch ( Figure 4G View Figure 4 ).
Oesophagus short; oesophagus: pharynx ratio varying from 14% in paratype MZU PL.00190 to 18.5% in the holotype. It is lined by ciliated, cuboidal to columnar epithelium with some insunk nuclei. The oesophagus is coated with a thick subepithelial muscle layer with circular fibres, followed by a thin muscle layer with longitudinal fibres (about 60 – 100 µm thick).
Pharynx and pharyngeal lumen lined by ciliated, cuboidal epithelium with insunk nuclei. Pharyngeal glands constituted by three secretory cell types: cells with fine granular erythrophil secretion; cells with fine granular xanthophil secretion and cells with amorphous cyanophil secretion. Cell bodies of pharyngeal glands located in the mesenchyme. Outer pharyngeal musculature (about 10 – 24 µm thick) comprised of subepithelial layer of longitudinal muscles, followed by a circular layer and more internally by longitudinal fibres. All layers become thinner towards pharyngeal tip and the longitudinal internal fibres become mixed with the circular fibres. Inner pharyngeal musculature (about 100 – 150 µm thick) comprises a thick subepithelial layer with circular fibres, mixed internally and externally with longitudinal fibres. Inner musculature gradually becomes thinner towards pharyngeal tip.
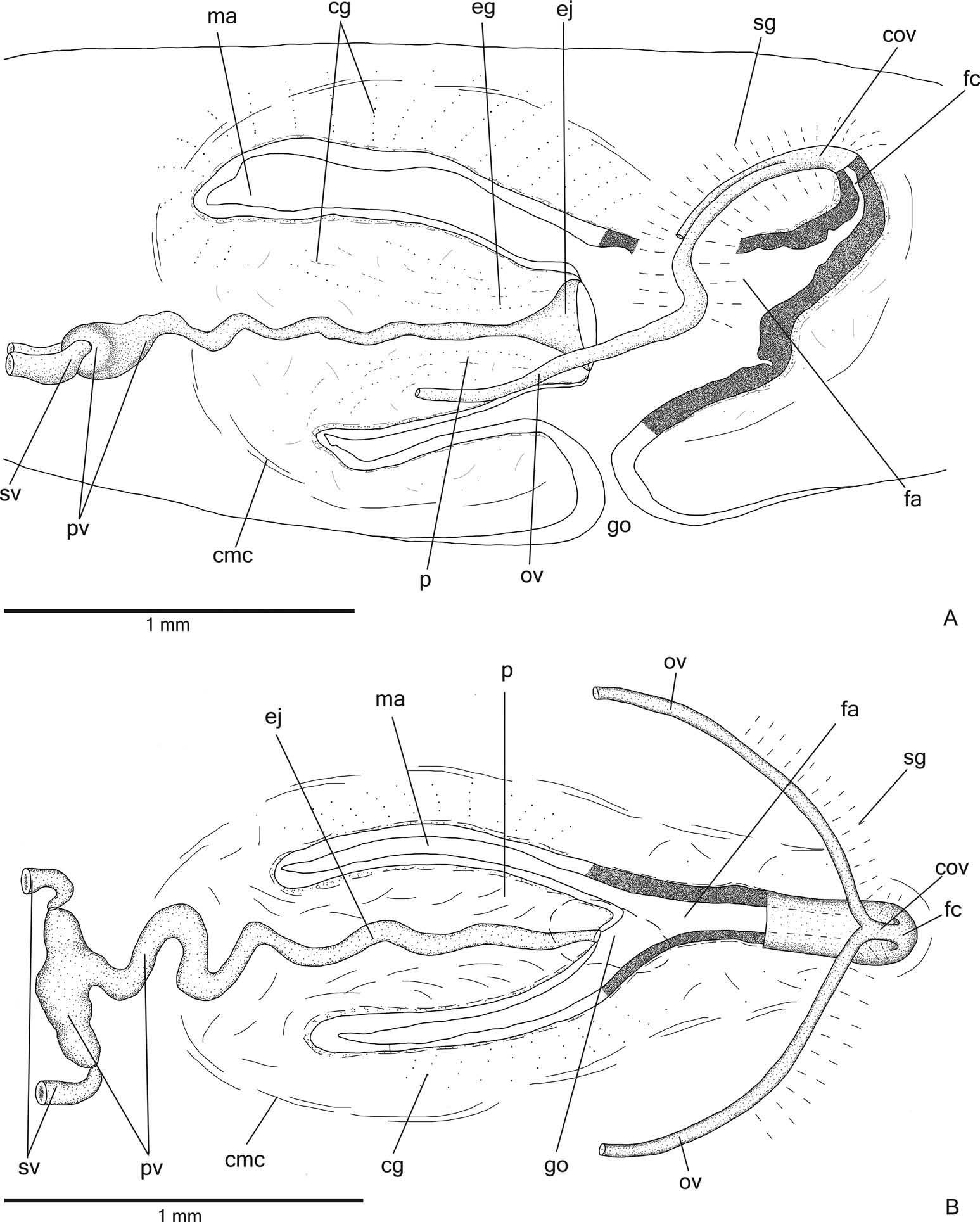
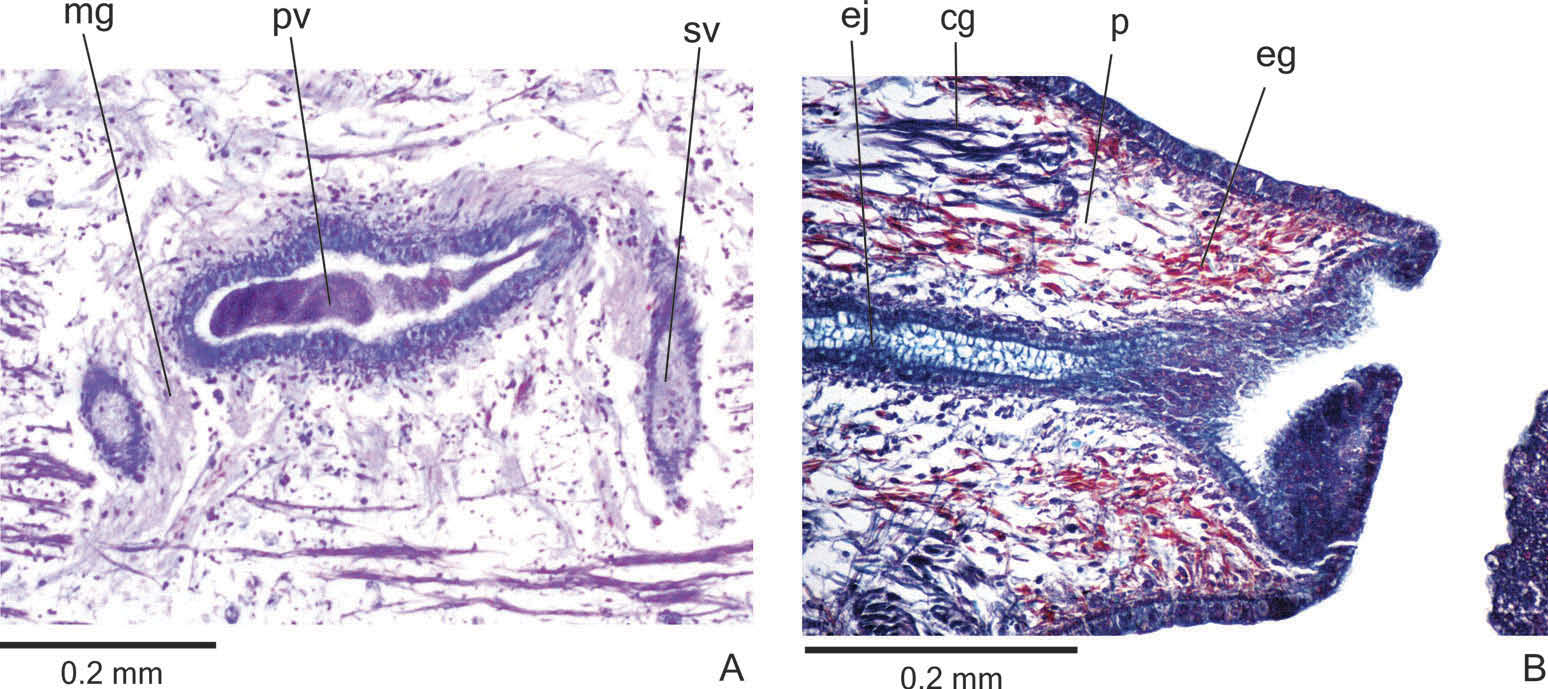
Reproductive organs. Testes in one irregular row on either side of the body, located beneath the dorsal transverse mesenchymal muscles ( Figure 4C, E View Figure 4 ). They begin slightly posterior to the ovaries, in the anterior third of the body, and extend to near the root of the pharynx ( Table 1). Sperm ducts medial to ovovitelline ducts, above fibres of the subintestinal transverse mesenchymal musculature, in pre-pharyngeal region. They form spermiducal vesicles posterior to pharynx. Distally, spermiducal vesicles enter laterally into the proximal expanded portion of the prostatic vesicle ( Figures 5A, B View Figure 5 , 7A View Figure 7 ). Extrabulbar prostatic vesicle, unpaired, located near the common muscle coat, with ample proximal portion and tubular distal portion. The proximal portion is laterally expanded and T-shaped ( Figures 5B View Figure 5 , 7A View Figure 7 ); it is located closer to the ventral epidermis than to the dorsal epidermis. The prostatic vesicle penetrates the common muscle coat, becoming sinuous, and continues inside the penis papilla as an ejaculatory duct ( Figure 5A View Figure 5 ). Ejaculatory duct slightly sinuous, opening through an expansion at the tip of the penis papilla ( Figure 5B View Figure 5 , 7B View Figure 7 ). Male atrium without folds, occupied by the conical and symmetric penis papilla with ventral insertion posteriorly displaced ( Figures 5A, B View Figure 5 , 6A, B View Figure 6 , Table 1).
Lining epithelium of sperm ducts cuboidal and ciliated; thin muscularis (about 2 – 4 µm) constituted of interwoven circular and longitudinal fibres. Prostatic vesicle lined with ciliated, tall columnar or pseudostratified epithelium. Muscularis of prostatic vesicle (about 10 – 20 µm thick) comprises circular fibres mixed with longitudinal and oblique fibres. Ejaculatory duct lined with ciliated, columnar epithelium, showing a pseudostratified appearance at the expanded portion ( Figure 7B View Figure 7 ). Muscle coat of ejaculatory duct thin (about 3 – 5 µm), mainly constituted of circular fibres. Both prostatic vesicle and ejaculatory duct receive openings from glands producing a fine granular, mixed secretion ( Figure 7A View Figure 7 ) as well as glands with amorphous cyanophil secretion. Granules with a mixed secretion have a cyanophil external part and an erythrophil internal core. Both glands with extrabulbar cell bodies.
Penis papilla lined with non-ciliated, pseudostratified epithelium. Penis glands produce numerous fine granular, xanthophil and erythrophil secretions as well as less numerous cyanophil secretions of two types (amorphous and fine granular secretion). All penis glands with cell bodies external to common muscle coat; their long necks run longitudinally through the papilla ( Figures 6A, B View Figure 6 , 7B View Figure 7 ), with numerous openings through its lining epithelium. Muscularis of the penis papilla (about 6 – 10 µm thick) composed of a subepithelial circular layer, followed by a longitudinal layer.
Epithelial lining of male atrium columnar to pseudostratified (about 20 – 150 µm thick), non-ciliated. Four types of glands empty through this epithelium: abundant cells with fine granular, cyanophil secretion and cells with cyanophil amorphous secretion; and few glands with fine granular, mixed secretion (cyanophil peripheral portion and erythrophil core) as well as scarce xanthophil glands. These glands have their cell bodies located in the mesenchyme, mainly anterior and laterally to the copulatory apparatus, or between the fibres of the common muscle coat. Numerous necks of both types of cyanophil glands concentrate their openings at the dorso-lateral wall of the male atrium ( Figure 6A, B View Figure 6 ). Their numerous gland necks are located between fibres of the stroma in the dorso-lateral walls of the male atrium. Muscularis of male atrium (about 4 – 8 μm thick) comprised of a subepithelial layer with circular fibres, followed by a longitudinal layer.
Vitellaria ( Figure 4C, F View Figure 4 ), situated between intestinal branches, opening into the ovovitelline ducts. Ovaries oval-elongate ( Figure 4H View Figure 4 ), measuring about 300 – 520 µm in diameter in the holotype and paratype MZU PL.00188, respectively. They are located dorsal to the ventral nerve plate, in the anterior third of the body ( Table 1). Ovovitelline ducts emerge dorsally from the median third of the ovaries ( Figure 4H View Figure 4 ) and run posteriorly immediately above the nerve plate. Lateral to the female atrium, ovovitelline ducts ascend postero-medially, to unite dorsal to the end of the median third or dorsal to the posterior third of the female atrium, thus forming the common glandular ovovitelline duct ( Figures 5A, B View Figure 5 , 6A View Figure 6 ). The female genital duct is dorso-anteriorly curved ( Figures 5A, B View Figure 5 , 6A View Figure 6 ). Female atrium, shorter than male atrium, funnel-shaped without folds ( Figures 5A, B View Figure 5 , 6A, B View Figure 6 , Table 1).
Ovovitelline ducts and common ovovitelline duct lined with ciliated, cuboidal to columnar epithelium and covered with intermingled circular and longitudinal muscle fibres (about 3 – 5 μm). Numerous shell glands with erythrophil secretion empty into common glandular ovovitelline duct as well as into the distal half of the ascending portion of the ovovitelline ducts ( Figures 5A, B View Figure 5 , 6A, B View Figure 6 ).
Female genital duct and atrium lined by tall columnar to pseudostratified epithelium ( Figure 6A View Figure 6 ) containing some lacunae. Female duct and atrium receive abundant cyanophil secretion of two types (amorphous and fine granular secretion), as well as erythrophil glands and few xanthophil glands. Their cell bodies are located between fibres of the atrial stroma or external to the common muscle coat. Muscularis of vagina and female atrium (about 15 µm thick) composed mainly of circular fibres interposed with some longitudinal fibres.
Gonoduct vertical at the sagittal plane. Male and female atria with ample communication, without separating folds ( Figures 5A, B View Figure 5 , 6A, B View Figure 6 ). Gonoduct lined with ciliated columnar epithelium, receiving the openings of numerous rhabditogen glands and cyanophil glands with amorphous secretion. Muscularis of gonoduct comprised of a subepithelial layer of circular fibres, followed by a longitudinal layer.
Common muscle coat with circular, longitudinal and oblique fibres; it is thin along both male and female atria. A stroma with sparse intermingled muscle fibres separates the atrial muscularis and common muscle coat.
Variability
Paratype MZU PL .000190 is at an initial stage of maturity, showing poorly developed testes and vitellaria, few shell glands and short female atrium. In paratype MZU PL .00187, the penis papilla is protruded towards the gonoduct and there is abundant cyanophil secretion in the male atrium and in the gonoduct; the epithelial lining of the female atrium is taller laterally than medially. The proximal portion of the prostatic vesicle contains sperm in paratype MZU PL .00189.
Comparative discussion
In accordance with the phylogenetic analyses, the external and internal characters of the new species herein described as Cratera ochra sp. nov. strongly support its inclusion into the genus Cratera Carbayo et al. The morphological characters concern the following features: monolobated eyes, ejaculatory duct forming a distal cavity in the penis papilla and funnel-shaped female atrium.
Cratera ochra sp. nov., with dorsal eyes, can be differentiated from Cratera pseudovaginuloides ( Riester, 1938) , Cratera yara (E.M. Froehlich, 1955) and Cratera cuarassu Carbayo & Almeida, 2015 , which have exclusively marginal eyes (E.M. Froehlich 1955; Froehlich 1956; Carbayo and Almeida 2015). It is also easily distinguished from Cratera steffeni Rossi et al., 2014 , in which the eyes are restricted to either side of the body ( Rossi et al. 2014). These four species and also Cratera crioula (E.M. Froehlich, 1955) , Cratera joia ( Froehlich, 1956) and Cratera anamariae Carbayo & Almeida, 2015 have different colour patterns in comparison to the new species herein described ( Riester 1938; E.M. Froehlich 1955; Froehlich 1956; Rossi et al. 2014; Carbayo and Almeida 2015). Cratera ochra sp. nov. has colour and eye patterns similar to those of Cratera tamoia (E.M. Froehlich, 1955) , but the new species can be mainly distinguished from the latter by its cylindrical pharynx, whereas the pharynx of C. tamoia is bell-shaped (E.M. Froehlich 1955).
With respect to the morphology of the copulatory apparatus, species of the genus Cratera have an extrabulbar prostatic vesicle, a penis papilla with a distal cavity and a funnel-shaped female atrium, among other characteristics. The prostatic vesicle has two portions, a proximal expanded section and a distal tubular and sinuous portion. In C. ochra sp. nov., similarly to C. steffeni , the proximal portion is characteristically T-shaped with the sperm ducts opening through its lateral walls. This form is particularly evident in horizontal sections of the copulatory apparatus. Other species of the genus, such as C. crioula , C. joia , C. pseudovaginuloides and C. tamoia , which were described as possessing a prostatic vesicle with proximal diverticula ( Riester 1938; E.M. Froehlich 1955; Froehlich 1956), may have a vesicle with a similar form.
In summary, C. ochra sp. nov. is mainly differentiated from its congeners by the combination of the following morphological characters: dorsal body surface with dispersed pigmentation forming two broad bands, eyes spreading over the dorsal surface, cylindrical pharynx.
Measures of intraspecific and interspecific variation of the COI gene, as well as the ABGD algorithm applied to the COI data set, supported C. ochra sp. nov. as a species different from its congeners. In addition, both phylogenetic methods (maximum likelihood and Bayesian inference) revealed similar topologies, placing C. ochra sp. nov. as the sister group of the clade formed by C. tamoia and C. crioula ( Figure 1 View Figure 1 ).
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
| PL |
Západoceské muzeum v Plzni |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
GEOPLANINAE Stimpson, 1857
| Rossi, Ilana, Amaral, Silvana Vargas, Ribeiro, Giovana Gamino, Cauduro, Guilherme Pinto, Fick, Israel, Valiati, Victor Hugo & Leal-Zanchet, Ana Maria 2015 |
Cratera ochra
| Rossi & Amaral & Ribeiro & Cauduro & Fick & Valiati & Leal-Zanchet 2015 |
Cratera Carbayo et al., 2013
| Carbayo et al. The 2013 |