Scinax x‑signatus ( Spix, 1824 )
|
publication ID |
https://doi.org/10.11606/1807-0205/2020.60.56 |
|
publication LSID |
lsid:zoobank.org:pub:FCF6CE24-EFB5-4BD8-856F-032740367771 |
|
DOI |
https://doi.org/10.5281/zenodo.4975570 |
|
persistent identifier |
https://treatment.plazi.org/id/03E787FA-6708-F17C-FE88-FC8E7028213A |
|
treatment provided by |
Carolina |
|
scientific name |
Scinax x‑signatus ( Spix, 1824 ) |
| status |
|
Scinax x‑signatus ( Spix, 1824) View in CoL
Hyla x‑signata Spix, 1824 View in CoL .
Hyla affinis Spix, 1824 View in CoL – Considered a synonym of Hyla x‑signata View in CoL by Hoogmoed & Gruber (1983). Sturaro & Peloso (2014) questioned this association based on the description and figure provided by Spix (1824). Our study of photographs of the holotype (ZSM 2945) indicates that the situation is uncertain. Only a study of the taxonomy of amazonian populations associated with Scinax x‑signatus View in CoL would allow to clarify the status of this nomen.
Hyla coerulea Spix, 1824 View in CoL – Considered a synonym of Hyla x‑signata View in CoL by Hoogmoed & Gruber (1983). Sturaro & Peloso (2014) questioned this association based on the description and figure provided by Spix (1824). Our study of photographs of the lectotype designated by Hoogmoed & Gruber (1983) (ZSM 2710-0-1) indicates that the situation is uncertain. Only a study of the taxonomy of amazonian populations associated with Scinax x‑signatus View in CoL would allow to clarify the status of this nomen.
Hyla rubra Daudin, 1802 View in CoL (part) – Duméril & Bibron, 1841. First treatment as a synonym of Hyla rubra Laurenti, 1768 View in CoL (not Daudin, 1802; see León, 1969; Rivero, 1969; Duellman & Wiens, 1993).
Scytopis xsignatus [sic] – Cope, 1870. First combination with Scytopis Cope, 1862 .
Hyla rubra var. x‑signata View in CoL – Peters 1872. First treatment as a variety of Hyla rubra Laurenti. View in CoL
Hyla rubra x‑signata View in CoL – Müller, 1927. First treatment as a subspecies of Hyla rubra Laurenti, 1768 View in CoL .
Hyla x‑signata x‑signata View in CoL – Lutz, 1973. First use as nominal subspecies.
Ololygon x‑signata – Fouquette & Delahoussaye, 1977. First combination with Ololygon Fitzinger, 1843 .
Scinax x‑signata View in CoL – Duellman & Wiens, 1992. First combination with Scinax Wagler, 1830 View in CoL .
Scinaxx‑signatus – Köhler & Böhme,1996.Gender change.
Neotype
CFBH 44688, adult male, campus of the Universidade Estadual de Santa Cruz – UESC, Salobrinho, Ilhéus, State of Bahia, Brazil [ 39°10′24″W, 14°47′52″S; about 30 m above sea level (a.s.l.)], collected 10 April 2018 by G. Novaes-e-Fagundes. urn:lsid:zoobank.org:act:
Referred specimens
Fifteen adults ( 12 males and three females) from eight localities in the State of Bahia, Brazil. CFBH 21071 (male), Povoado Senote, Caetité ( 42°28′48″W, 14°04′55″S); MHNJCH 1014 (male), Floresta Nacional Contendas do Sincorá, Contendas do Sincorá ( 41°07′04″W, 13°55′20″S); MZUESC 20683 (male), Condomínio Parque Universitário, Salobrinho, Ilhéus ( 39°10′43″W, 14°47′44″S); CFBH 44687 (male), Fazenda Lagoa Nova, Irajuba ( 39°59′57″W, 13°12′19″S); CFBH 18797 (male), Fazendas Santo Onofre and Cana Brava, Maracás (approx. 40°25′23.58″W, 13°23′29.30″S); MHNJCH 1701 (male), near Fazendas Santo Onofre and Cana Brava, Maracás ( 40°26′26.23″W, 13°21′59.10″S); MHNJCH 1698-1700 (males), Assentamento do Cumbe, Maracás ( 40°27′38″W, 13°26′40″S); MZUESC 14890, 14893 (males), and 14891 (female), Companhia de Pesquisa de Recursos Minerais – CPRM, Morro do Chapéu ( 41°09′28″W, 11°32′56″S or 41°06′26″W, 11°29′34″S); MZUESC 15894 and 17503 (females), Praça Municipal, Potiraguá ( 39°57′30″W, 15°37′07″S); and UFMG 4787 (male), Route Sebastião Laranjeiras-Candiba, Sebastião Laranjeiras (approx. 42°56′21.34″W, 14°33′13.74″S).
Diagnosis (based on neotype and referred specimens)
Scinax x‑signatus is a species of Scinax , as it shares three synapomorphies of this genus: webbing between toes I and II that does not extend beyond the subarticular tubercle of toe I; origin of the m. pectoralis abdominalis through well-defined tendons; and m. pectoralis abdominalis overlapping m. obliquus externus ( da Silva, 1998; Faivovich, 2002; Faivovich et al., 2005). A single synapomorphy is known for the S.ruber Clade: tadpoles with the vent tube above the margin of the lower fin ( Faivovich, 2002; Faivovich et al., 2005). While tadpoles unequivocally associated to S. x‑signatus remain unknown, this species was associated to the S. ruber Clade by having the unique combination of external vocal sac and presence of pectoral fold [internal vocal sac and pectoral fold absent in most species of the S. catharinae Clade; in few species where the vocal sac is external, the pectoral fold is absent ( e.g., S. garibaldiae , S. rizibilis ); otherwise, in the two cases where the pectoral fold is present, the vocal sac is internal ( S.agilis and S.melanodactylus ); J.Faivovich & K. Araujo-Vieira, pers. obs.; see also Bokermann, 1964; Cruz & Peixoto, 1982; Faivovich, 2002; Lourenço et al., 2014, 2019].
Scinax x‑signatus can be differentiated from all other species of the S. ruber Clade by the combination of the following characters: (1) male SVL 34.5-38.4 mm, n = 13; (2) snout rounded in dorsal view and profile; (3) pointed tubercles on lower jaw absent; (4) vocal sac subgular, weakly bilobate; (5) spicule-shaped papillary epidermal projections on the nuptial pads and pectoral region present in males; (6) pectoral glands present in males; (7) dorsal color pattern with large irregular dark blotches, commonly with dark X-shaped mark composed of one or two pairs of inverted parenthesis-like blotches; (8) hidden surfaces of thighs dark with irregular pale blotches, yellow in living specimens; (9) iris yellowish golden or bronze with a median black streak; (10) physiological chlorosis absent; and (11) advertisement call composed of a single, multipulsed note, 0.11- 0.25 s duration, 6-14 pulses/note, 52-64 pulses/s.
Comparisons with other species of Scinax ruber Clade
The SVL in males of Scinax x‑signatus (34.5-38.4 mm, n = 13) distinguishes it from the larger species S. castroviejoi and S.eurydice (SVL males 44.0-52.0 mm; De la Riva, 1993; Bokermann, 1968), and from the smaller species S. altae , S. auratus , S. cabralensis , S. caldarum , S. cruentomma , S. danae , S. exiguus , S. fuscomarginatus , S. juncae , S. karenanneae , S. lindsayi , S. madeirae , S. maracaya , S. ruberoculatus , S. rupestris , S. staufferi , S. strussmannae , S. tymbamirim , S. villasboasi , and S. wandae (SVL males 15.7-29.0 mm; Lutz, 1968, 1973; Duellman, 1970, 1986; Pyburn & Fouquette, 1971; Cardoso & Sazima, 1980; Duellman & Wiens, 1993; Pyburn, 1992, 1993; Drummond et al., 2007; Nunes & Pombal, 2010, 2011; Nunes et al., 2012; Brusquetti et al., 2014; Araujo-Vieira et al., 2015; Ferrão et al., 2018a, b).
The snout rounded in dorsal view and profile differentiates Scinax x‑signatus from S. alter , S. auratus , S. cretatus , S. crospedospilus , S. imbegue , S. juncae , and S. tymbamirim (sub-elliptical with a pointed tip in dorsal view and slightly acute in profile), S.fuscovarius (roundly acute in dorsal view and protruding in profile), S. caldarum , S. curicica , S. duartei , S. maracaya , S. rossaferesae , and S. tigrinus (sub-elliptical or subovoid in dorsal view and slightly acute in profile), S. squalirostris (pointed in dorsal view and acute in profile), and species of the S. rostratus group (elongate pointed in dorsal view and acute with or without a fleshy proboscis in profile). Furthermore, the absence of pointed tubercles on the lower jaw differentiates S. x‑signatus from almost all species of the S. rostratus Group; exceptions are S.kennedyi and S.rostratus ( e.g., Duellman, 1972 a, 1973; Pyburn, 1973; Lescure & Marty, 2000; Lima et al., 2005; this study).
The presence of a weakly bilobate subgular vocal sac in Scinax x‑signatus distinguishes it from S.camposseabrai (bilobate subgular vocal sac; see also Caramaschi & Cardoso, 2006: fig. 1) and from the remaining species of the S. ruber Clade with single subgular vocal sac;exceptions are S.acuminatus , S.dolloi , S.funereus , S.fuscovarius , S.hayii , S.karenanneae , S.montivagus , S.onca , S.oreites , S.pachycrus , S.perereca , S. ruberoculatus , and S.tsachila , that have a weakly bilobate subgular vocal sac ( e.g., Cei, 1980; Duellman & Wiens, 1993; Pyburn, 1993;Ferrão et al., 2017, 2018a; this study).
The presence of spicule-shaped papillary epidermal projections on the nuptial pad and pectoral region in males differentiates Scinax x‑signatus from all other species of the S. ruber Clade, except for S. fuscovarius (see also Luna et al., 2018: fig. 10A, C). The presence of pectoral glands in males differentiates S. x‑signatus from most species of the S. ruber Clade, except for S. funereus , S.fuscovarius , S.nasicus , S.onca , and S.similis , and species of the S. uruguayus Group ( e.g., Müller & Hellmich, 1936; Lutz, 1973; Cei, 1980; this study).
The dorsal pattern with large irregular dark blotches, commonly with dark X-shaped marks composed of one or two pairs of inverted parenthesis-like blotches, distinguishes Scinax x‑signatus from S. altae , S. alter , S. auratus , S. boesemani , S. caldarum , S. cretatus , S. crospedospilus , S. curicica , S. cuspidatus , S. duartei , S. exiguus , S. fuscomarginatus , S. imbegue , S. juncae , S. madeirae , S. oreites , S. pachycrus , S. quinquefasciatus , S. ruber , S. squalirostris , S. staufferi , S. tsachila , S. tymbamirim , and S. villasboasi (variable number of dorsal and/or lateral stripes; e.g., Duellman, 1970; Lutz, 1973; Duellman & Wiens, 1993; Pugliese et al., 2004; Nunes et al., 2012; Brusquetti et al., 2014; Ron et al., 2018; this study), and S. blairi , S. cabralensis , S. chiquitanus , S. danae , S. iquitorum , S. lindsayi , S. maracaya , and S. strussmannae (scattered or homogeneously distributed spots and/or irregular blotches; e.g., Fouquette & Pyburn, 1972; Cardoso & Sazima, 1980; De la Riva, 1990; Drummond et al., 2007; Ferrão et al., 2018b; this study).
The hidden surfaces of thighs dark colored with light irregular pale blotches, yellow in living specimens differentiate Scinax x‑signatus from S. altae , S. auratus , S. baumgardneri , S. boesemani , S. cretatus , S. crospedospilus , S. cruentomma , S. cuspidatus , S. danae , S. elaeochroa , S. exiguus , S. fuscomarginatus , S. ictericus , S. iquitorum , S. madeirae , S. manriquei , S. pachycrus , S. ruberoculatus , S. staufferi , S. strussmannae , S. squalirostris , S. tsachila , S. villasboasi , S. wandae , and species of the S. uruguayus Group (hidden surfaces of thighs uniform, light or dark colored; e.g., Rivero, 1961; Duellman, 1970, 1986; Lutz, 1973; De la Riva, 1990; Duellman & Wiens, 1993; Barrio-Amorós et al., 2004; Nunes & Pombal, 2011; Brusquetti et al., 2014; Ferrão et al., 2018a, b; Ron et al., 2018; Baldo et al., 2019; this study), S. funereus , S. onca , and S. iquitorum (hidden surfaces of thighs with horizontal or irregular dark blotches; Duellman, 1971; Ferrão et al., 2017; Moravec et al., 2009; this study), and from species of the S. rostratus Group (hidden surfaces of thighs uniform light or marked with bold dark and light mottling or broad vertical bars; Duellman, 1972 a, 1973; Henle, 1991; Lescure & Marty, 2000; Lima et al., 2005; this study).
The yellowish golden or bronze iris, with a median black streak, distinguishes Scinax x‑signatus from S. cruentomma (silvery bronze iris, with a median red streak; Duellman et al., 1972b), S. ruberoculatus (bicolored, reddish upper half and grey lower half; Ferrão et al., 2018a), and species of the S. uruguayus Group (bicolored, golden upper half and dark brown to black lower half; Baldo et al., 2019). The absence of physiological chlorosis in S. x‑signatus distinguishes it from S. boesemani , S. caprarius, S. cruentomma , S. cuspidatus , S. elaeochroa , S. funereus , S. ictericus , S. iquitorum , S. karenanneae , S. manriquei , S. onca , S. strussmannae , and S. tsachila (present in these species; León, 1969; Lutz, 1973; Pyburn, 1993; La Marca, 2004;Moravec et al., 2009;Cole et al., 2013; Melo-Sampaio & Souza, 2015; Ferrão et al., 2017, 2018b; Acosta-Galvis, 2018; Ron et al., 2018;Taboada et al., 2020).
The advertisement call composed of a single multipulsed short note (0.11- 0.25 s), with 6-14 pulses/note, and pulse rate of 52-64 pulses/s differentiates Scinax x‑signatus from S. castroviejoi and S. eurydice (two or three multipulsed notes; De la Riva, 1993; De la Riva et al., 1994; Pombal et al., 1995a; Magrini et al., 2011; Mângia et al., 2017), S. alter , S. curicica , and S. perereca (note duration 0.28- 4.5 s and 21-152 pulses/note; Pombal et al., 1995a, b; Pugliese et al., 2004), S. cruentomma , S. fuscomarginatus , and S. strussmannae (17-90 pulses/note and 113-272 pulses/s; De la Riva et al., 1994; Duellman, 1972b; Brusquetti et al., 2014; Carvalho et al., 2015;Ferrão et al., 2018b), S. exiguus (23-90 pulses/note; Carvalho et al., 2017), S. madeirae (note duration 0.72- 1.16 s and 104-145 pulses/s; Brusquetti et al., 2014), S. staufferi (100-130 pulses/s; León, 1969), S. wandae (note duration 0.44- 0.69 s and 70-108 pulses/note; Pyburn & Fouquette, 1971; Duellman, 1986; Pombal et al., 2011), and from some large species of the S. rostratus Group: S. boulengeri and S. proboscideus (80-230 pulses/s; León, 1969; Duellman, 1972a), S. jolyi (note duration 2.5 s and 180 pulses/note; Lescure & Marty,2000), S.kennedyi (note duration 0.66- 2.9 s; Pyburn, 1973),and S.sugillatus (note duration 0.28- 0.60 s and 110-140 pulses/s; Duellman, 1973).
Description of the neotype
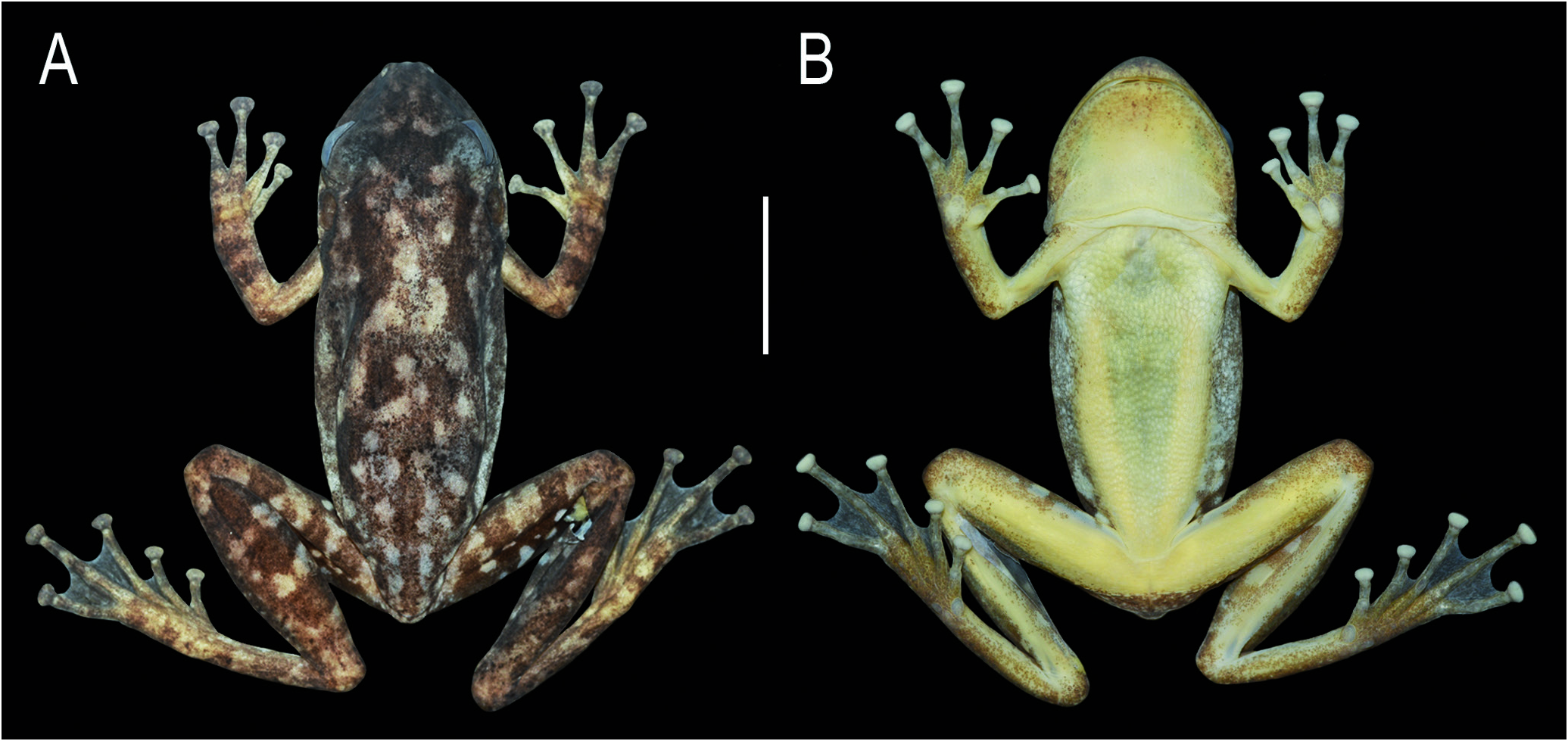
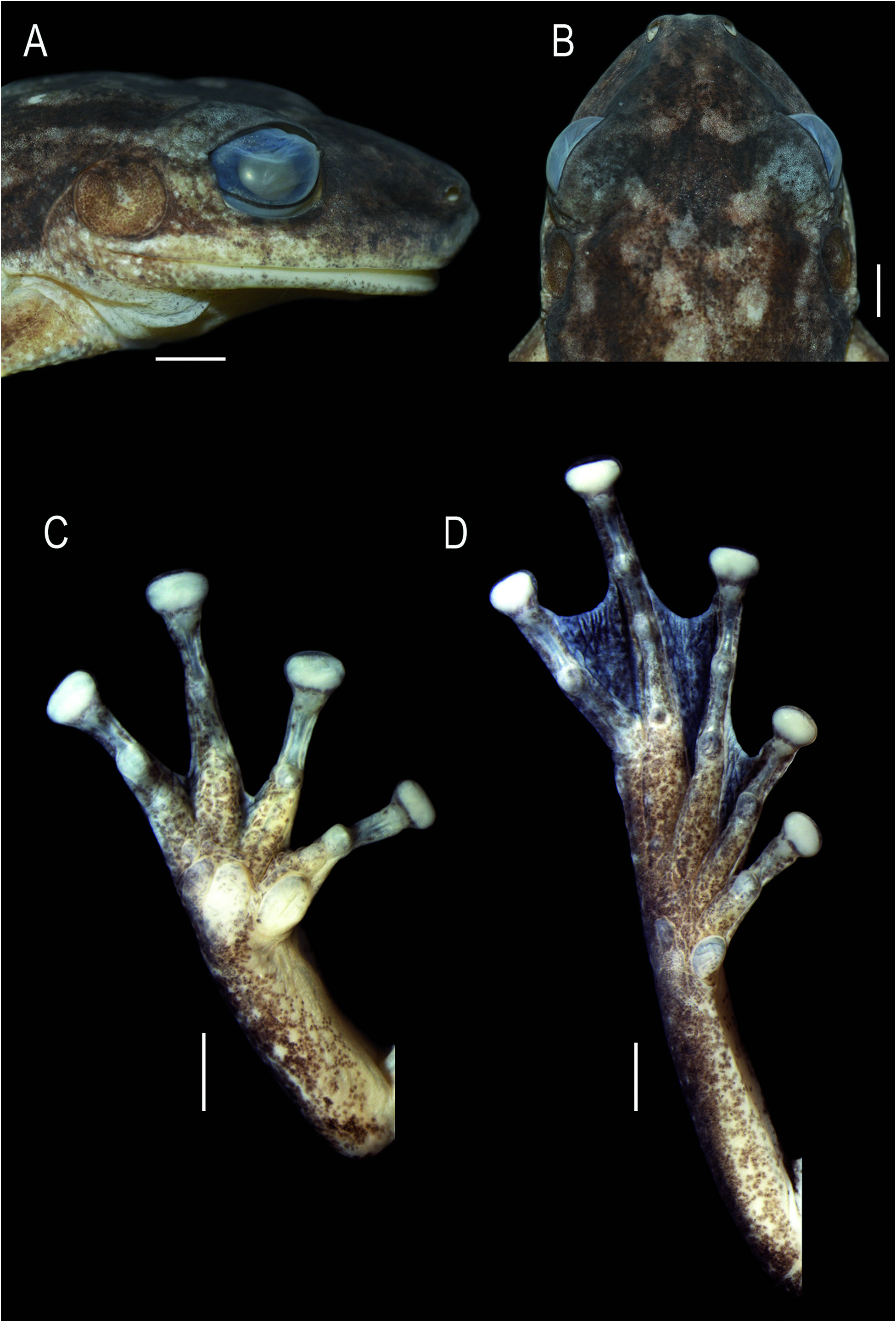
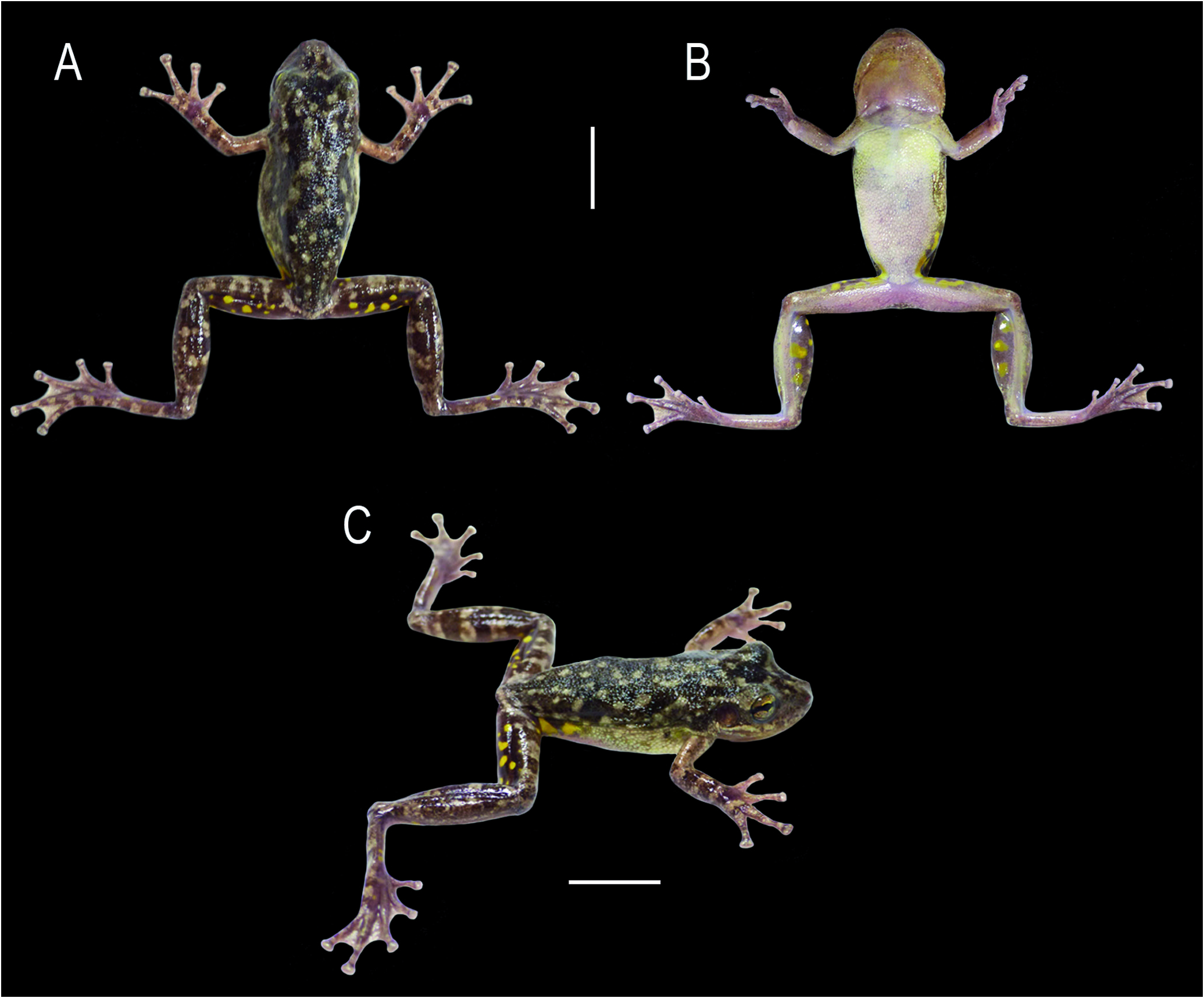
Head as wide as long, HL 35.1% and HW 33.2% of SVL ( Fig. 2 View Figure 2 ). Snout rounded in dorsal view and profile, with a low protuberance on the tip ( Fig. 3A, B View Figure 3 ). Nostrils dorsolateral, elliptical, protruded; IND 39.7% of IOD. Canthus rostralis marked, convex. Loreal region slightly concave. Eyes large, protuberant, ED 94.7% of IOD and 92.3% of END. Pupil horizontal, subelliptical. Tympanum rounded, separated from eye by a distance almost half TD; TD 75.0% of ED. Tympanic annulus rounded, with the posterior upper portion hidden by the supratympanic fold. Supratympanic fold evident, from the posterior upper portion of the tympanum to the insertion of the forearm. Vocal sac subgular, weakly bilobate, externally evident by the loose skin, not occupying space between head and body, and ventrally not reaching the pectoral fold ( Fig. 2B View Figure 2 ). Pectoral fold present, with pre- and postaxillar elements. Vocal slits present, nearly parallel to the mandible, originating laterally to the tongue and running towards the corner of the mouth. Tongue ovoid, free laterally and posteriorly, slightly notched posteriorly. Vomerine teeth in two slightly separated convex series, bearing five (right) and four (left) teeth. Choanae oval.
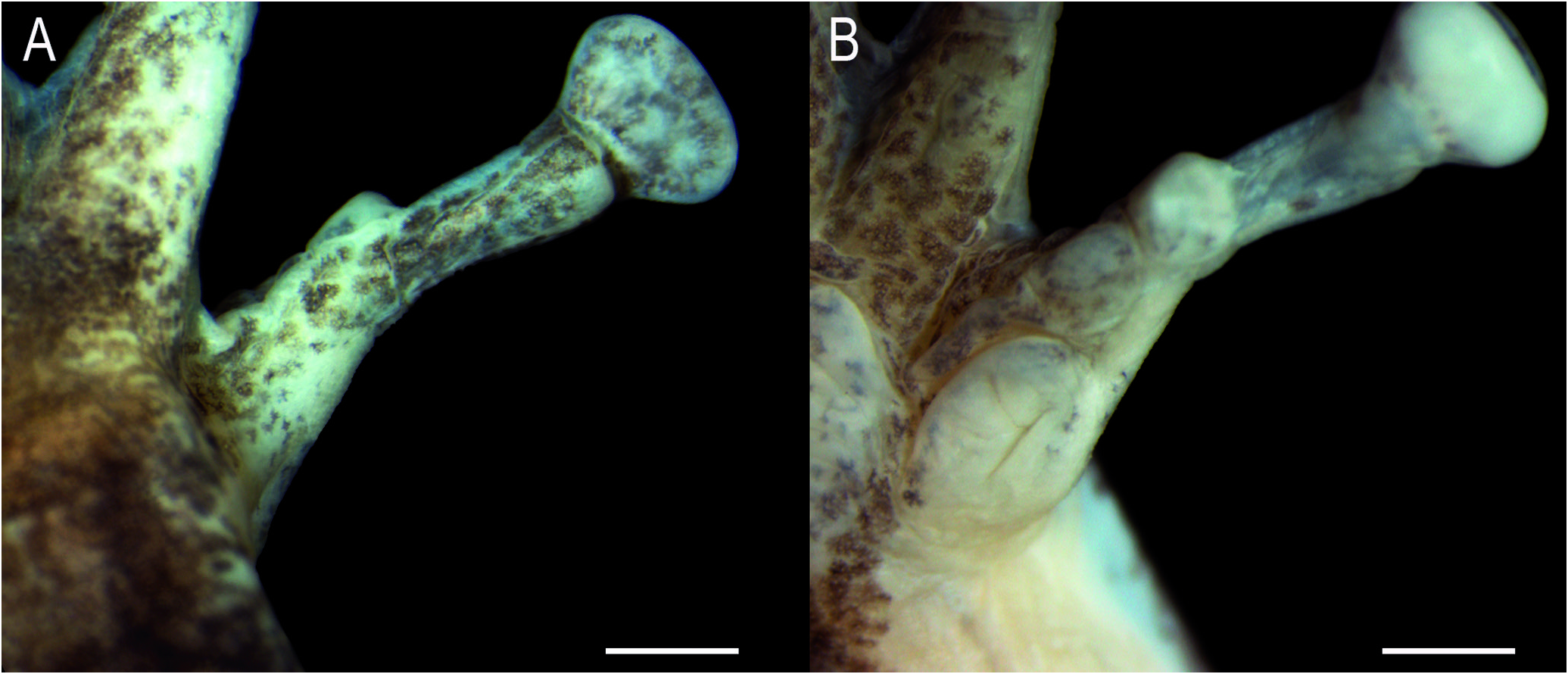
Axillary membrane absent. Upper arm more slender than forearm. A series of small, flat, ulnar tubercles on the forearm. Fingers short and slender, fringed ( Fig. 3C View Figure 3 ). Relative finger length II<III≈V<IV. Discs elliptical, wider than long, 3FD 59.2% of TD; disc of Finger II smaller than others. Subarticular tubercles single, conical on fingers II and III; rounded and quadrangular on fingers IV and V. Supernumerary tubercles small, single, rounded. Inner metacarpal tubercle single, elliptical; outer metacarpal tubercle flat, nearly triangular, bilobate. Webbing absent between fingers II and III; basal between fingers III, IV, and V. Slightly thickened, light-colored nuptial pad, covering Metacarpal II dorsomedially, and ventrally extending from the base of inner metacarpal tubercle, obscuring its outer margin, to the subarticular tubercle ( Fig. 4A, B View Figure 4 ). Glandular acini on inner margin of fingers II-III; on Finger II from the distal margin of nuptial pad to the disc. Spicule-shaped papillary epidermal projections on the nuptial pad, margins of fingers II-III ( Fig. 4A, B View Figure 4 ), and dorsum of fingers II-V. Hindlimbs robust; TL 49.0% of SVL, FL 39.8% of SVL. Toes slender, fringed (Fig. 3D). Relative toe length I<II<III≈V<IV. Discs elliptical, wider than long, slightly smaller than discs of fingers, 4TD = 3FD. Subarticular tubercles single, conical, rounded; supernumerary tubercles small, single, rounded. Inner metatarsal tubercle single, elliptical; outer metatarsal tubercle single, slightly marked, two thirds smaller than inner tubercle.Webbing formula I 2-2⁺ II 1¹ / ⁴-2⁺ III 1-2¹ / ³ IV 2⁺-1 V. Fringe on lateral margin of Toe V extends along the margin of the sole by a poorly developed ridge that reaches the distal portion of the metatarsus.Ventrolateral margin of tarsus smooth; heel slightly granular.
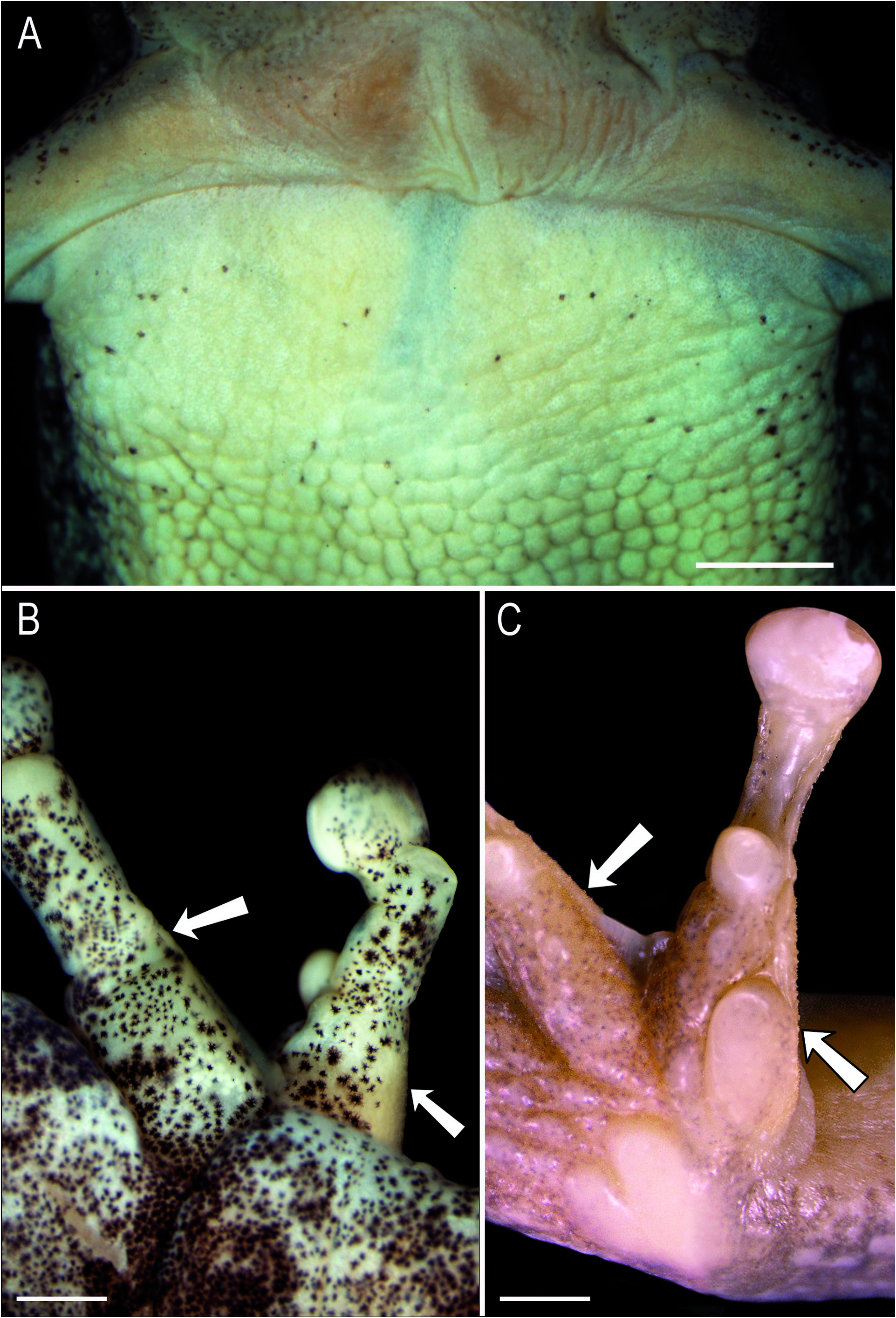
Cloacal opening directed posteriorly at upper level of thighs. Skin on dorsum of head, upper eyelid, trunk, and limbs smooth, with scattered granules. Posterior corner of eyes, around tympanum and forearm insertion, supratympanic fold, and flanks granular. Vocal sac, hidden surfaces of limbs, and inguinal region smooth; other ventral surfaces and subcloacal area densely covered with rounded, flat granules. Pectoral region and inner margin of upper- and forelimbs with glandular acini, covered with spicule-shaped papillary epidermal projections.
Measurements (mm): SVL 36.7; HL 12.9; HW 12.2; IND 2.5; IOD 3.8; ED 3.6; END 3.9; TD 2.7; FL 14.6; TL 18.0; 3FD 1.6; 4TD 1.6. Coloration in life: The description is based on the freshly euthanized specimen ( Fig. 5 View Hindlimbs ). Dorsal color dark brown, with two pairs of large, irregular, black blotches on the suprascapular and sacral regions, and scattered, small, round or irregular, light blotches; interocular region with an inverted triangle-shaped, black marking ( Fig. 5A View Hindlimbs ). Upper lip light with diffuse brown blotches anteriorly, and a white stripe on the infraorbital region extending to posterior margin of the tympanum. Loreal region brown with small, irregular, black dots; dark brown canthal line. Post-orbital dark brown line from anterior corner of the eyes, upper margin of tympanum, to the middle of the flanks. Flanks light with irregular, dark brown blotches. Dorsal surfaces of discs, fingers, toes, forearms, and tarsus brownish gray with transverse, brown bars; upper arms uniform; shanks and thighs with large dark brown blotches.Toe webbing covered by brown melanophores. Iris grayish bronze with thin black reticulations, thin yellow halo bordering the pupil, and a median black streak.
Soles and palms light brown; glandular pectoral region yellowish white; other ventral areas creamy white, immaculate,but margins of gular region, around forearm insertion and knees, tarsus, and shanks finely spotted with brown (Fig. 5B). Inguinal region yellow, with irregular dark blotches. Hidden surfaces of thighs and shanks brown, with small to medium-size, rounded or irregular yellow blotches (Fig. 5C).White bones.
Coloration in preservative: Paler than in the fresh specimen.The coloration on the glandular pectoral region, inguinal region, and hidden surfaces of thighs and shanks faded to light beige or cream white.
Variation See Table 1 for measurements of the available specimens. Vomerine teeth vary from 5 to 8. In some individuals, the medial constriction of the vocal sac is barely evident externally. Snout rounded in dorsal view and profile, with or without a low protuberance on the tip. Toe webbing formula varies as follows: I (2⁺-2⁻) – (2¹ / ²-2⁻) II (1¹ / ²-1) – (2¹ / ²-2⁻) III (1¹ / ²-1) – (2¹ / ²-2) IV (2⁺-2⁻) – (1⁺-1) V.
Dorsal skin similar to that of neotype, with scattered or uniformly distributed granules. Ulnar and tarsal tubercles protuberant or inconspicuous. Three or four, low, rounded tubercles can be present next to the tibio-tarsal articulation; the distal one is elongated in some individuals.
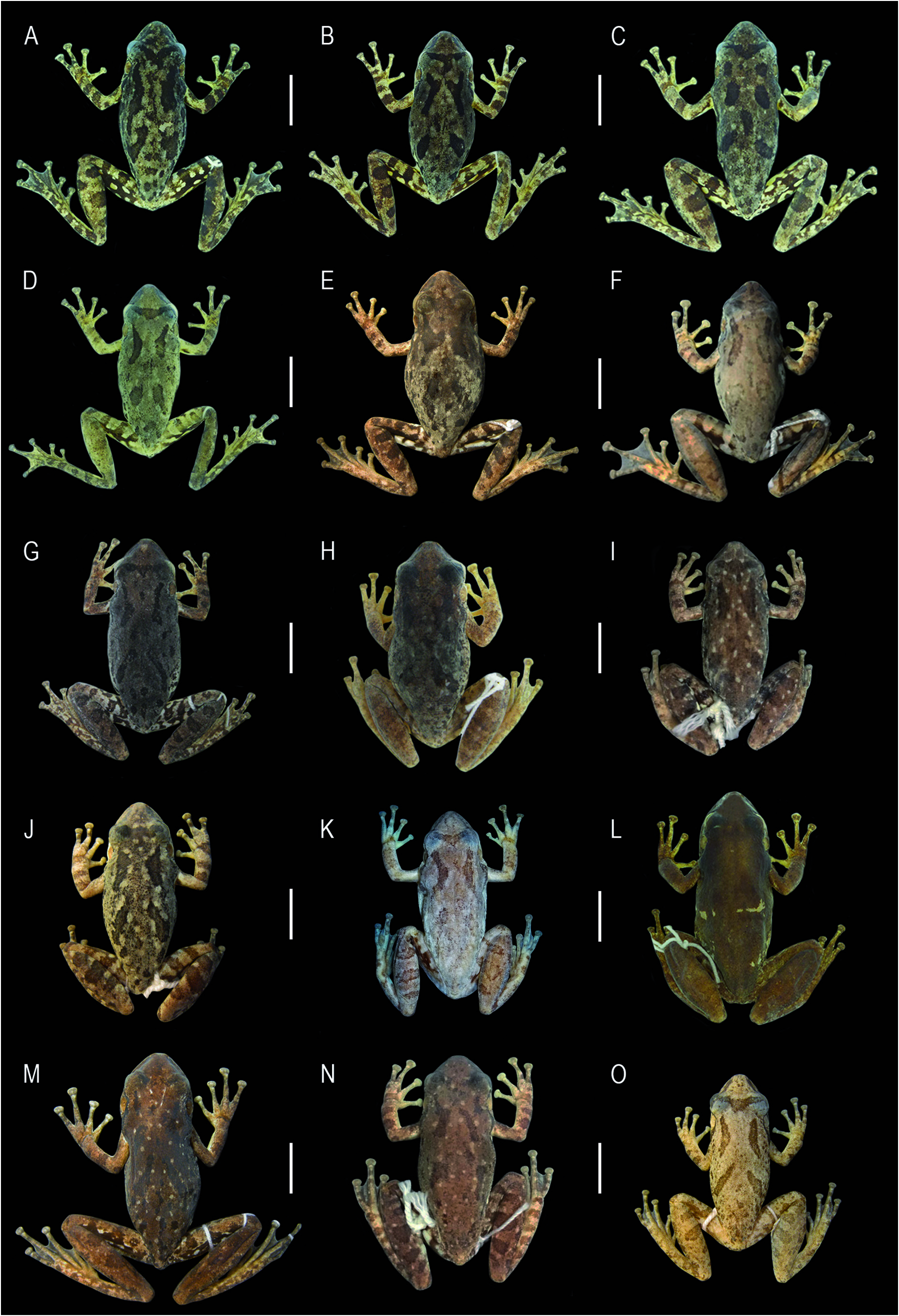
In freshly euthanized specimens, dorsal color varies from beige or gray to dark brown, with large irregular brown to black blotches, and small white blotches ( Fig. 6 View Figure 6 ). In males, pectoral region light yellow and abdomen yellowish beige; in females, cream white. Inguinal region light yellow or yellow, with irregular dark blotches; hidden surfaces of thighs, shanks, and tarsi brown to black, with small to large, rounded or irregular yellow blotches. Flanks light yellow or cream white; axillae yellow in some individuals. Iris yellowish golden or bronze, with many thin, dark reticulations, and a median black streak. In life, overall coloration similar to fleshly euthanized specimens. Still, dark and light tones are more intense and brighter, especially yellow coloration on inguinal region and hidden surfaces of hindlimbs ( Fig. 7 View Figure7 ). Iris iridescent yellowish golden or bronze ( Fig. 7 View Figure7 A-E).
In preservative, dorsal pattern varies from light beige or light gray to dark brown, with large, irregular dark blotches, usually with X-shaped marks composed of one or two pairs of inverted parentheses-like blotches, with or without light blotches ( Fig. 8 View Figure 8 ). Small, dark brown, irregular blotches on the dorsum of all individuals. Interocular marking can be inverted triangle, T-shaped, or W-shaped, sometimes fragmented. Upper lip light with diffuse brown blotches, sometimes with a white infraorbital stripe that extends to the tympanum. Dark canthal line present in all specimens. Post-orbital line varies in extension, reaching posteriorly level of forearm insertion or middle of flanks. Dark blotches on flanks and inguinal region rounded or irregular. Small to large, rounded or irregular light blotches on hidden surfaces of hindlimbs. Ventral surfaces from cream white to light beige, finely or conspicuously covered with brown spots in some individuals. Dark coloration predominates on dorsum of specimen MHNJCH 1014 ( Fig. 8L View Figure 8 ); however, this resulted from the fixation process in 10% formalin. In life, this specimen showed the X-shaped mark and other dorsal blotches common to the other specimens.
The occurrence of glandular tissue in the pectoral region has been considered a secondary sexually dimorphic character occurring in males ( Lutz, 1973), and this is corroborated here ( Fig. 9A View Figure 9 ). This is also the case of the spicule-shaped papillary epidermal projections on the nuptial pad ( Fig. 9B, C View Figure 9 ), inner margin of upper- and forearms, and pectoral region. The glandular areas (acini) on the inner margins of upper- and forearms, and fingers II-III (excluding the nuptial pad) are absent in some specimens ( e.g., MHNJCH 1014, 1698-1700); when present, spicule-shaped papillary epidermal projections also occur on these areas. Spicule-shaped projections can be present, scattered, and apparently not associated to acini on fingers II-V in some individuals. Although our sample of females is small (three individuals), females tend to be larger than males ( Table 1 View Table 1 ).
Advertisement call
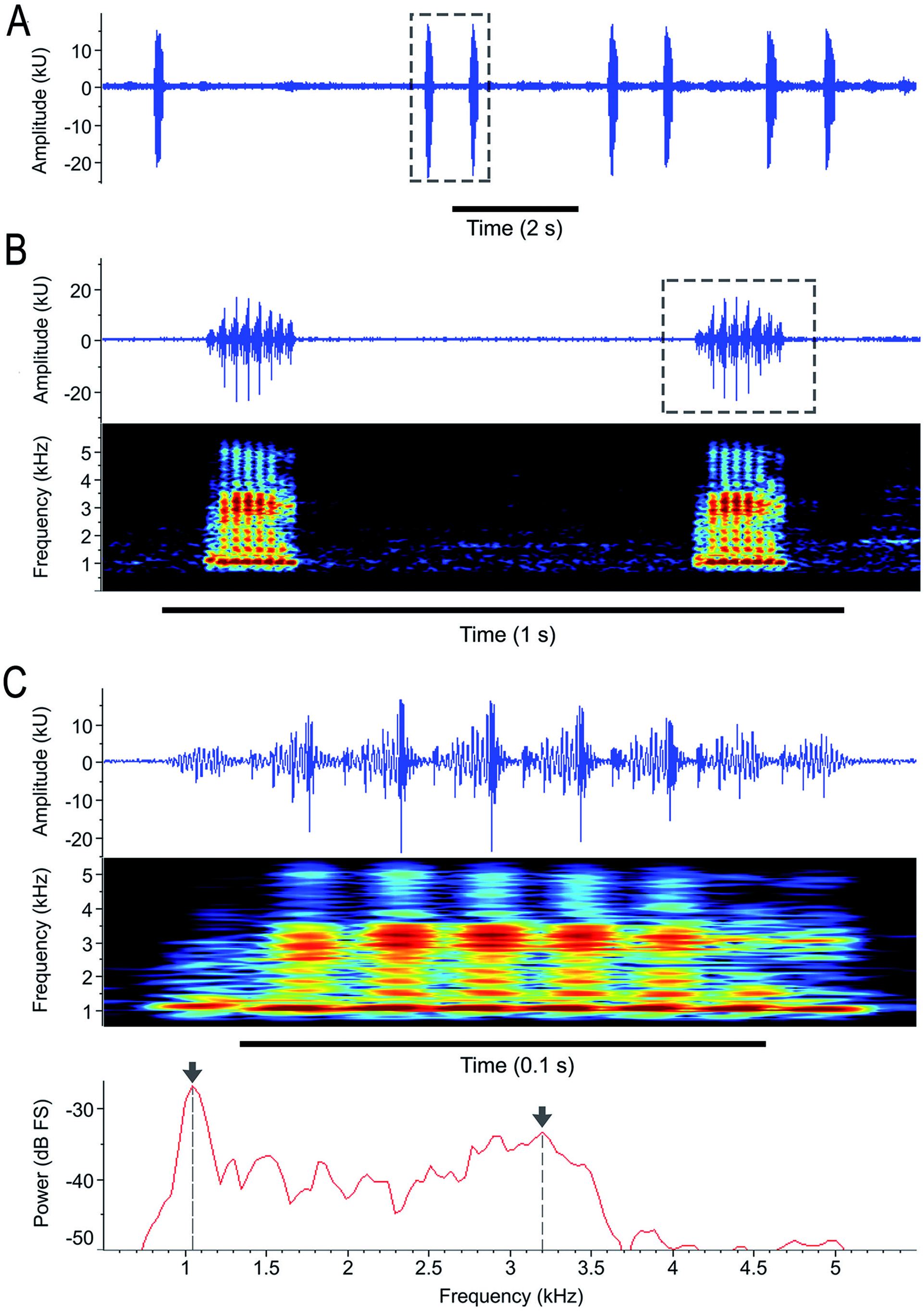
The advertisement call of Scinax x‑signatus consists of a single multipulsed note,emitted at a highly variable repetition rate (2-105 notes/minute; Table 2 View Table2 ; Fig. 10A View Figure 10 ), which is likely affected by conspecific chorus density, since the longer intervals between notes (up to 25.3 s; Table 2 View Table2 ) were observed in the recording of the neotype, which was calling alone with no nearby conspecific. Notwithstanding, much longer intervals are the exception, and the notes are repeated at faster rates (29-105 notes/minute; Table 2 View Table2 ), but never composing a stereotyped series.
Note duration is 0.11- 0.25 s ( Table 2 View Table2 ; Fig. 10 View Figure 10 A-C); each note is composed of 6-14 pulses with modulating amplitude increasing from the first third, reaching the maximum amplitude around the middle of the note,and gradually decaying towards the last pulse ( Fig. 10B, C View Figure 10 ). Pulse rate is 52-64 pulses/s and pulse duration 0.013 - 0.018 s ( Fig. 10B, C View Figure 10 ).
Calls have a broadband spectrum (BW90% 2250-2799 Hz; Table 2 View Table2 ; Fig. 10C View Figure 10 ). The power spectrum is usually biphasic, with two main emphasized frequency bands ( Fig. 10C View Figure 10 ). The lower band ( i.e., the low-frequency band) comprises most of the power of the spectrum, with the dominant frequency ranging between 904-1359 Hz ( Fig. 10C View Figure 10 ), and also including part of the freq5% (861-991 Hz; Table 2 View Table2 ). The upper band ( i.e., the high-frequency band) has less power than the first one, surrounding the freq95% (3188-3704 Hz; Table 2 View Table2 ), with its peak frequency between 2885-3618 Hz ( Fig. 10C View Figure 10 ). Between the two power spectrum bands, there is a low-power“valley” (or gap) around 2.0-2.5 kHz ( Fig. 10C View Figure 10 ). The dominant frequency does not alternate between the lower and upper bands, remaining in the lower band.
Notes on calling site and calling behavior
Males of Scinax x‑signatus call near lentic water bodies, either natural or artificial (such as pools and tanks). They usually call from the ground, either uncovered or hidden among the vegetation. Less often, they call perched at low heights (below 1.5 m; rarely above that height) on the vegetation inside or at the margins of water bodies. Other species of Scinax found calling syntopically with S. x‑signatus are Scinax sp. aff. hayii , S. auratus , S. eurydice , and S. pachycrus . Scinax x‑signatus seems to tolerate some degree of light and sound disturbance; as we recorded the neotype inside the University Campus, with plenty of artificial light and crowd voices as background noise.
Phylogenetic analysis
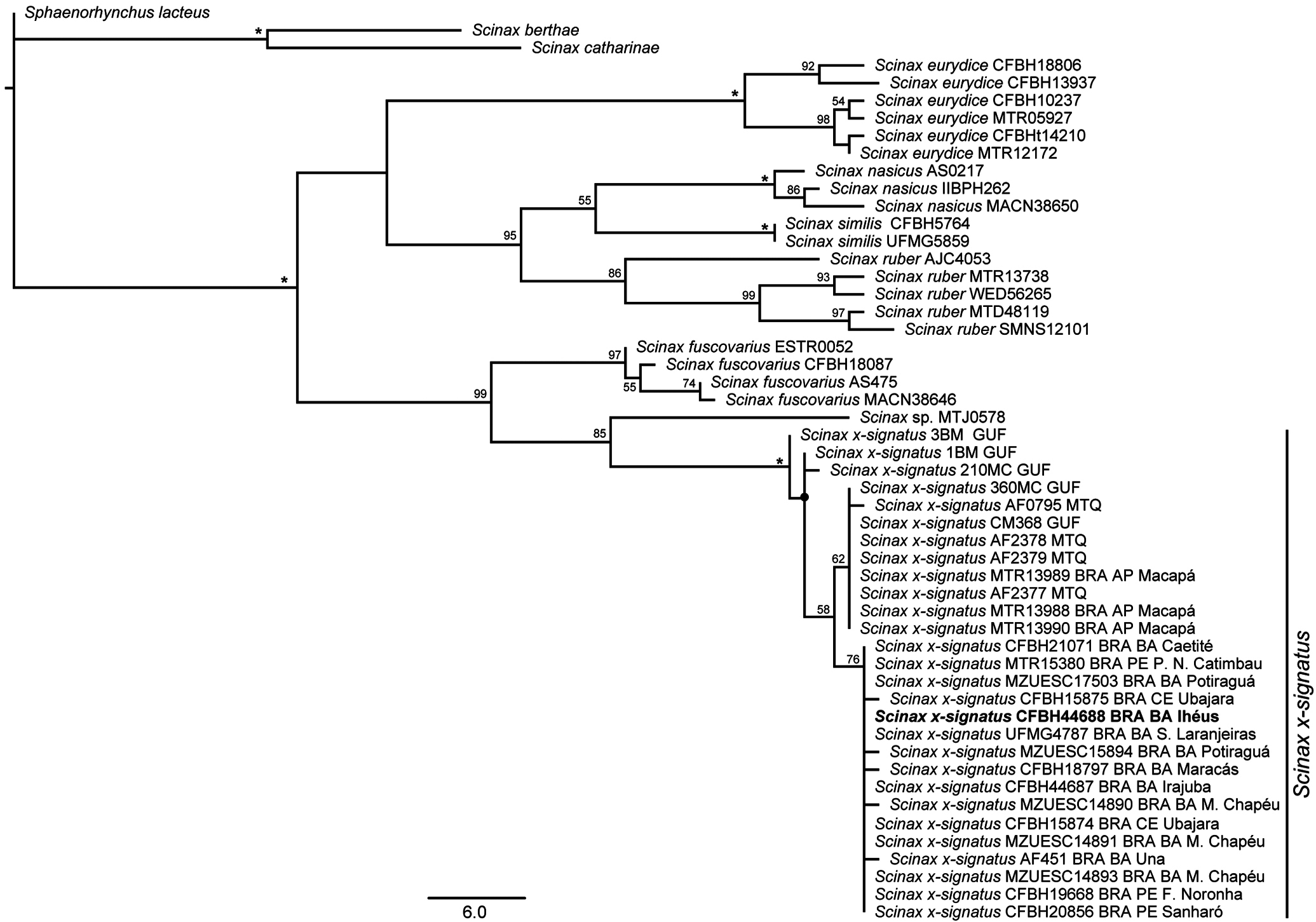
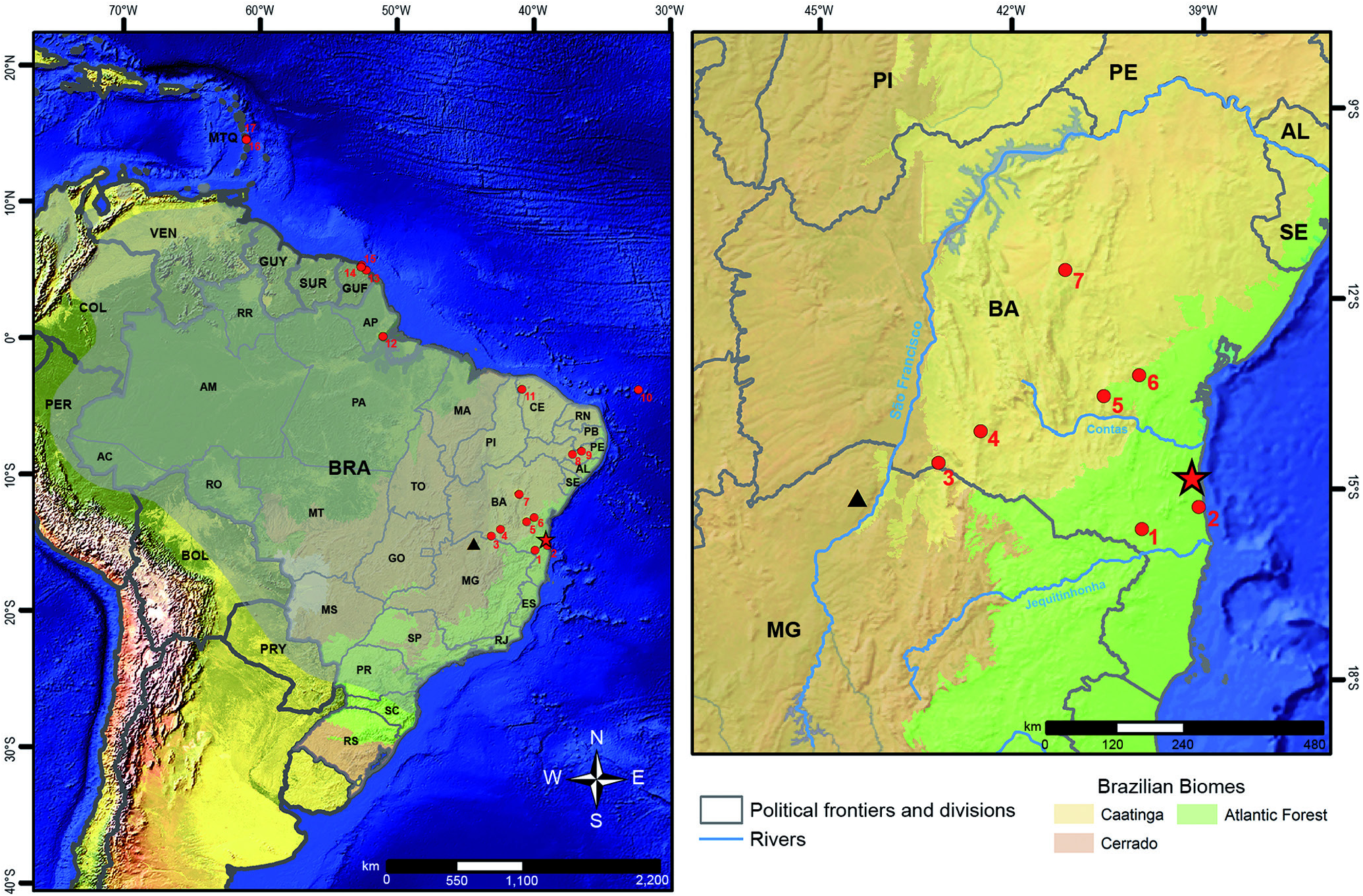
The two most parsimonious trees (length 468) recovered all specimens considered in the literature as Scinax x‑signatus closely related with the neotype and our referred specimens (100% jackknife; Fig. 11 View Figure 11 ). These include specimens from Bahia, Ceará, and Pernambuco (NE Brazil) and Amapá (N Brazil),and from French Guiana and Martinique ( Fig. 12 View Figure 12 ). The selected fragment of the mitochondrial ribosomal gene 16S rRNA showed uncorrected pairwise distances of 0.2-1.7% among the 28 individuals of S. x‑signatus ( Table 3 View Table3 ). The maximum value (1.7%) is between specimens from Kourou and Ile Royale ( French Guiana), and those from Ubajara ( Ceará, NE Brazil), Fernando de Noronha, and Sanharó ( Pernambuco, NE Brazil); the geographic distances between these points are approx. 1,700 km (see distances between points 8-9 and 14-15 in Fig. 12 View Figure 12 ).
Scinax x‑signatus is moderately supported (85% jackknife) as sister taxon of Scinax sp. (as S. x‑signatus “ Scinax _64” in Vacher et al., 2020) from Parque Nacional Cavernas do Peruaçu, Januária, N Minas Gerais, Brazil. Uncorrected pairwise distances between S. x‑signatus and Scinax sp.are 6.9-10.2%, with a sequence divergence of 8.0% between one specimen of S. x‑signatus (UFMG 4787) from Sebastião Laranjeiras ( Bahia, NE Brazil) only distant approx. 170 km ENE from the locality of this candidate species in N Minas Gerais (see Fig. 12 View Figure 12 ). The voucher specimen of Scinax sp. (MTJ0578) was not available for morphological study, and therefore we are not aware of any diagnostic characters for this candidate species. The clade S. x‑signatus + Scinax sp. is well-supported (99% jackknife) as sister taxon of S. fuscovarius , followed by a poorly supported clade (<50% jackknife) composed of S. eurydice , S. nasicus , S. ruber , and S. similis .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Scinax x‑signatus ( Spix, 1824 )
| Araujo-Vieira, Katyuscia, Caramaschi, Ulisses, Novaes-e-Fagundes, Gabriel, Orrico, Victor G. D. & Faivovich, Julián 2020 |
Scytopis
| Cope 1862 |
Ololygon x‑signata
| Fitzinger 1843 |
Ololygon
| Fitzinger 1843 |
Scinax x‑signata
| Wagler 1830 |
Scinax
| Wagler 1830 |
Hyla affinis
| Spix 1824 |
Hyla coerulea
| Spix 1824 |
Hyla rubra
| Daudin 1802 |
Hyla rubra
| Laurenti 1768 |
Hyla rubra
| Laurenti 1768 |