Allobates carajas, Simões & Rojas & Lima, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4550.1.3 |
|
publication LSID |
lsid:zoobank.org:pub:16D8B01C-30BF-4D41-97F7-040146C8DEEB |
|
DOI |
https://doi.org/10.5281/zenodo.5925164 |
|
persistent identifier |
https://treatment.plazi.org/id/03A587BC-FFEF-FF83-84D3-441BD5BD7E04 |
|
treatment provided by |
Plazi |
|
scientific name |
Allobates carajas |
| status |
sp. nov. |
Allobates carajas View in CoL sp. nov.
Figures 2–10 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 , 14 View FIGURE 14
Allobates View in CoL gr. marchesianus Neckel-Oliveira et al. 2012 p View in CoL . 79.
Allobates View in CoL sp. Carajas Grant et al. 2017 p. S29, Fig. 21.
Allobates sp. (Carajás BR) Simões et al. 2018 p. 125, Fig. 9 View FIGURE 9 .
Holotype. INPA-H 38643 (field code APL 20621). An adult male collected by P.I. Simões and D. Rojas, on 18 February 2014, in an area of streamside dense canopy forest in the Serra Sul region of Floresta Nacional de Carajás, in the municipality of Parauapebas, state of Pará, Brazil (06°23’34.7” S, 50°19’09.8” W). GoogleMaps
Paratopotypes. Eight adult specimens (two females, six males). Females: INPA-H 38635, 38642 (field codes APL 20620, 20622, respectively). Males: INPA-H 38633, 38637, 38640, 38641, 38646, 38649 (field codes APL 20617, 20625, 20624, 20623, 20618, 20626). One juvenile specimen: INPA-H 38647 (field code APL 20619). All collected by P.I. Simões and D. Rojas between 18–20 February 2014 at the same locality as the holotype.
Paratypes. Nine adult specimens (three females, six males). All collected by P.I. Simões and D. Rojas, within Floresta Nacional de Carajás , Parauapebas, state of Pará, Brazil. Trilha do Lago: Female: INPA-H 38638 (field code APL 21110) . Males: INPA-H 38639 , 38644 , 38645 , 38648 , 38650 (field codes APL 20629, 21109 , 20627 , 21108 , 20628 , respectively) . Collected between 22–24 February 2014 (coordinates 06°02’41.1” S, 50°05’29.7” W). N1 Trail: Female: INPA-H 38634 (field code APL 21105) GoogleMaps . Male: INPA-H 38636 (field code APL 21106) . Collected on 23 February 2014 (coordinates 06°03’06.3” S, 50°15’39.9” W). Road to Águas Claras: Female: INPA-H 38624 (field code APL 20608) GoogleMaps . Thirteen tadpoles: INPA-H 38632 (field code APL 20610) . Collected on 16 February 2018 (coordinates 06°13’00.9” S, 50°20’14.4” W).
Etymology. The specific epithet is a noun in singular nominative and refers to the species type locality at Floresta Nacional de Carajás.
Generic placement. The new species was assigned to Allobates based on overall similarity with other species of the genus and presence of the following diagnostic phenotypic characters proposed by Grant et al. (2006, 2017): (1) Finger IV reaching distal half of distal subarticular tubercle of Finger III, (2) webbing absent on postaxial side of Toe I, (3) webbing absent on preaxial side of Toe II, (4) webbing absent on postaxial side of Toe II, (5) webbing absent on preaxial side of Toe III, (6) webbing present on preaxial side of Toe IV, (7) oblique lateral line absent or diffuse, and (8) pale ventrolateral stripe present.
Definition. Allobates carajas is characterized by: (1) skin texture of dorsum smooth, weakly granular posteriorly, small granules more prominent from mid to posterior dorsum; (2) paired dorsal digital scutes present; (3) distal tubercle present on Finger IV; (4) discs on fingers I–IV moderately expanded; (5) dermal lateral fringes and basal webbing absent on fingers; (6) metacarpal ridge absent; (7) Finger III not swollen in male or female specimens; (8) carpal pad absent; (9) excrescences on thumbs of males absent; (10) thenar tubercle conspicuous; (11) black gland absent on arm; (12) tarsal keel present, tubercle-like, strongly curved; (13) discs on toes I-V moderately expanded; (14) basal webbing present between toes III and IV; (15) metartarsal fold absent; (16) cryptic, predominantly brown dorsal and lateral coloration; one to four transverse dark brown bars or blotches may be present on dorsal surface of thigh; pale dorsolateral stripe absent; a dark brown lateral stripe surrounds the whole body; pale oblique lateral stripe absent; pale ventrolateral stripe present, with a diffuse lower margin from behind the eyes to groin, iridescent white in live specimens, indistinct from background color of abdomen in preserved specimens; pale paracloacal mark absent; (17) dark throat-collar absent; (18) throat cream to white in preserved male specimens, with a variable number of melanophores; uniformly cream to white in preserved female specimens; (19) ventral surfaces uniformly yellow in life in male and female specimens; throat and vocal sac pinkish to translucent or peppered with a variable number of melanophores in live male specimens, uniformly yellow in female specimens; (20) iris dark with metallic gold flecks and a golden pupil ring; (21) large intestine unpigmented; (22) testis unpigmented; (23) mature oocytes not pigmented; (24) median lingual process absent; (25) tympanum inconspicuous to the naked eye; (26) vocal sac single; (27) maxillary teeth present; (28) advertisement calls characterized by the emission of notes in at least four different temporal arrangements: continuous calls with notes separated either by (i) regular or (ii) irregular silent intervals, (iii) emission of notes arranged in trills, and (iv) emission of isolated notes between long silent intervals. Temporal and spectral properties of notes are similar among call arrangements, note duration ranging between 0.02– 0.06 s, dominant frequency between 4.75–5.38 kHz) and frequency bandwidth of notes between 0.53–0.92 kHz.
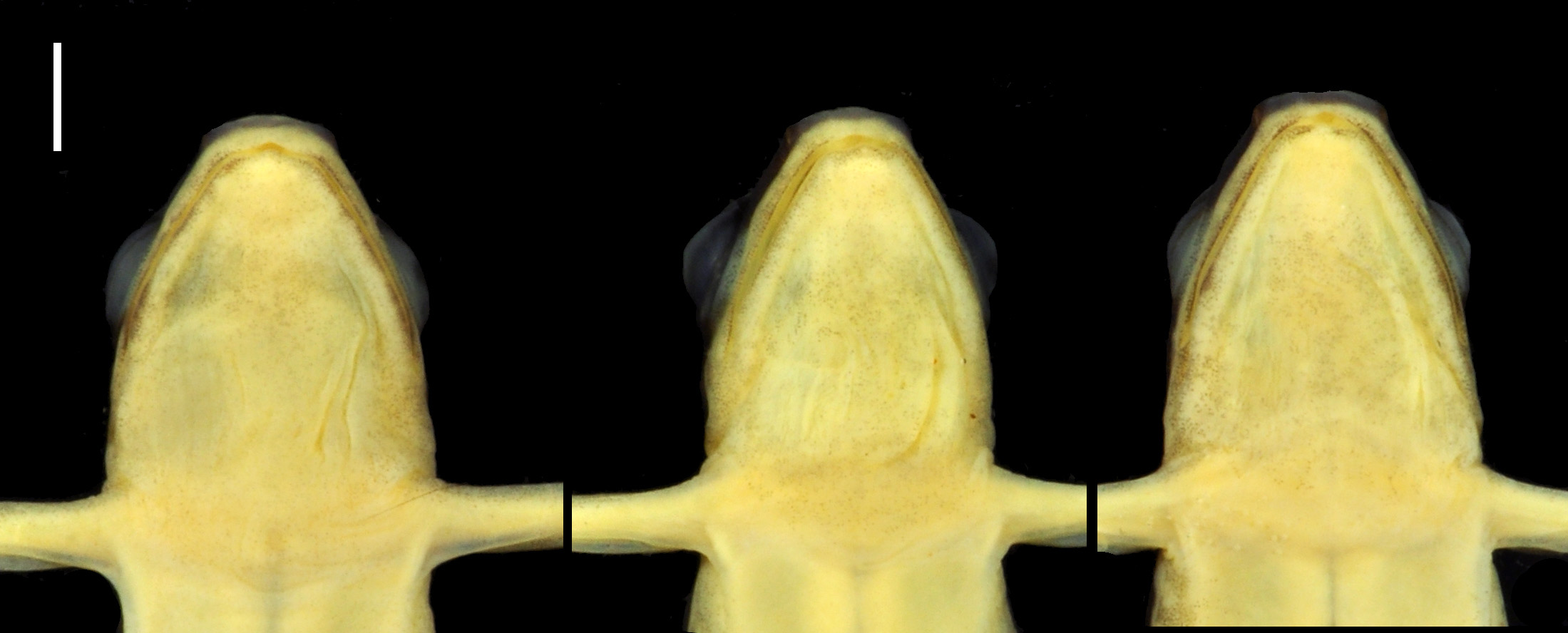
Description of the holotype. Adult male of SVL = 17.3 mm, in good state of preservation, with a piece of muscle ventrally cut from left thigh, preserved as tissue sample. Measurements of the holotype are presented in Table 1. Body robust, head wider than long (HL/HW = 0.94), head length 0.30 times the SVL ( Fig. 2 View FIGURE 2 ). Eye diameter larger than distance from anterior corner of the eye to nostril (EN/ED = 0.64) ( Fig. 3 View FIGURE 3 ). Nares located posterolaterally to tip of snout, directed laterally, visible in lateral, ventral and anterior views. Distance between nostrils 0.43 times the HW. Snout truncate in dorsal view. Canthus rostralis straight from nostril to anterior corner of the eye in dorsal view, rounded in cross section. Loreal region vertical. Tympanum round, 0.32 times ED. Margins of tympanum indistinct to the naked eye (visible under magnification) ( Fig. 3 View FIGURE 3 ). Maxillary teeth present, concealed by inner surface of upper lip, detectable under 20X magnification or by moving a wire probe along the maxillary surface. Tongue longer than wide, anterior third attached to the mouth floor. Median lingual process absent. Choanae round, positioned anteriorly to eye bulge. Vocal sac single, covering most of the medial and posterior portions of the subgular region. Lateral vocal slits conspicuous.
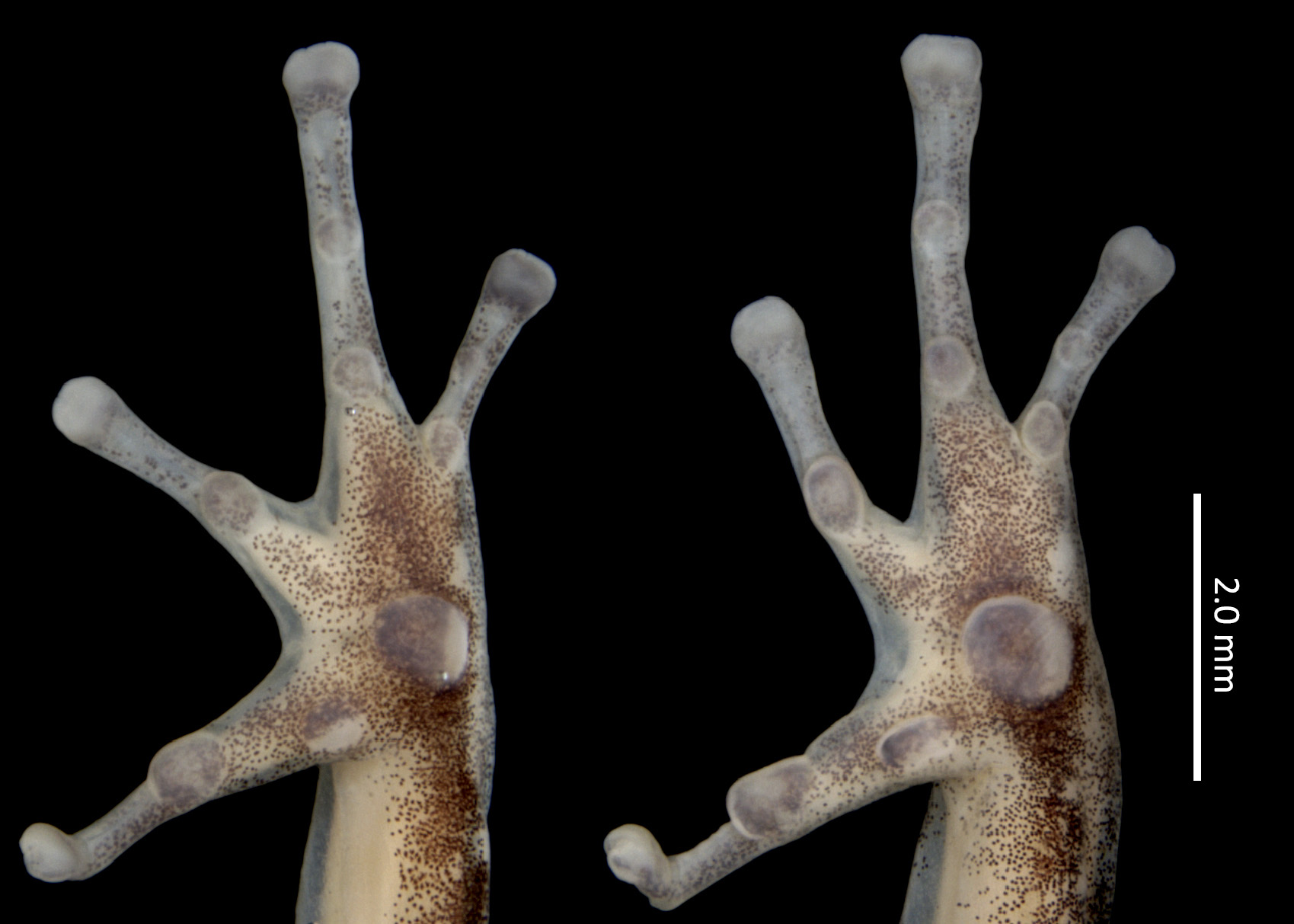
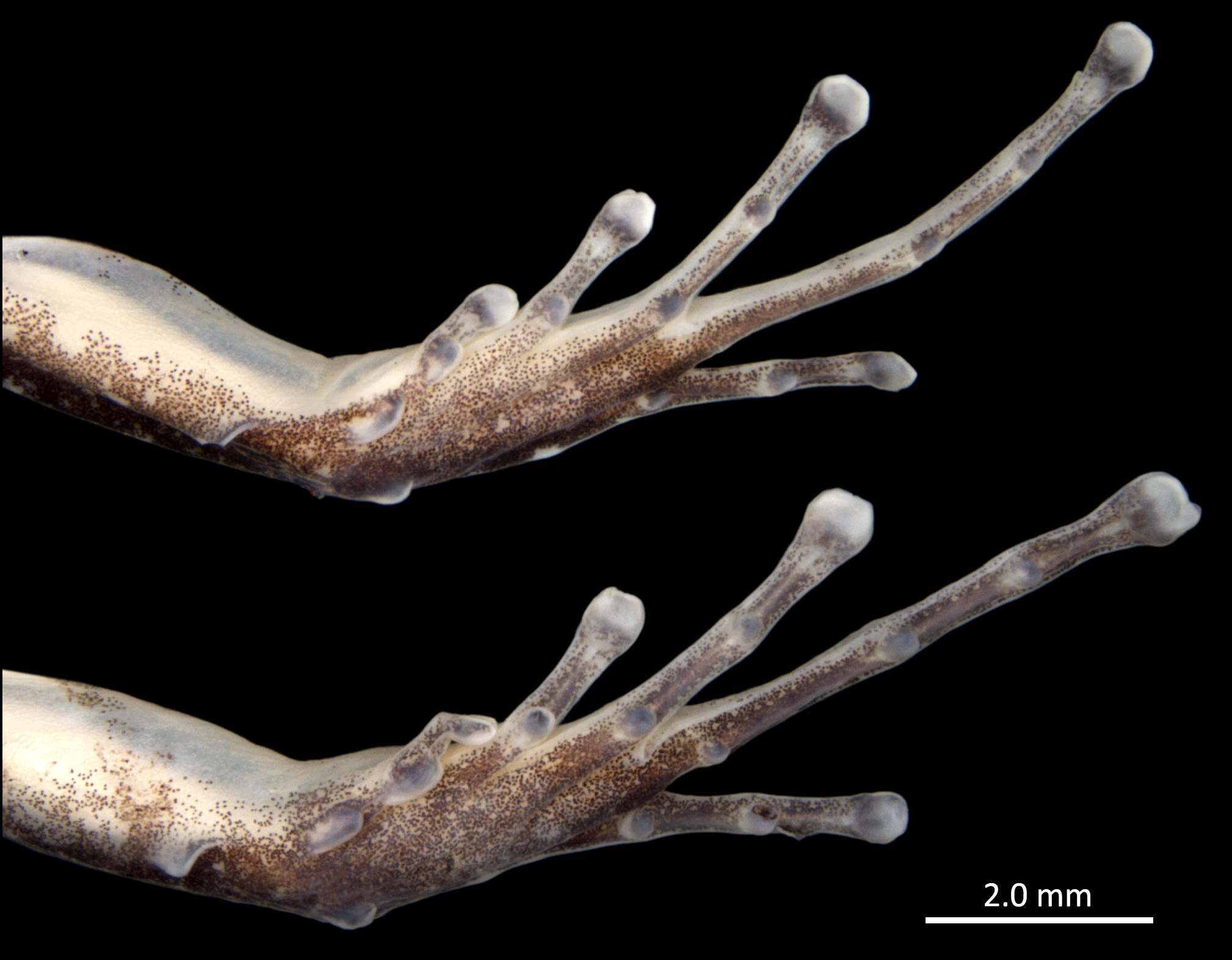
Palmar tubercle round to slightly elliptical. Thenar tubercle present, elliptical, evident in ventral view, less conspicuous in profile. Maximum diameter of thenar tubercle 58% of maximum diameter of palmar tubercle ( Fig. 4 View FIGURE 4 ). Subarticular tubercles of fingers III and IV round, small, not exceeding the width of phalanges. Subarticular tubercle on Finger II round, protuberant, 1.3 times larger than thenar tubercle in maximum diameter. Subarticular tubercle on Finger I very protuberant, elliptical, 1.4 times wider than thenar tubercle in maximum diameter. Distal subarticular tubercle present on Finger IV. Supernumerary tubercles absent. Metacarpal ridge absent. Finger fringes and hand webbings absent. Length of Finger II equivalent to approximately 96% of Finger I’s length. Tip of Finger IV reaching mid length of distal subarticular tubercle of Finger III when fingers are juxtaposed. Relative lengths of fingers: IV <II <I <III. Finger III not swollen. Discs of fingers I–IV moderately expanded, width of discs corresponding to 1.5, 1.5, 1.6 and 1.5 times the width of their respective adjacent phalanges ( Fig. 4 View FIGURE 4 ).
Tibia length approximately half the SVL (TL/SVL = 0.46). Tarsal keel present, tubercle-like, strongly curved at its proximal end, flattening and straightening towards metatarsal tubercle, but not reaching it ( Fig. 5 View FIGURE 5 ). Preaxial edge of tarsus smooth, not fringed. Metatarsal fold absent. Basal webbing present between toes III and IV. Basal webbing absent between other toes. Relative lengths of toes: I <II <V <III <IV. Discs of toes I–V moderately expanded, width of discs corresponding to 1.4, 1.5, 1.7, 1.7 and 1.6 times the width of adjacent phalanges, respectively.
Skin on dorsum smooth, weakly granular only posteriorly, from urostyle region to about mid body. Skin smooth laterally and ventrally. Dermal flap absent above cloaca ( Fig. 2 View FIGURE 2 ).
Color in alcohol of holotype. Dorsal surface of body tan to light brown, conspicuously lighter from tip of snout to the level of the anterior corner of the eye and dorsolaterally, from the level of posterior corner of the eye to the urostyle region ( Fig. 2 View FIGURE 2 ). Skin dark gray above orbits. Distinct longitudinal brown mark on dorsum, hourglassshaped, extending centrally from the level of anterior corner of the eye (where it forms a triangle-shaped interorbital blotch) to the urostyle region. Longitudinal tan brown mark with diffuse outer edges, merging with the light brown background of dorsum. Pale dorsolateral stripe absent. Lateral surface of body characterized by a solid dark brown stripe, extending from tip of snout to groin, surrounding the body ( Fig. 3 View FIGURE 3 ). Solid dark brown stripe broadening only slightly from posterior margin of the eye towards groin. Solid dark brown stripe faded posteriorly, on the inguinal region, but not forming a vertical bar or oblique line. Pale ventrolateral stripe indistinct from cream to white background of ventrolateral surfaces of body ( Fig. 3 View FIGURE 3 ). Ventral surfaces cream to white, with a few dark brown melanophores scattered only on chin and throat. Tongue is cream-colored.
Background color of upper arm, forearm and hand cream to pale in dorsal view, with evenly scattered brown melanophores, more densely grouped on wrist and fingers. Tips of fingers brown. Paired scutes on discs of fingers I, II and III cream to gray, brown on Finger IV. Upper arm cream in ventral view, continuous with color pattern of abdomen. Outer lateral edge of upper arm with a thin brown line. Forearm cream, solid brown only along its outer edge in ventral view. Carpal and metarcarpal regions ventrally brown, densely pigmented ( Fig. 4 View FIGURE 4 ).
Area immediately around vent brown. Pale paracloacal mark indistinct. Instead, a pale transversal bar crosses the proximal portion of thigh dorsally, continuous with dorsolateral light brown color of dorsum ( Fig. 2 View FIGURE 2 ). Background color of thigh light brown. Thigh crossed proximally by two transverse dark brown bars in dorsal view (see Variation in type series below). Inner and outer dorsolateral surfaces of thigh brown. Dorsal surface of shank same color as thigh, with three dark brown blotches present medially (the distal one forming a transverse dark brown bar). Dorsal surface of tarsal region lighter than overall color of legs, with irregular dark brown blotches. Toes brown, with irregularly distributed melanophores. Paired scutes on toes dark brown to gray. Ventral surfaces of thigh and shank cream to translucent, free of melanophores. Ventral surfaces of tarsal and metatarsal regions uniformly dark brown. Toes brown, densely pigmented in ventral view ( Fig. 5 View FIGURE 5 ).
Variation in type series. Measurements of specimens in the type series pare presented in Table 1. In average, females are larger than males, but all measurements overlap in range between male and female paratypes. Longitudinal hourglass-shaped brown mark on dorsum variable in width, lightness and conspicuousness among specimens in the type series ( Fig. 6 View FIGURE 6 ). A faint dark brown transverse bar may be present medially on forearm of some specimens ( Fig. 6 View FIGURE 6 ; Fig. 8C View FIGURE 8 ). Dorsal surface of thigh medially and distally crossed by 1–4 dark brown transverse bars. Transverse bars on dorsal surface on shank varying between 0–3 ( Fig. 6 View FIGURE 6 ). Throat pigmentation variable on males, dark brown melanophores varying from a few and sparsely scattered (INPA-H 38645 and the holotype), to moderately to densely scattered from chin to pectoral region ( Fig. 7 View FIGURE 7 ; Fig. 8B, C View FIGURE 8 ). Females with a few brown melanophores sparsely scattered only on chin and along the upper lip.
Testes cream colored. Smaller oocytes uniformly pigmented, reddish-brown. Mature oocytes uniformly cream to white. Large intestine unpigmented.
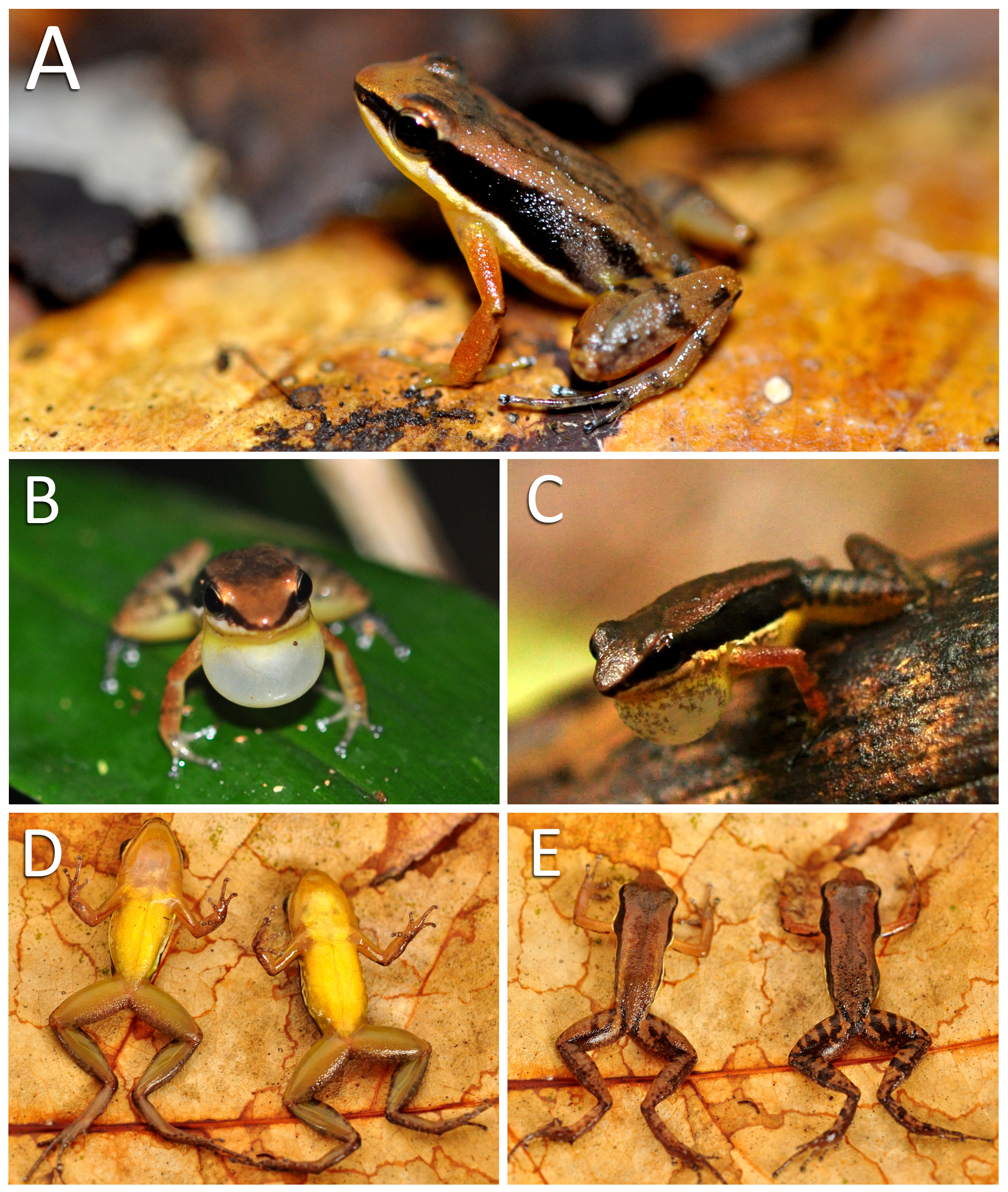
Color in life. Dorsum dark brown centrally, forming hourglass or rhomboid patterns on a tan brown background. Head dorsally tan brown from tip of snout to the level of the eye. Area between the eyes dark brown forming a triangular shape which connects with a rhomboid shape posteriorly. Iris golden, with dark brown reticulation. Dorsum laterally tan brown, darker on the urostyle region, where it is flanked by a thin black stripe. Pale dorsolateral stripe absent ( Fig. 8A View FIGURE 8 ). Lateral surface of body surrounded by a uniformly dark brown stripe from tip of snout to groin. Faint areas may be present posterolaterally on the dark brown stripe on the inguinal region, but never forming a solid pale oblique lateral line. Ventrolateral stripe iridescent white, unbroken, extending along the lower border of dark brown stripe from upper lip to groin ( Fig. 8 View FIGURE 8 ). Ventrolateral surface of body with iridescent white blotches, same color as ventrolateral stripe, on brown background, merging ventrally with the yellow color of chest and abdomen. Throat uniformly yellow in females. Throat and vocal sac pinkish to translucent in live male specimens ( Fig. 8D View FIGURE 8 ); when inflated vocal sac of males uniformly white to translucent ( Fig. 8B View FIGURE 8 ) or peppered with a variable number of melanophores ( Fig. 8C View FIGURE 8 ). Remaining ventral surfaces of body uniformly yellow, paler medially on the abdomen, where underlain by peritoneum.
Dorsal surface of upper and forearm uniformly tan brown, yellowish only around arm-body insertion. A faint dark brown transverse bar may be present medially on forearm ( Fig. 8B, C View FIGURE 8 ). Upper arm yellow to translucent, darker than chest in ventral view, blood vessels visible through skin. Forearm, carpal and metacarpal regions dark brown in ventral view. Fingers dark brown in ventral view, light gray brown in dorsal view. Paired scutes on finger discs iridescent white.
Surfaces immediately adjacent to vent uniformly dark brown. Light paracloacal marks absent. Instead, dorsal surface of thigh is proximally crossed by alternating tan brown and dark brown transverse bars. Dorsal surface of thigh medially and distally crossed by a variable number of dark brown transverse bars ( Fig 8E View FIGURE 8 ). Dorsal surface of shank same color as thigh, with a medial transverse dark brown bar. Smaller dark brown blotches may be present on shank, beside the transverse bar. Ventral surface of thigh and shank solid greenish-yellow, darker than yellow shades of abdomen ( Fig. 8D View FIGURE 8 ). Dorsal surface of tarsal region tan brown, with one or two transverse dark brown stripes. Tarsal and plantar regions brown in ventral view. Toes with tan brown and dark brown patterning. Paired scutes on toe discs iridescent white.
Color in life of juveniles same as that of adults, except for the lack of yellow colors on ventral surfaces, which are gray to translucent ( Fig. 14C, D View FIGURE 14 ).
.
Description of larvae. Morphometric measurements of tadpoles are presented in Table 2. A tadpole at developmental stage 34 is shown in Fig. 9 View FIGURE 9 . The following description is based on nine tadpoles at developmental stages 31–34: Body ellipsoid, slightly round anteriorly and posteriorly in dorsal view, slightly flattened in lateral view ( Fig. 9 View FIGURE 9 ). Body length 36–40% and tail length 59–64% of TL; body wider than deep, BH 70–77% of BW; HWLE 72–90% of BW; snout truncate to round in dorsal view, round in lateral view; END approximately same length as ED (END/ED = 0.8–1.2); eyes dorsal and directed laterally; IOD 26–31% of HWLE. Small nares located dorsolaterally and directed anterolaterally, visible in dorsal and lateral views; internarial distance 18–23% of HWLE. Fleshy ring present on the inner margin of nostrils, round, straight, not ornamented. Spiracle sinistral, free from the body, tubular, 1.0– 1.4 mm in length, attaching to body ventrolaterally at mid-body length ( Fig. 9 View FIGURE 9 ). Gut coiled, with its axis directed to the left side of body and located near the spiracular tube, visible through skin. Vent tube fused to ventral fin, 1.1–1.8 mm in length, dextral. Dorsal fin begins at body-tail insertion, dorsal edge shallow and straight anteriorly (along approximately 10% of its length), deeper posteriorly, reaching maximum depth after approximately two thirds of tail’s length. Dorsal fin slightly deeper than lower fin. Tail tip slightly acuminate. Caudal musculature dorsally reaching up to two thirds of body length from body-tail insertion to the level of spiracle ( Fig. 9 View FIGURE 9 ).
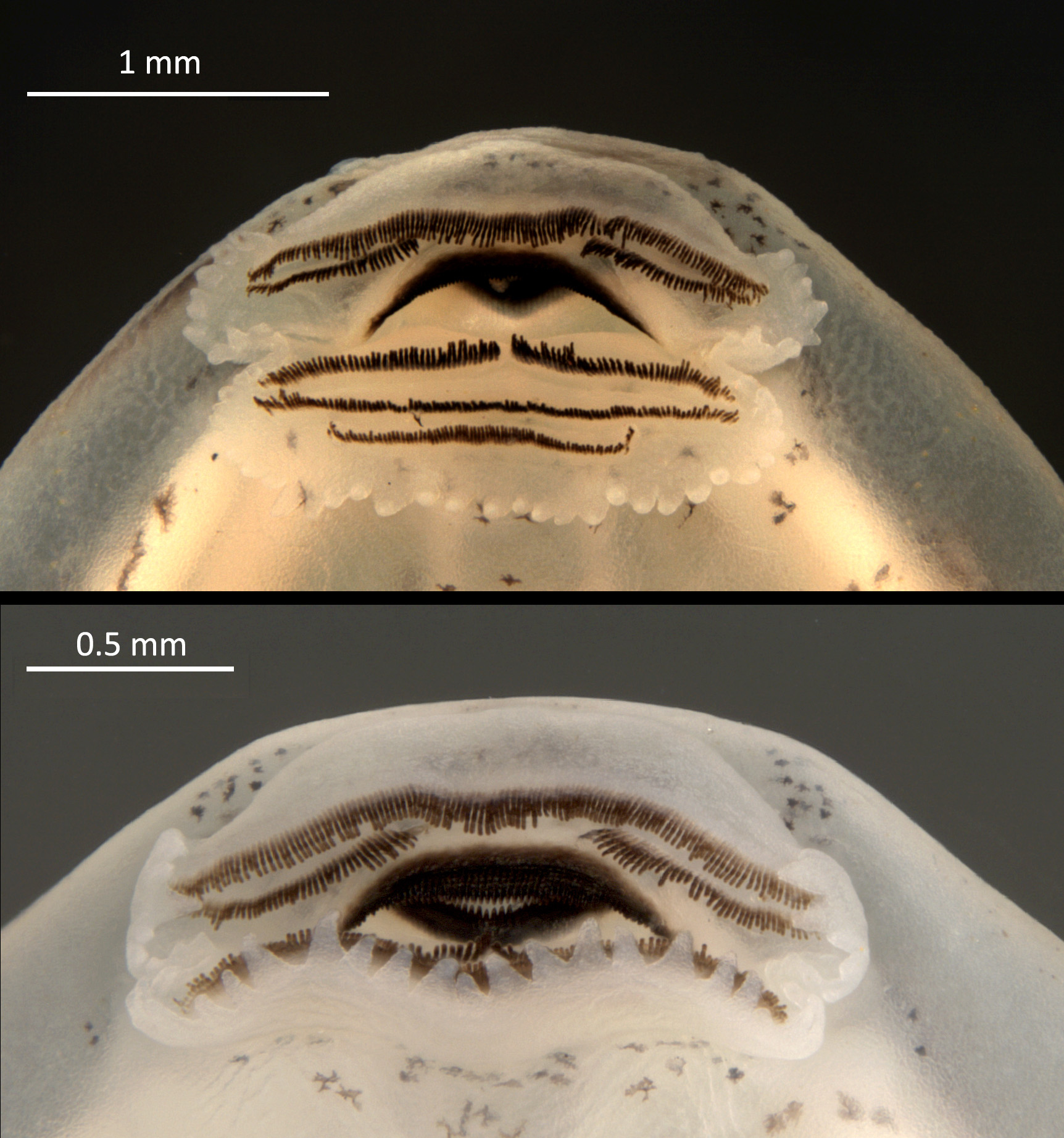
Oral disc located anteroventrally, emarginate laterally, transversely elliptical, 1.7–2.3 mm wide, corresponding to approximately 35% of body width at the level of spiracle ( Fig. 10 View FIGURE 10 ). Anterior labium with a group of 5–8 short, pyramidal papillae distributed in a single row on each side of labium’s lateral margins. Groups of marginal papillae on each side of the upper labium split by a medial gap 1.1–1.6 mm long, corresponding to approximately 70% of oral disc’s width. Posterior labium with a single row of marginal papillae variable in length, pointing at the same direction. Papillae short and round to pyramidal on the outer lateral folds of labium (4–7 papillae on each side). Longer pyramidal papillae (6–9 papillae on each side) distributed medially on posterior labium, in a single line ( Fig. 10 View FIGURE 10 ). Submarginal papillae, absent.
Upper jaw sheath arch-shaped, longer than lower jaw sheath, with no medial notch. Lower jaw sheath Ushaped, deeper than upper jaw sheath. Cutting edge of upper and lower jaw sheaths serrated; serrations extending along the entire length of both sheaths. Length of tooth rows A-1=A-2=P-1=P-2, 1.2–1.7 mm. Tooth rows A-1, P- 2, P-3 complete; tooth row A-2 interrupted by a medial gap, 0.3–0.8 mm ( Fig. 10 View FIGURE 10 ). Tooth row P-1 with a narrow medial gap (<0.1 mm), evidenced by a break between the underlying tooth ridges. Row P-3 shorter than other rows, 1.0– 1.4 mm.
Background color of lateral, dorsal and anteroventral surfaces of body cream, with large darker areas formed by the aggregation of brown melanophores, more densely grouped on dorsum and flanks ( Fig. 9 View FIGURE 9 ). Darker brown color appears on dorsum between the diverging sections of caudal musculature. A pale, unpigmented patch, is present below the eye, slightly larger in area than the eyeball. Ventral surface of body posteriorly transparent and immaculate; intestines visible through skin. Tail muscle cream; tail fins transparent with scattered irregular blotches of brown melanophores distributed more densely over tail muscle and upper fin ( Fig. 9 View FIGURE 9 ).
Comparison with tadpoles of other species. Tadpoles of Allobates caeruleodactylus ( Lima & Caldwell, 2001), A. grillisimilis Simões, Sturaro, Peloso & Lima, 2013 , A. marchesianus ( Melin, 1941) , A. subfolionidificans ( Lima, Sanchez & Souza, 2007) and A. tapajos Lima, Simões & Kaefer, 2015, differ from those of A. carajas in having distinctively elongate papillae on posterior labium (papillae round to pyramidal in A. carajas ). Additionally, A. caeruleodactylus and A. marchesianus have distinct dark transversal bars on tail (absent in A. carajas ) ( Caldwell et al. 2002a; Simões et al. 2013b; Lima et al. 2007; 2015). Labial tooth rows P-1, P-2 and P-3 are sub-equal in tadpoles of A. sumtuosus ( Morales, 2002) ( Simões & Lima 2012); row P-3 is lacking in tadpoles of A. granti ( Kok et al. 2006) (all rows present in A. carajas , with P-3 distinctively shorter than P-1 and P-2). Row A-2 shorter than A- 1 in tadpoles of A. brunneus ( Cope, 1887) ( Lima et al. 2009); row A-2 longer than A- 1 in A. magnussoni Lima, Simões & Kaefer, 2014 (rows A-1 and A-2 with the same length in A. carajas ). Tadpoles of A. goianus ( Bokermann, 1975) have large dark blotches distally on tail and short and round papillae medially on posterior labium (pigments distributed over tail muscle and upper fin along the whole length of tail, papillae on medial posterior labium pyramidal). Tadpoles of A. hodli Simões, Lima & Farias, 2010 , have short and round papillae medially on posterior labium (papillae on medial posterior labium pyramidal). Tadpoles of A. paleovarzensis ( Lima, Caldwell, Biavati & Montanarin, 2010) have a distinct dark brown longitudinal bar extending from the snout to the eye, and towards mid-body (longitudinal dark brown bar absent in A. carajas ). Tadpoles of A. nidicola ( Caldwell & Lima, 2003) and A. masniger ( Morales, 2002) are endotrophic and develop entirely in a terrestrial nest, and lack oral discs (and associated mouthparts) and spiracles ( Caldwell & Lima, 2003; Albertina P. Lima, pers. observation).
Advertisement calls. SVL of captured recorded males ranged between 17.6–18.5 mm (average 17.7 mm, n = 5). By inspecting the waveforms and spectrograms of calls of ten males recorded in Carajás, we identified four types of temporal arrangement of notes ( Fig. 11 View FIGURE 11 ). In Serra Sul, notes were emitted either grouped in discrete trills (n = 2 males) or continuously, separated by regular silent intervals (n = 1 male). At Trilha do Lago, most recorded males (n = 3) emitted isolated notes, separated by long, irregular silent intervals. In this location, two other males emitted notes continuously, but notes were separated by short, irregular silent intervals. A sixth male emitted notes grouped in trills, but turned to a continuous regular call at the end of the recording. A single male was recorded at N1 Trail while emitting notes arranged in trills.
Considering all recordings, notes had an average duration of 0.04 ± 0.01 s (range 0.02– 0.06 s) and were emitted at an average dominant frequency of 5101.9 ± 236.1 Hz (range 4752.8–5384.9 Hz). Notes had an ascending frequency modulation ( Fig. 12 View FIGURE 12 ). Average lower frequency of notes was 4655.1 ± 199.9 Hz (range 4402.3–4937.9 Hz) and average upper frequency of notes was 5418.8 ± 232.6 Hz (range 5107.3–5742.0 Hz). In average, notes occupied a frequency bandwidth of 763.7 ± 1129 Hz (range 533.1–920.0 Hz).
When notes were emitted continuously (n = 5 males), silent intervals between notes was extremely variable, ranging between 0.22– 3.40 s (mean 0.76 ± 0.62 s; n = 100 silent intervals). When arranged in discrete trills (n = 4 males / 23 trills), trills were formed by 4–22 notes (mode = 8 notes) and their duration ranged between 1.49– 7.05 s (mean = 4.38 ± 1.61 s). Trills were emitted between irregular, generally long silent intervals, ranging between 3.03– 17.95 s (mean 7.22 ± 3.60 s). Silent interval between notes within a trill ranged between 0.24– 0.91 s (mean 0.40 ± 0.14 s; n = 69 silent intervals).
Comparison with advertisement calls of other species. Advertisement calls of A. caeruleodactylus , A. magnussoni , A. masniger , A. nidicola , and A. subfolionidificans are formed by a single note, emitted continuously between irregular ( A. caeruleodactylus , A. subfolionidificans ) or regular ( A. magnussoni , A. masniger , A. nidicola ) inter-note silent intervals, not arranged in discrete note trills. Dominant frequency of notes in A. carajas is lower than that of notes in calls of A. caeruleodactylus (dominant frequency 5.54–6.64 kHz) ( Lima & Caldwell 2001). Note duration in calls of A. carajas is generally longer than that in calls of A. magnussoni (note duration 0.047– 0.104 s). Additionally, A. magnussoni frequently emits calls formed by note-pairs ( Lima et al. 2014). Silent interval between notes in the continuous calls of A. nidicola and A. masniger are always regular, ranging between 0.206– 0.315 s and 0.253– 0.425 s, respectively ( Caldwell & Lima 2003; Kaefer et al. 2012). Continuous calls of A. carajas can not be distinguished from those of A. subfolionidificans by their spectral or temporal parameters, overlapping in all measurements ( Lima et al. 2007).
Some Allobates species alternate between two types of advertisement calls, emitting notes continuously or arranged in bouts or trills. When emitting trills of notes, silent interval between notes of A. carajas is longer than that in trills of A. marchesianus (0.12– 0.21 s) ( Caldwell et al. 2002b).
Continuous calls and note trills of A. carajas overlap in temporal parameters with those of A. olfersioides , however, notes in calls of A. olfersioides occupy a narrower frequency bandwidth (frequency bandwidth range 0.08–0.41 kHz) ( Forti et al. 2017). Dominant frequency of notes in calls of A. carajas is lower than that of notes in calls of A. sumtuosus (dominant frequency 5.60–6.48 kHz) ( Simões et al. 2013a).
Advertisement calls of some Allobates species are constituted exclusively by note trills. However, in comparison to A. carajas , note trills are generally longer and formed by a larger number of notes in calls of A. bacurau (call duration 6.92– 11.07 s, 60–81 notes) ( Simões 2016). Trills of A. crombiei ( Morales, 2002) are formed by a larger number of notes, emitted between much shorter silent intervals (25–59 notes, interval between notes 0.045– 0.069 s) ( Lima et al. 2012). Note trills and silent interval between notes within a trill are shorter in calls of A. grillissimilis (trill duration 0.12– 0.30 s, interval between notes 0.010–0.043) ( Simões et al. 2013b). Minimum trill duration in A. carajas (1.49 s) only slightly overlapping with maximum trill duration in A. trilineatus (trill duration 0.97– 1.55 s). Minimum interval between notes in trills of A. carajas (0.24 s) much longer than maximum interval between notes in A. trilineatus (0.07– 0.09 s) ( Grant & Rodríguez 2001).
Advertisement calls of A. flaviventris Melo-Sampaio, Souza & Peloso, 2013 , and A. hodli are emitted as trills of note-pairs or couplets, not as trills of single notes ( Simões et al. 2010; Melo-Sampaio et al. 2013; Lima et al. 2014). Advertisement calls of A. femoralis ( Boulenger, 1884) and A. myersi ( Pyburn, 1981) are emitted as trills of three, four, six or eight notes ( Amézquita et al. 2006; Simões & Lima 2011).
Note trills of A. carajas more closely resemble those of A. brunneus , A. goianus , A. paleovarzensis , and A. tinae , overlapping in all call parameters. However, in general, notes in trills of A. goianus are shorter and emitted between shorter silent intervals (note duration 0.030– 0.051s, interval between notes 0.020– 0.048 s) ( Carvalho et al. 2016). Notes in trills of A. paleovarzensis are split by generally shorter silent intervals and emitted with a lower dominant frequency (interval between notes 0.065– 0.266 s, dominant frequency 4.05–4.93 kHz) ( Lima et al. 2010). Note trills of A. tinae are generally formed by a small number of notes (2–9 notes) ( Melo-Sampaio et al. 2018). Additionally, the ability to emit continuous calls is not reported for A goianus , A. paleovarzensis or A. tinae . Continuous calls or note trills of A. brunneus can not be distinguished from those of A. carajas . However, notes emitted in continuous calls of A. brunneus are always regularly spaced, with silent intervals between notes varying between 0.35– 0.42 s ( Lima et al. 2014).
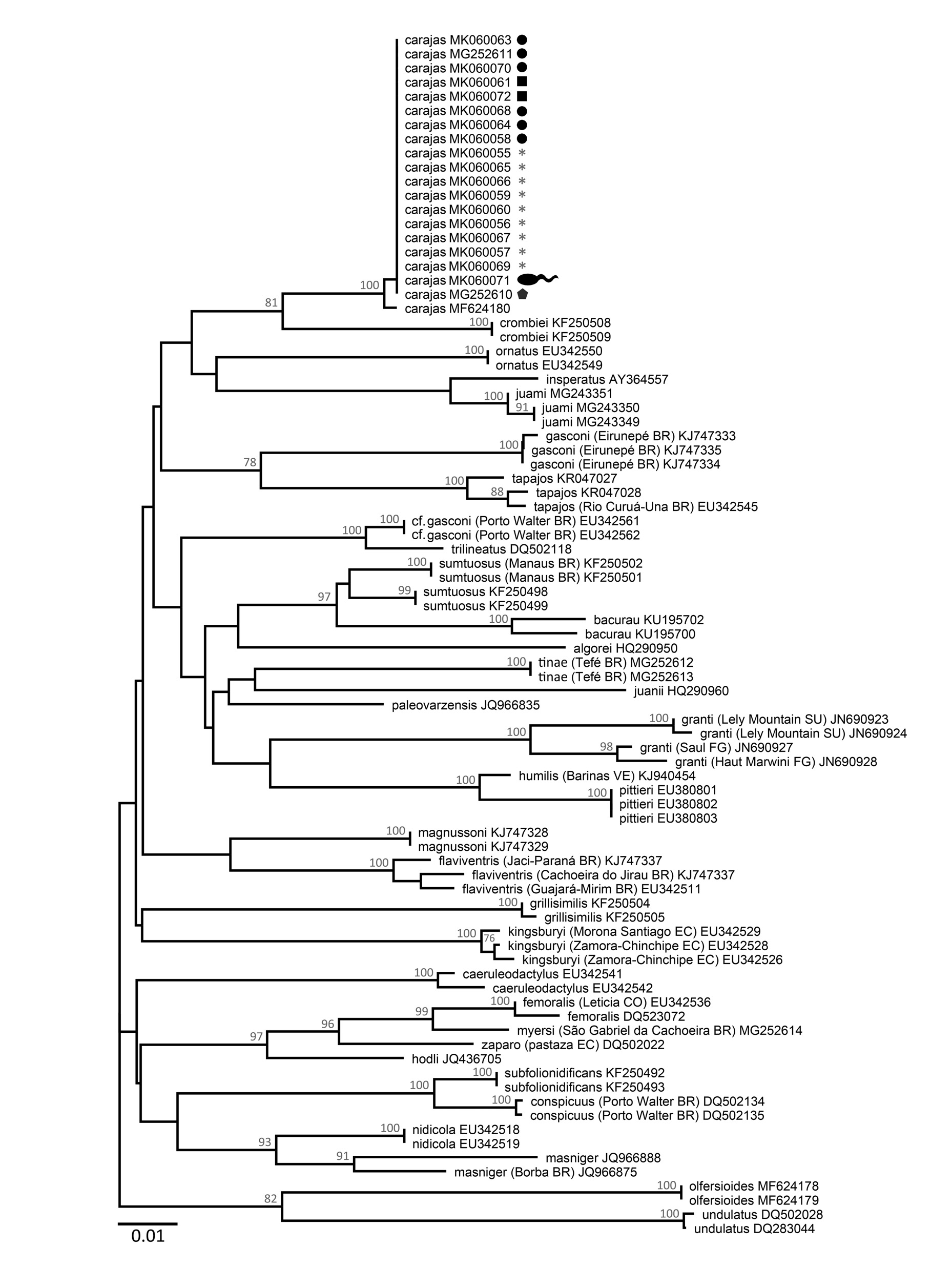
DNA barcoding. All adult specimens (n = 19) and the single tadpole sequenced shared the same mitochondrial 16S rRNA haplotype ( Fig. 13 View FIGURE 13 ) and formed a well-supported group with a second haplotype found in an earlier study that sampled this species in Carajás ( Grant et al. 2017). This group is nested within Allobates inhabiting cis- Andean South America for which 16S rDNA sequences were available ( Fig. 13 View FIGURE 13 ). Two sequences of A. crombiei from Cachoeira do Espelho, Pará, Brazil, are the most similar to those of A. carajas , with high bootstrap support ( Fig. 13 View FIGURE 13 ). Average uncorrected pairwise and Kimura-2-parameter (K2P) genetic distances between A. crombiei and A. carajas are 5 and 6%, respectively ( Table 3).
Average genetic distances between A. carajas and the remaining samples analyzed is ḵ 7 or 8% (uncorrected pairwise and K2P distances, respectively). Average uncorrected pairwise genetic distances estimated between A. carajas and other cryptically colored Allobates with hourglass-shaped marks on dorsum ( A. flaviventris , A. gasconi , A. magnussoni , A. undulatus ) are ḵ 8% (ḵ9 % considering K2P genetic distances) ( Table 3).
Diagnosis. Allobates carajas is distributed in eastern Brazilian Amazonia, south of the Amazon River. Hence, we compare the new species with all Allobates species occurring in Brazil and with one additional species from Venezuela, Allobates undulatus ( Myers & Donnelly, 2001) , which is morphologically similar to A. carajas . Character states of the new species are in parentheses throughout the diagnosis.
Live specimens of Allobates femoralis , A. hodli and A. myersi have bright yellow, orange or red flash marks on dorsal surface of thigh, and black and white marbling on the surface chest and abdomen (bright colored marks absent on dorsal surface of thigh, abdominal surfaces solid yellow or pale in live and preserved specimens of A. carajas ). Minimum SVL reported for individuals of these species 22.2 mm in a male of A. hodli (largest A. carajas male = 18.5 mm).
Allobates bacurau Simões, 2016 View in CoL , A. caeruleodactylus View in CoL , A. conspicuus ( Morales, 2002) View in CoL ; A. fuscellus ( Morales, 2002) View in CoL , A. grillisimilis View in CoL , A. juami Simões, Gagliardi-Urrutia, Rojas-Runjaic & Castroviejo-Fisher 2018 View in CoL , A. marchesianus View in CoL , A. masniger View in CoL , A. nidicola View in CoL , A. paleovarzensis View in CoL , A. subfolionidificans View in CoL , A. sumtuosus View in CoL , A. tapajos View in CoL , A. tinae Melo-Sampaio, De Oliveira & Prates, 2018 View in CoL , A. vanzolinius ( Morales, 2002) View in CoL lack rhomboid or hourglass-shaped brown marks centrally on dorsum (dorsum with distinct longitudinal brown mark, hourglass-shaped, extending centrally from the level of anterior corner of the eye to the urostyle region in A. carajas View in CoL ).
Allobates bacurau View in CoL and A. grillisimilis View in CoL are also distinguished from A. carajas View in CoL by their smaller size, reaching a maximum SVL = 16.0 mm, in a female A. grillisimilis View in CoL (minimum SVL of A. carajas View in CoL = 16.5 mm) and by the predominantly white to translucent ventral surfaces, with no shades of yellow in life (ventral surfaces yellow in live A. carajas View in CoL ). Fingers sky-blue in live males of A. caeruleodactylus View in CoL (fingers brown in male A. carajas View in CoL ). Allobates caeruleodactylus View in CoL also has a diffuse dark brown lateral stripe (solid dark brown lateral stripe). Allobates conspicuus View in CoL has a well-defined pale dorsolateral stripe and pale paracloacal mark (dorsolateral stripe absent, pale paraclacal mark indistinct). Ventral surfaces of male A. fuscellus View in CoL dark gray, transverse dark bars absent on dorsal surfaces of thigh (ventral surfaces yellow in life, white to translucent in preserved specimens, transverse bars present on dorsal surface of thigh). Dark pigments absent on ventral surfaces of male A. juami View in CoL (dark melanophores present on throat, or at least on chin in male A. carajas View in CoL ). Pale dorsolateral stripe present and well-defined, pale ventrolateral line absent in A. marchesianus View in CoL (pale dorsolateral stripe absent, pale ventrolateral stripe present in A. carajas View in CoL ). Ventral surfaces of male A. marchesianus View in CoL dark to light gray ( Caldwell et al. 2002b) (yellow in life, white to translucent in preserved A. carajas View in CoL ). Throat black or dark brown in male A. masniger View in CoL and A. nidicola View in CoL (throat pinkish to translucent in life, white in preserved specimens, with scattered melanophores in male A. carajas View in CoL ). Throat pinkish gray in life, gray in preserved males of A. paleovarzensis View in CoL (throat pinkish to translucent in life, white in preserved specimens). Allobates paleovarzensis View in CoL also lacks transverse dark bars on dorsal surface of thigh (dorsal surface of thigh with a variable number of transverse dark bars). Ventral surfaces solid white in live males of A. subfolionidificans View in CoL (ventral surfaces predominantly yellow in live males of A. carajas View in CoL ). Dorsal color of thighs bluish gray in live A. sumtuosus View in CoL , with no transverse dark bars (dorsal surface of thigh tan brown with a variable number of transverse dark bars). Some specimens of A. tapajos View in CoL may have brown dorsal markings forming approximately rhomboid patterns, but throat and vocal sac are golden yellow in live males (pinkish to translucent) and dark brown lateral stripe is diffuse posteriorly (dark brown lateral stripe solid, not diffuse). Throat and vocal sac golden yellow in live males of A. tinae View in CoL (pinkish to translucent) and no transverse dark bars on dorsal surface of thighs of males or females (dorsal surface of thigh with a variable number of transverse dark bars). Minimum SVL of male A. vanzolinius View in CoL (19.6 mm) larger than maximum SVL of A. carajas View in CoL (18.5 mm). Allobates vanzolinius View in CoL also lacks transverse dark bars on dorsal surface of thigh (dorsal surface of thigh with a variable number of transverse dark bars).
X-shaped dark brown mark present on dorsum and C-shaped pale paracloacal mark present adjacent to vent of Allobates olfersioides ( Lutz, 1925) ( Verdade & Rodrigues 2007) (hourglass-shaped mark present on dorsum, pale paracloacal mark absent in A. carajas ).
Allobates brunneus View in CoL , A. crombiei View in CoL , A. flaviventris View in CoL , A. gasconi ( Morales 2002) View in CoL , A. goianus View in CoL , A. magnussoni View in CoL and A. undulatus ( Myers & Donnelly, 2001) View in CoL have distinct diamond-shaped or hourglass patterns on dorsum and could be potentially mistaken for A. carajas View in CoL . However, lateral brown stripe surrounding body and pale ventrolateral line are diffuse posteriorly in A. brunneus View in CoL ( Lima et al. 2009) (lateral stripe dark brown and solid, pale ventrolateral stripe continuous). Allobates crombiei View in CoL lacks a distal subarticular tubercle on Finger IV, and male specimens lack dark pigments on throat ( Morales 2002; Lima et al. 2012) (distal subarticular tubercle present on Finger IV, males with throat conspicuously peppered by dark melanophores in A. carajas View in CoL ). Live specimens of A. flaviventris View in CoL and A. magnussoni View in CoL with an interrupted or diffuse pale ventrolateral stripe, formed by iridescent-white dots and blotches, males with pinkish-white chest and silvery-white anterior abdomen, yellow colors projecting only marginally from posterior to anterior abdomen (pale ventrolateral stripe unbroken, abdomen uniformly yellow in live male specimens of A. carajas View in CoL ). Maximum SVL of male A. gasconi View in CoL (16.3 mm) shorter than minimum SVL of A. carajas View in CoL (16.5 mm). Pale dorsolateral stripe present in A. gasconi View in CoL (absent in A. carajas View in CoL ). Allobates goianus View in CoL with a conspicuous pale paracloacal mark, evident in live and preserved specimens and basal webbing present between toes II-III ( Carvalho et al. 2016) (pale paracloacal mark absent, basal webbing absent between toes II-III). Minimum SVL of A. undulatus View in CoL (20.0 mm) larger than maximum SVL of A. carajas View in CoL (19.1 mm). Additionally, lateral stripe surrounding body black and broad, covering whole flank posterior to arm in A. undulatus View in CoL (lateral stripe dark brown, limited inferiorly by pale ventrolateral stripe)
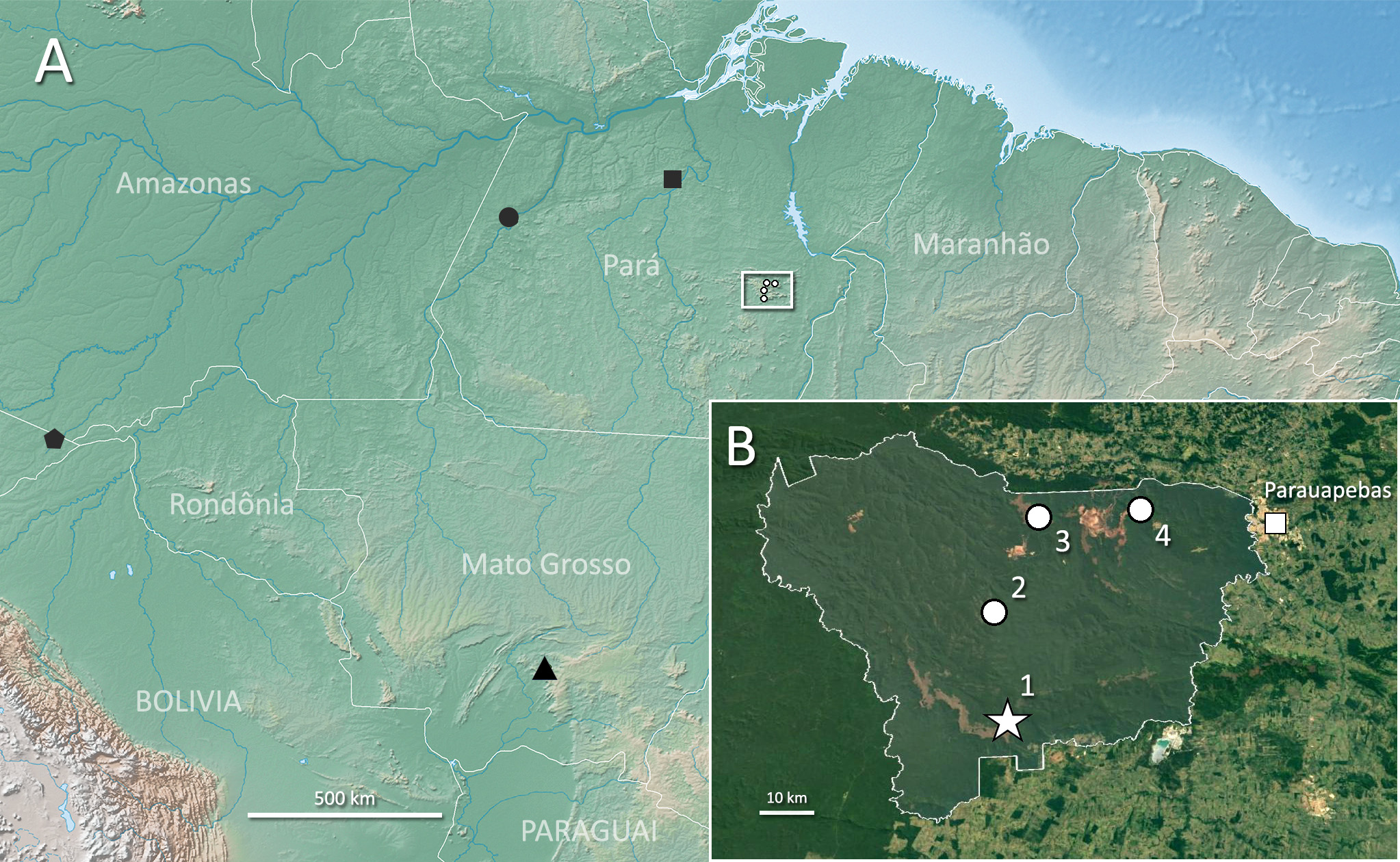
Geographic distribution. The current distribution of Allobates carajas View in CoL encompasses forested areas of Floresta Nacional de Carajás, where the species was detected in at least four locations on its southern, northern and central regions ( Fig. 1 View FIGURE 1 ). During fieldwork in Carajás, we did not observe any specimens of A. carajas View in CoL in canga (open xerophytic vegetation growing on exposed rocky terrain) or swampy environments. Given the increasing isolation of the forested environments of Carajás due to mining and cattle ranching along its borders, and the paucity of anuran surveys in neighboring forest remnants, the presence of the new species outside of Carajás is uncertain.
Natural history notes. In Carajás, Allobates carajas was found exclusively in well-preserved dense-canopy forest habitat. At Serra Sul, males called atop rocks, in areas where the leaf litter layer on the forest floor was deeper, or near the entrance of dens alongside rocky walls. Calling sites were better characterized at Trilha do Lago. Average calling site height was 23.5 cm (range 1.0–41.0 cm, n = 11). Males called while perched on green leaves (n = 3) or twigs (n = 3) in the lower understory vegetation, perched on the blades of leaves of stemless palms (n = 2), on the leaf litter of the forest floor (n = 2) or atop termite nests (n = 1). Also at Trilha do Lago, a single jelly nest was found on the upper surface of a broadleaf herb, less than 20 cm from the calling site of a male ( Fig. 14 View FIGURE 14 ). The jelly was transparent and contained 17 white-yolked eggs, ranging from 1.9 to 3.0 mm in maximum diameter (average = 2.2 mm).
Juvenile specimens were common in the leaf litter of Serra Sul, and shared the same color pattern of the adult specimens, except for the lack of yellow shades on ventral surfaces of body and limbs ( Fig. 14 View FIGURE 14 ). Water accumulated in holes and on the concave surfaces of rocks, providing isolated water bodies adequate for tadpole deposition, but no Allobates tadpoles were found. Tadpoles were also absent in phytotelmata or rain puddles in all sampling sites. These facts suggest that the peak of the reproductive season must have occurred earlier than February in that year.
Tadpoles were only found at the bottom of a shallow valley that crossed the access road to Serra Sul (50°20’14.4” W, 06°13’00.9” S), where water accumulated in a single pool in a forested habitat. A female specimen (INPA-H 38624) was captured near the pool, while males were heard calling at least 20 m far from the site. DNA barcoding analyses confirmed these tadpoles as belonging to A. carajas (see DNA barcoding above). In life, tadpoles had yellow and pinkish brown coloration, merging with the background color of the leaf litter at the bottom of the pool ( Fig. 14 View FIGURE 14 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Allobates carajas
| Simões, Pedro Ivo, Rojas, Diana & Lima, Albertina P. 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
Allobates carajas
| Simões & Rojas & Lima 2019 |
carajas
| Simões & Rojas & Lima 2019 |
Allobates carajas
| Simões & Rojas & Lima 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
Allobates carajas
| Simões & Rojas & Lima 2019 |
A. carajas
| Simões & Rojas & Lima 2019 |
A. juami Simões, Gagliardi-Urrutia, Rojas-Runjaic & Castroviejo-Fisher 2018
| Simoes, Gagliardi-Urrutia, Rojas-Runjaic & Castroviejo-Fisher 2018 |
A. tinae
| Melo-Sampaio, De Oliveira & Prates 2018 |
juami
| Simoes, Gagliardi-Urrutia, Rojas-Runjaic & Castroviejo-Fisher 2018 |
tinae
| Melo-Sampaio, De Oliveira & Prates 2018 |
A. juami
| Simoes, Gagliardi-Urrutia, Rojas-Runjaic & Castroviejo-Fisher 2018 |
A. tinae
| Melo-Sampaio, De Oliveira & Prates 2018 |
Allobates bacurau Simões, 2016
| Simoes 2016 |
bacurau
| Simoes 2016 |
Allobates bacurau
| Simoes 2016 |
| 2015 |
| 2015 |
| 2015 |
magnussoni
| Lima, Simões & Kaefer 2014 |
A. magnussoni
| Lima, Simões & Kaefer 2014 |
A. magnussoni
| Lima, Simões & Kaefer 2014 |
A. grillisimilis
| Simoes, Sturaro, Peloso & Lima 2013 |
flaviventris
| Melo-Sampaio, Souza & Peloso 2013 |
grillisimilis
| Simoes, Sturaro, Peloso & Lima 2013 |
A. grillisimilis
| Simoes, Sturaro, Peloso & Lima 2013 |
A. grillisimilis
| Simoes, Sturaro, Peloso & Lima 2013 |
A. flaviventris
| Melo-Sampaio, Souza & Peloso 2013 |
A. flaviventris
| Melo-Sampaio, Souza & Peloso 2013 |
marchesianus
| Neckel-Oliveira et al. 2012 |
A. marchesianus
| Neckel-Oliveira et al. 2012 |
A. marchesianus
| Neckel-Oliveira et al. 2012 |
A. marchesianus
| Neckel-Oliveira et al. 2012 |
hodli
| Simoes, Lima & Farias 2010 |
olfersioides
| Verdade & Rodrigues 2007 |
A. conspicuus (
| Morales 2002 |
A. fuscellus (
| Morales 2002 |
A. vanzolinius (
| Morales 2002 |
A. gasconi (
| Morales 2002 |
A. undulatus (
| Myers & Donnelly 2001 |
Allobates
| Zimmermann & Zimmermann 1988 |
Allobates
| Zimmermann & Zimmermann 1988 |
Allobates
| Zimmermann & Zimmermann 1988 |