Hannia greenwayi Vari 1978
|
publication ID |
https://doi.org/10.11646/zootaxa.4869.4.5 |
|
publication LSID |
lsid:zoobank.org:pub:0B47C656-573A-4E61-A8E7-8B8F78BE20D0 |
|
DOI |
https://doi.org/10.5281/zenodo.4569812 |
|
persistent identifier |
https://treatment.plazi.org/id/03AE87D9-FFAF-FFA8-FF48-FA5FFB32FD18 |
|
treatment provided by |
Plazi |
|
scientific name |
Hannia greenwayi Vari 1978 |
| status |
|
Hannia greenwayi Vari 1978 View in CoL
Fig. 1 View FIGURE 1 , 6 View FIGURE 6 , 8 View FIGURE 8 and 9 View FIGURE 9 , Tables 4 View TABLE 4 , 5 View TABLE 5 and 6 View TABLE 6
English vernacular name: Greenway’s grunter
Aboriginal language names: Boornda (Gooniyandi language), Emana/Wanggari (Ngarinyin language)
Hannia greenwayi Vari, 1978 View in CoL — Allen, 1982: 46, pl. 10; Merrick & Schimda, 1984: 223; Pollard & Burchmore, 1986: 626; Lane & McComb, 1988: 131; Allen, 1989: 155, 173; Paxton et al., 1989: 532; Allen & Leggett, 1990: 537; Eschmyer et al., 1990: 174; Wager, 1996; Axelrod et al., 1997: 1063 image 3; Allen et al., 2002: 219; Morgan et al., 2002: 47; Morgan et al., 2002: 150; Morgan et al., 2005: 6; Hoese et al., 2006: 1334; Davis et al., 2011: 270; Morgan et al., 2011: 16 View Cited Treatment ; Davis et al., 2012: 1166; Jackson et al., 2012: 100; Davis et al., 2014: 208; Morgan et al., 2014: 267, 270; Thorburn et al., 2014; 368; Shelley et al., 2018a: 124.
Hannia greenwayi View in CoL I—Shelley et al. 2018b: 849–851, 855–856; Shelley et al. 2019b: 2427, Shelley et al. 2020: 1732–1734 View Cited Treatment ).
Holotype: WAM P.25380-002 [physical specimen destroyed (see remarks), photo and description presented in Vari
1978], 80.9 mm SL, Hann River at Moll Gorge, 15 mi. northeast of the Mount House homestead, Western Australia,
16° 58’ 36.1”S, 125° 59’ 51.5”E, G.J. Nelson, W.H. Butler and D.E. Rosen, 28 April 1969.
Paratypes: 100 specimens (15 examined), AMNH 35641 About AMNH (52), not examined, and WAM P.25380-001 [ex AMNH] (48, 5 deteriorated/destroyed) , 5 specimens ( 50.5–86.1 mm SL) examined for all characters, extra 44 specimens ( 43.5–86.1 mm SL) x-rayed for vertebral and dorsal/anal fin spine and cray counts, all collected with holotype .
Non-type material examined (all characters): 9 specimens, 58.5–97.2 mm SL. NMV A 31776-003 About NMV to 006 About NMV (4), 62.2–93.7 mm SL, downstream of waterfall on the Glenelg River, Western Australia, 15° 41’ 47.7”S, 125° 0’ 29.6”E, 100 m elevation, JJS & MCL, 20 July 2014, gill net; GoogleMaps NMV A 31740-007 About NMV to 009 About NMV and 015 to 016 (5), 58.5–97.2 mm SL, Calder River at Bachsten Track crossing, Western Australia, 16° 3’ 17.7”S, 125° 12’ 49.6”E, 200 m elevation, JJS & MCL, 8 February 2014, electrofisher GoogleMaps .
Non-type material examined (x-rayed for osteological comparisons only): 31 specimens, 31.1–91.6 mm SL. NMV A 31740-017 About NMV (4), 49.1–91.6 mm SL, Calder River at Bachsten Track crossing, Western Australia, 16° 3’ 17.7”S, 125° 12’ 49.6”E, 200 m elevation, JJS & MCL, 8 February 2014, electrofisher; GoogleMaps NMV A 31746-014 About NMV to 024 About NMV (11), 51.9–63.0 mm SL, Plain Creek , at Dillie Gorge , Western Australia, 16° 44’ 3”S, 125° 22’ 59”E, 370 m elevation, JJS & MCL, 23 June 2013, electrofisher; GoogleMaps NMV A 31765-011 About NMV to 018 About NMV (11), 31.1–87.1 mm SL, Fitzroy River , Diamond Gorge , Western Australia, 17° 44’ 18”S, 126° 2’ 25”E, 165 m elevation, JJS & MCL, 21 June 2013, gill net; GoogleMaps NMV A 32034-001 About NMV (5), 53.2–61.2 mm SL, McRae River , lower, Western Australia, 15° 39’ 45”S, 124° 54’ 50”E, 55 m elevation, JJS & MCL, 22 July 2014, gill net GoogleMaps .
Diagnosis: Hannia greenwayi can be distinguished from its congener by being smaller in size (recorded up to 110 mm SL) and by a combination of the following characters: a straight head profile between the posterior of occiput and base of first dorsal spine. Pectoral fin rays usually 13, preopercular spines 10.5 (0–16), and postorbital length 32.5 (26.5–36.6) % HL. Further, discrimination is provided by a combination of the following characters: dorsal fin rays usually 10, fourth spine and third ray longest; anal fin rays usually 8, second anal fin spine and pelvic fin ray longest; lateral line scales usually 38, scales below lateral line usually 11; pre-dorsal scales usually 10; caudal circumpeduncular scales usually 16. Body depth at dorsal fin origin 33.3 (28.6–42.0) % SL; body depth at anal fin origin 27.2 (20.5–32.4) % SL; snout length 39.0 (35.9–42.2) % HL; jaw width 29.1 (22.1–34.6) % HL; pectoral fin length 19.3 (15.0–22.5) % SL; dorsal fin length 56.4 (51.8–59.7) % SL; pelvic fin length 22.0 (19.2–23.9) % SL; caudal peduncle depth 12.0 (10.8–13.5) % SL; longest anal fin spine length 16.1 (11.1–20.5) % SL.
Description: Based on 15 ( 6 type and 9 non-type) specimens assessed for all characters, 61.8–166.0 mm SL, and 31 additional, non-type specimens that were x-rayed for osteological comparison only. See Table 4 View TABLE 4 for frequency distributions of variable meristic characters, and Table 5 View TABLE 5 and Table 6 View TABLE 6 for summaries of meristic and morphometric variation respectively. Dorsal fin spines XIII* (XI–XIII), rays 10* (9–11); anal fin spines III*, rays 8* (7–9); caudal fin rays 9+8+7+10 (9+8+5–9+5–9, n=45); pectoral fin rays 13 (13–15); pelvic spines I*, rays 5; vertebrae 10+15=25 (n=45); lateral line scales 38 (34–42) [36*]; scales above lateral line 7 (5–8) [6*]; scales below lateral line 11* (11–12); pre-dorsal scales 10 (8–13); cheek scale rows 5 (3–7) [4]; caudal circumpeduncular scales 16 (14–20); gill rakers on first arch 9+16=25 (8–9+14–16=22–25); opercular spines 2*; preopercular spines 10.5 (0–16) [15].
Dorsal profile straight at 45° to the horizontal plane from snout to occiput, straight from posterior of occiput to base of first dorsal spine, continuing straight or slightly concave to base of last dorsal ray, then straight (running horizontal) to caudal fin base. Ventral profile almost evenly arched: straight or slightly convex at 35° to the horizontal plane from snout to pelvic fin base, slightly concave to base of last anal ray, then straight (running horizontal) to caudal fin base. Lateral line continuous from behind head to base of caudal fin, and approximately parallel with dorsal profile. Body depth at dorsal fin origin 33.3 (28.6–42.0) % SL; body depth at anal fin origin 27.2 (20.5–32.4) % SL; caudal peduncle length 16.3 (14.8–19.6) % SL; caudal peduncle depth 12.0 (10.8–13.5) % SL; pre-anus length 63.7 (59.5–67.3) % SL. Head length 34.8 (32.8–36.5) % SL; head pointed; snout length 39.0 (35.9–42.2) % HL; upper-jaw length 29.7 (25.8–33.5) % HL, jaws equal; jaw width 29.1 (22.1–34.6) % HL. Mouth terminal, gape oblique. Maxillary reaching to vertical through posterior nostril.
Jaws with outer row of large, conic teeth of same size with 4 (3–5) rows of smaller teeth on upper jaw and 4 (3–4) rows on lower jaw, embedded in a sturdy gum; no teeth on vomer or palatines. Teeth in outer row of upper jaw 32 (25–44) number increasing with fish size, 4.3 (2.7–6.9) / mm when standardised with lower jaw length; teeth on outer row of lower jaw 26 (17–36), number only increasing slightly with fish size, 3.6 (1.6–6.4) / mm when standardised with lower jaw length. Nostrils set wide apart; separated by a distance equal to 0.5 eye diameter. Lower jaw U-shaped when viewed from below; upper jaw usually with fleshy, narrow, discontinuous lip fold, but occasionally continuous. Eye diameter 27.1 (22.5–31.4) % HL; inter-orbital width 25.9 (22.2–28.6) % HL, smooth, with slightly elevated bony ridge above each orbit; postorbital length 32.5 (26.5–36.6) % HL. Lacrimal with 4–6 serrations along posteroventral edge, overhung by skin. Preoperculum with 10.5 (0–16) spines; longest and most robust on apex of preopercle, reduced in size dorsally and anteriorly, anteriormost spine situated behind posterior edge of orbit. Lower opercular spine longer and more robust than upper opercular spine; not extending beyond edge of opercular lobe. Posttemporal not exposed; covered with skin and scales. Cleithrum exposed on posterior edge; covered with skin and scales elsewhere; serrate posteriorly. Supracleithrum covered by a thin layer of skin.
Dorsal fin origin vertically above pelvic fin origin, posterior to vertical plane through pectoral fin origin, terminates immediately posterior to base of last dorsal ray; pre-dorsal fin length 45.4 (43.5–47.4) % SL. Dorsal fin base with 1 sheath scale, length 49.8 (45.8–57.3) % SL, fin length 56.4 (51.8–59.7) % SL; spinous dorsal arched, 4 th (3 th– 5 th) dorsal spine longest, length 16.5 (14.1–18.3) % SL, spines progressively shorter from first and last respectively; last dorsal fin spine fully linked to first dorsal fin ray by membrane; 3 rd (2 nd– 5 th) dorsal fin ray longest, length 13.8 (10.6–15.6) % SL, margin of rayed portion rounded to tip of last ray. Anal fin origin immediately anterior to vertical plane through base of last dorsal fin spine, terminates immediately anterior to base of last ray, pre-anal fin length 69.7 (64.1–73.8) % SL. Anal fin base with 2 sheath scales, length 15.1 (12.5–18.4) % SL, fin length 22.2 (18.4–25.7) % SL; 2 nd anal fin spine longest, length 16.1 (11.1–20.5) % SL; 2 nd (2 nd– 4 th) anal fin ray longest, length 14.7 (10.3–18.3) % SL; subsequent rays progressively shorter; distal margin of rayed portion rounded.
Caudal fin slightly forked; upper caudal lobe length 25.3 (22.4–29.3) % SL; middle caudal ray length 20.4 (16.9–24.7) % SL. Pre-pectoral fin length 33.7 (26.5–37.3) % SL, pectoral fin length 19.3 (15.0–22.5) % SL, pre-pelvic fin length 34.9 (26.1–43.2) % SL, pelvic fin spine 12.9 (10.6–15.0) % SL; 1 st pelvic ray longest, 21.4 (19.2–24.0) % SL; pelvic fin length 22.0 (19.2–23.9) % SL.
Colouration when fresh: As described for H. wintoni sp. nov. ( Fig. 8b View FIGURE 8 ).
Colouration when preserved: As described for H. wintoni sp. nov.
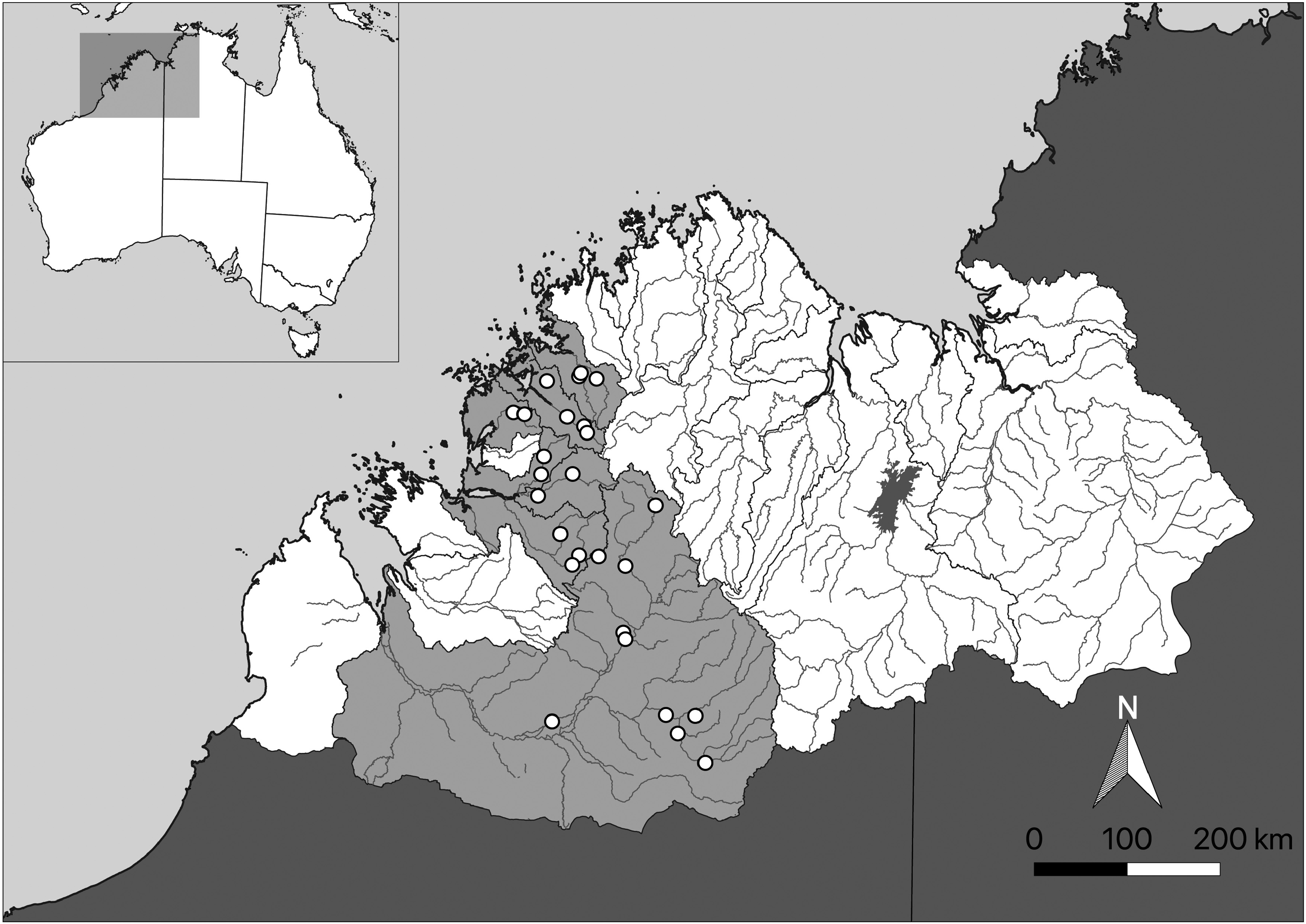
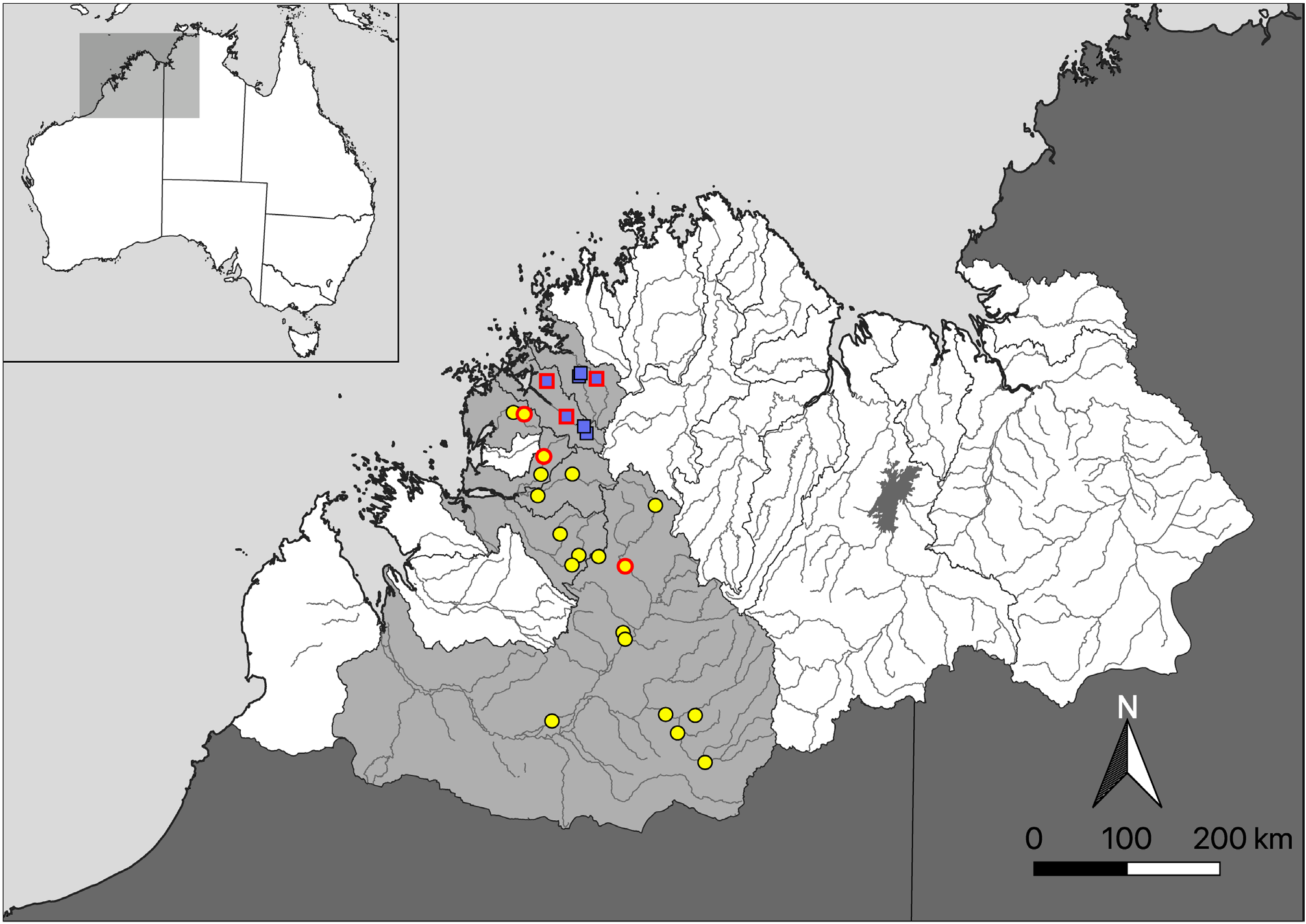
Distribution: Known to occur throughout the Fitzroy, Isdell, Charnley, Calder, Sale and Glenelg rivers, all draining the west coast of the Kimberley region ( Fig. 6 View FIGURE 6 ).
Etymology: The species was named in honour of James C. Greenway, Jr., of the Department of Ornithology, the American Museum of Natural History, who provided financial support for the American Museum of Natural History expedition to Western Australia, which collected the type series.
Ecology: Prefers slow to moderate flowing streams in clear or slightly turbid water over sandy to rocky substrates ( Fig. 9 View FIGURE 9 ). The omnivorous diet consists mainly of insect larvae and to a lesser degree, algae.
Remarks: The holotype had been allowed to dry out while on loan. It had deteriorated to such an extent that it was considered “totally useless” (Glen Moore, pers. comm. 2017) and had been discarded by Barry Hutchins in 1990 ( Hutchins & Smith 1991). The photograph and meristic and morphometric measurements of the holotype presented in Vari (1978), however, remain informative.
In Shelley et al. (2018a), gill rakers on the upper limb of the first gill arch and scale rows above the lateral line were presented as distinguishing characters between the Hannia species based on preliminary analysis. However, following further data collection and analysis, these characters were found to be uninformative.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Hannia greenwayi Vari 1978
| Shelley, James J., Delaval, Aurélien, Le Feuvre, Matthew C., Dempster, Tim, Raadik, Tarmo A. & Swearer, Stephen E. 2020 |
Hannia greenwayi
| Shelley, J. J. & Swearer, S. E. & Dempster, T. & Adams, M. & Le Feuvre, M. C. & Hammer, M. P. & Unmack, P. J. 2020: 1732 |
| Shelley, J. J. & Unmack, P. J. & Dempster, T. & Le Feuvre, M. C. & Swearer, S. E. 2019: 2427 |
Hannia greenwayi Vari, 1978
| Shelley, J. J. & Morgan, D. L. & Hammer, M. P. & Le Feuvre, M. C. & Moore, G. I. & Gomon, M. F. & Allen, M. G. & Saunders, T. 2018: 124 |
| Davis, A. M. & Unmack, P. J. & Pusey, B. J. & Pearson, R. G. & Morgan, D. L. 2014: 208 |
| Morgan, D. L. & Unmack, P. J. & Beatty, S. J. & Ebner, B. C. & Allen, M. G. & Keleher, J. J. & Donaldson, J. A. & Murphy, J. 2014: 267 |
| Davis, A. M. & Unmack, P. J. & Pusey, B. J. & Johnson, J. B. & Pearson, R. G. 2012: 1166 |
| Jackson, S. & Finn, M. & Featherstone, P. 2012: 100 |
| Davis, A. M. & Pearson, R. G. & Pusey, B. J. & Perna, C. & Morgan, D. L. & Burrows, D. 2011: 270 |
| Morgan, D. L. & Allen, G. R. & Pusey, B. J. & Burrows, D. W. 2011: 16 |
| Hoese, D. F. & Bray, D. J. & Paxton, J. R. & Allen, G. R. 2006: 1334 |
| Morgan, D. L. & Thorburn, D. & Fenton, J. & Wallace-Smith, H. & Goodson, S. 2005: 6 |
| Allen, G. R. & Midgley, S. H. & Allen, M. G. 2002: 219 |
| Morgan, D. L. & Allen, M. G. & Bedford, P. & Horstman, M. 2002: 47 |
| Morgan, D. L. & Allen, M. G. & Bedford, P. & Horstman, M. 2002: 150 |
| Axelrod, H. R. & Burgess, W. E. & Pronek, N. & Walls, J. G. 1997: 1063 |
| Allen, G. R. & Leggett, R. 1990: 537 |
| Allen, G. R. 1989: 155 |
| Paxton, J. R. & Hoese, D. F. & Allen, G. R. & Hanley, J. E. 1989: 532 |
| Lane, J. A. K. & McComb, A. J. 1988: 131 |
| Allen, G. R. 1982: 46 |