Allobates bacurau
sp. nov.
Figures 1–9
Holotype. INPA-H
35398
(original field number
APL 12647
). An adult male collected by P.I. Simões on 4th of December 2007, at
Estrada do Miriti, in the outskirts of the city of Manicoré, on the right bank of the Madeira River
, State of Amazonas, Brazil (05°52'05" S, 61°17'13" W). Advertisement calls of holotype recorded at 11:30 h and 25.2°C.
GoogleMaps
Paratypes. INPA-H 35397, 35399–35409 (original field numbers APL 12644–12646, 12648–12656), ten males, two females. Collected by P.I. Simões on 4th–5th of December 2007, proceeding from the same locality.
Etymology. The specific epithet is in reference to the Portuguese word “
bacurau
” (modified from the original native Tupi word “wakura’wa”), which designates several species of nighthawks (Family Caprimulgidade). Inhabitants of Manicoré have historically entitled themselves “Povo Bacurau” (“The Nighthawk People”). The origin of this habit is disputed, but it possibly relates to the nickname of a notable citizen, Mr. Francisco A. Vieira Nunes (1939–1997), a writer and politician who gained prestige as a social worker and activist for Hansen’s disease awareness. The epithet is applied as a noun in apposition.
Diagnosis.
Allobates bacurau
is characterized by: (1) skin texture of dorsum granular, flat granules scattered from the level of tympanum to the urostyle region, more evident from mid to posterior dorsum; (2) paired dorsal digital scutes present; (3) distal tubercle absent on Finger IV; (4) discs on fingers II and III unexpanded, discs of fingers I and IV weakly expanded; (5) dermal lateral fringes and basal webbing absent on fingers; (6) metacarpal ridge absent; (7) Finger III swollen in male specimens, width of swelling variable among males; Finger III not swollen in females; (8) carpal pad absent; (9) male excrescences absent on thumbs; (10) thenar tubercle conspicuous; (11) black gland absent on arm; (12) tarsal keel present, tubercle-like, strongly curved; (13) disc of Toe I weakly expanded; discs of toes II, III and IV moderately expanded; (14) basal webbing present between toes III and IV; (15) metartarsal fold absent; (16) cryptic external coloration; background color of dorsum tan brown, with darker brown spots scattered from the level of tympanum to posterior dorsum; large diamond, “X” or hourglass-shaped marks absent on dorsum; dorsal surface of thigh grayish brown in life, light brown in preserved specimens, with no transverse bars; dorsal surface of arm yellow to dark copper in life, cream in specimens preserved for at least five years; pale dorsolateral stripe present,well defined, variable in width among specimens; a dark brown lateral stripe surrounds the whole body, reaching leg-body insertion; pale oblique lateral stripe absent; pale ventrolateral stripe present, partially diffuse, extending from behind the eyes to groin, cream in preserved specimens, iridescent white in live individuals; small, brown irregular blotches or marbling present ventrolaterally from snout to groin; pale paracloacal mark present; (17) dark throat-collar absent; (18) color of throat generally cream in preserved specimens, appearing light brown in specimens with higher concentration of melanophores on throat skin; density of melanophores on throat variable among specimens; throat light to dark gray in life; (19) central abdominal region immaculate, white to translucent in live male and female specimens; throat region with a variable number of melanophores in both sexes; chest and posterior third of throat light to dark gray in live males, white to translucent in live females, with parietal peritoneum projecting anteriorly from the level of chest and visible through skin; (20) iris metallic gold with tiny black flecks and a light gold pupil ring; (21) large intestine unpigmented; (22) testis unpigmented; (23) mature oocytes pigmented, pigments dark brown; (24) median lingual process absent; (25) tympanum inconspicuous; (26) vocal sac single; (27) maxillary teeth absent or inconspicuous; (27) habit is diurnal, males calling during daytime; (28) advertisement calls characterized by distinctively long trills (7–11 s) formed by approximately 60–80 short notes, emitted at an average rate of seven notes per second; (29) very small snout-to-vent length (SVL), adult males 14.4 mm on average (range 14.0– 14.7 mm), adult females 14.8 mm on average (range 14.7–14.9 mm).
The new species is assigned to genus
Allobates
by the combination of characters 14, 17, 22, 24, 27 and 29.
Species comparisons. The new species has a small geographic distribution in central Brazilian
Amazonia
(see Geographic distribution section below), located at least 600 km from the nearest country border. Thus, we restrict comparisons to
Allobates
species occurring in Brazil and to three additional species distributed outside the country, but which closely resemble the new species in external morphology.
Allobates bacurau
is distinguished from
A. femoralis ( Boulenger 1884)
,
A. myersi ( Pyburn 1981)
and
A. hodli Simões, Lima & Farias 2010
by its smaller body size and by lacking bright aposematic colors on body and dorsal surface of thighs (maximum SVL of adult males = 14.8 mm in
A. bacurau
, minimum SVL of adult males of remaining species = 22.2 mm, in
A. hodli
; yellow, orange or bright-red flash marks present on thighs of these large species).
Allobates bacurau
is distinguished from
A. brunneus ( Cope 1887)
,
A. crombiei ( Morales 2002)
,
A. gasconi ( Morales 2002)
,
A. flaviventris Melo-Sampaio, Souza & Peloso 2013
,
A. goianus ( Bokermann 1975)
and
A. magnussoni
Lima, Simões & Kaefer 2014 by lacking a wavy-edged or hourglass-shaped dark brown pattern on dorsum. Maximum body size of
A. bacurau
also smaller than minimum body size registered among specimens of
A. crombiei
,
A. flaviventris
and
A. magnussoni
(maximum SVL of adult males = 14.8 mm in
A. bacurau
, minimum SVL of males of the latter three species = 16.0 mm, reported for
Allobates magnussoni
). Most specimens of
A. olfersioides ( Lutz 1925)
with X-shaped dark-brown patterns on dorsum (dorsum solid tan-brown with tiny darker brown spots in
A. bacurau
);
A. olfersioides
distributed along the Brazilian Atlantic forest, not reaching the Amazon basin.
Allobates bacurau
is distinguished from
A. masniger ( Morales 2002)
,
A. nidicola
( Caldwell and Lima 2003) and
A. paleovarzensis
Lima, Caldwell, Biavati & Montanarin 2010 by its smaller body size (minimum SVL of adult males among the three species = 17.9 mm reported for
A. masniger
; maximum SVL of adult males = 14.8 mm in
A. bacurau
). Pronounced sexual dimorphism in color of throat and chest of live specimens of
A. masniger
,
A. nidicola
and
A. paeovarzensis
. Throat and chest of
Allobates masniger
and
A. nidicola
black to gray in males and uniformly yellow in females, fading to pale in preserved females. Throat of
A. paleovarzensis
grayish violet in live males, yellowish in live females (throat light gray to dark gray in live male and female
A. bacurau
).
A. bacurau
is distinguished from
A. fuscellus ( Morales 2002)
by its smaller body size (minimum SVL in males of
A. fuscellus
= 15.8 mm) and by uniformly pale to translucent color of ventral surfaces of preserved specimens, darker only on distal parts of limbs and throat (ventral surfaces uniformly dark gray in preserved specimens of
A. fuscellus
). Minimum SVL of adult male
A. vanzolinius ( Morales 2002)
21.5 mm, much larger than maximum SVL of
A. bacurau
males (14.8 mm).
Considering smaller species,
Allobates bacurau
is distinguished from
A. caeruleodactylus
( Lima & Caldwell 2001) by lacking sky-blue colors on fingers of live specimens and by the presence of defined pale dorsolateral and ventrolateral stripes (fingers sky-blue at least distally, pale dorsolateral and ventrolateral stripes absent in
A. caeruleodactylus
).
A. bacurau
is distinguished from
A. conspicuus ( Morales 2002)
by lacking transverse bars on dorsal surface of thigh and by the presence of a partially diffuse pale ventrolateral stripe (thigh with transverse dark brown bars and continuous pale ventrolateral stripe present in
A. conspicuus
).
A. bacurau
is distinguished from
A. grillisimilis Simões, Sturaro, Peloso & Lima 2013
by lacking transversal dark brown bars on the dorsal surface of thigh (dark brown bars absent or present in variable number on dorsal surface of thigh in
A. grillisimilis
), by upper arm golden yellow in live specimens (upper arm olive brown in
A. grillisimilis
) and by throat in variable shades of gray in live male specimens (throat white to translucent, with a few melanophores distributed only on chin in male
A. grillisimilis
).
Allobates bacurau
is distinguished from
A. marchesianus (Melin 1941)
by lacking basal webbing between Toes II and III and by the absence of strong sexual dimorphism in color of ventral surfaces of preserved and live specimens (basal webbing present between Toes II and III in
A. marchesianus
; throat and chest of males strongly pigmented, appearing dark gray; ventral surfaces of preserved females uniformly cream, bright yellow in life).
Allobates bacurau
is distinguished from
A. subfolionidificans
( Lima, Sanchez & Souza 2007) by the presence of melanophores on throat and by the presence of a pale and conspicuous dorsolateral stripe in preserved specimens (throat unpigmented, uniformly cream or white, pale dorsolateral stripe absent in
A. subfolionidificans
). Preserved specimens of
A. bacurau
are distinguished from
A. tapajos
( Lima, Simões & Kaefer 2015) by a solid dark brown lateral stripe surrounding the body, by a partially diffuse pale ventrolateral line and pale marbling on the ventrolateral surface of body (dark brown lateral stripe with a diffuse lower edge; pale ventrolateral stripe and pale lateral marbling absent in
A. tapajos
). Live males of
A. tapajos
also with golden yellow throat and chest (throat and chest gray in
A. bacurau
).
Preserved specimens of
Allobates bacurau
are almost identical to specimens of
Allobates sumtuosus ( Morales 2002)
, but
A. sumtuosus
lacks dark pigments (melanophores) on throat, which appear uniformly white, light gray or translucent (throat always pigmented
A. bacurau
, appearing lighter or darker gray depending on melanophore coverage). The two species are also distinguished by their color in life. Throat, chest and abdominal surfaces of female
A. sumtuosus
uniformly light yellow; the same surfaces are uniformly light gray to translucent in male
A. sumtuosus
(throat gray, chest and abdomen light gray to translucent in live male and female
A. bacurau
). Dorsal surface of thigh uniformly bluish gray in live
A. sumtuosus
, occasionally with a few brown irregular spots (dorsal surface of thigh grayish brown, sometimes with dark brown blotches extending from the lateral surfaces in
A. bacurau
).
Three
Allobates
species distributed in forested areas of the Guiana Shield and eastern Andean Piedmont of Cordillera de Merida and Cordillera Oriental de Colombia outside Brazil resemble
A. bacurau
morphologically.
A. algorei Barrio-Amorós & Santos 2009
is distinguished from
A. bacurau
by the presence of a dark brown transverse stripe on thigh (absent in
A. bacurau
), by a dark brown lateral stripe fading posteriorly (dark brown lateral stripe solid around whole body in
A bacurau
) and by a light yellow posterior abdomen in live specimens (posterior abdomen light gray to translucent in
A. bacurau
).
A. amissibilis Kok, Hölting & Ernst 2013
, from Guyana, is distinguished from
A. bacurau
by the slightly larger size of males (minimum SVL of male
A. amissibilis
16.3 mm), by an absent or thin and diffuse pale dorsolateral stripe (pale dorsolateral stripe unbroken and well defined in live and preserved
A. bacurau
), by the presence of a broad oblique lateral stripe extending from groin to mid-body (oblique lateral stripe absent in
A. bacurau
), and by color of throat in live specimens, pinkish gray in males, cream to yellow in females (throat gray in live male and female specimens of
A. bacurau
).
A. granti (Kok, MacCulloch, Gaucher, Poelman, Bourne, Lathrop & Langlet 2006)
from French Guiana differ from
A. bacurau
in lacking a pale dorsolateral line (present in
A. bacurau
) and by the ventral color of live females (throat of female
A. granti
pale, free of melanophores, abdomen yellowish; throat of female
A. bacurau
gray, with scattered melanophores, abdomen white to translucent).
Allobates bacurau
can be distinguished from other species of cryptically colored
Allobates
by means of its advertisement calls, formed by long trills of notes. See “
Call
description and comparisons” section below for comparisons with advertisement calls of similar congeneric species.
Description of holotype. Measurements of the holotype are presented in Table 1. Body is robust, head wider than long (HL/HW = 0.84), head length 0.29 times the SVL (Fig1A, B; Fig. 2A
View FIGURE 2
). Eye diameter larger than distance from anterior corner of the eye to nostril (EN/EL = 0.63). Nares located posterolaterally to tip of snout, directed laterally, visible in dorsal, ventral, lateral, and anterior views. Distance between nostrils 0.82 times that of HW. Canthus rostralis slightly convex from tip of snout to nostril, straight from nostril to anterior corner of the eye. Loreal region vertical. Tympanum round, 0.37 times the maximum diameter of the eye. Margins of tympanum indistinct to the naked eye, visible under a dissecting microscope, more conspicuous anteroventrally. Maxillary teeth absent, not visually detectable under 40X magnification or when a wire probe is moved along the maxillary surface. Tongue conspicuously longer than wide, with anterior tip attached to the mouth floor. Median lingual process absent. Choanae round, positioned anterodorsally to eye bulge. Vocal sac single, corresponding to most of the area of the medial and posterior subgular region. Lateral vocal slits conspicuous.
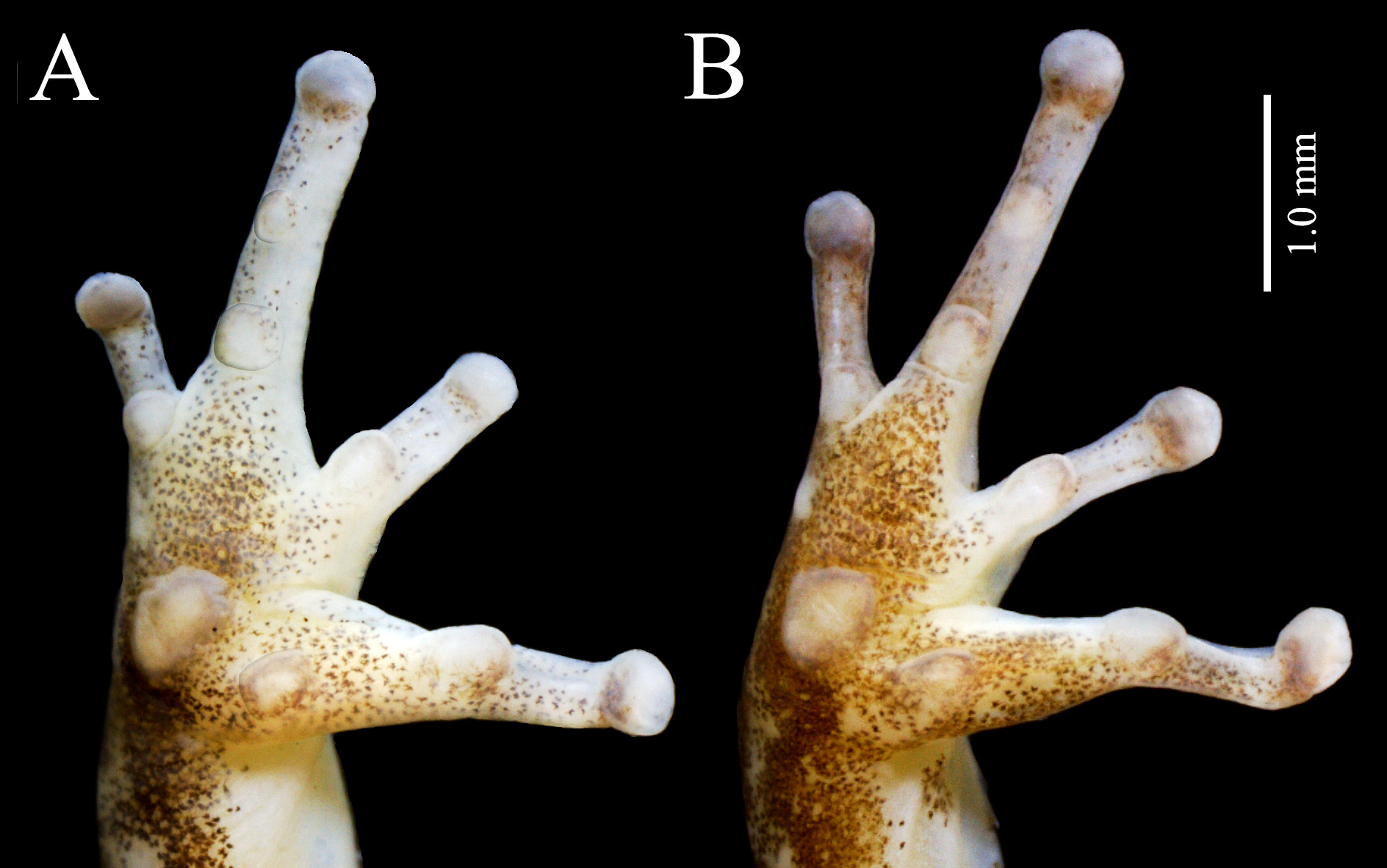
Palmar tubercle elliptical, with the longer width aligned towards the base of Finger II. Thenar tubercle conspicuous, oval, evident in ventral view and in hand profile. Maximum diameter of thenar tubercle 68% of maximum diameter of palmar tubercle ( Fig. 3A
View FIGURE 3
). Subarticular tubercles of Fingers II, III and IV round, small, never exceeding the width of phalanges. Subarticular tubercle on Finger I elliptical, protuberant, 1.2 times larger than thenar tubercle in maximum diameter. Distal subarticular tubercle absent on Finger IV. Supernumerary tubercles absent. Carpal and metacarpal ridges absent. Fringes or webbings absent on fingers. Length of Finger II equivalent to approximately 85% of Finger I´s length. Tip of Finger IV does not reach distal subarticular tubercle of Finger III when fingers are juxtaposed. Relative lengths of fingers: IV = II <I <III. Finger III swollen; swelling preaxial. Discs of fingers II and III with about the same width of that of underlying phalanges. Discs of fingers I and IV weakly expanded, width of discs corresponding to 1.1 and 1.2 times the width of adjacent phalanx ( Fig. 3A
View FIGURE 3
).
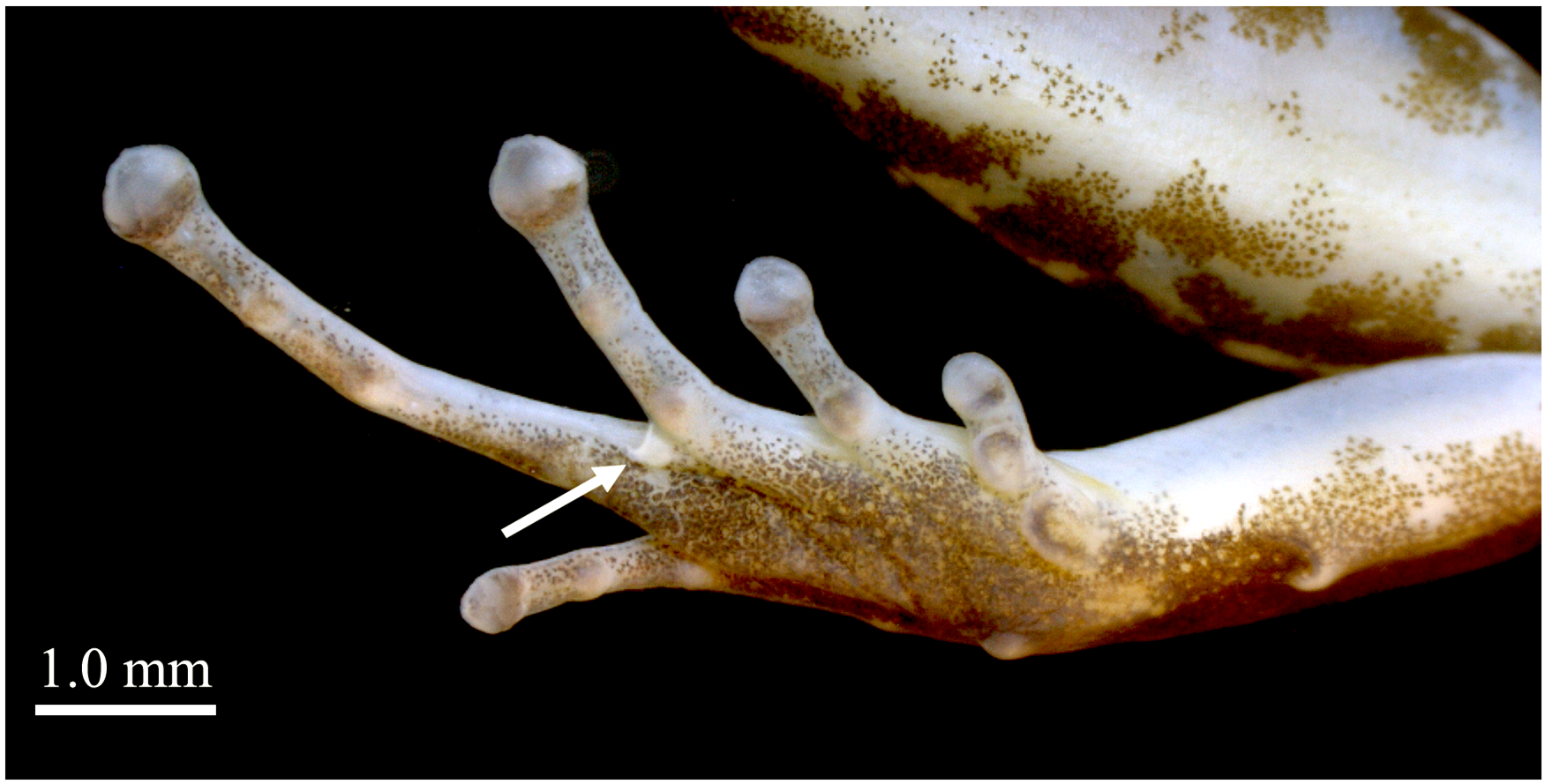
Tibia length corresponding to approximately half the SVL (TL/SVL = 0.52). Tarsal keel present, tubercle-like, strongly curved at its proximal end, flattening and straightening towards metatarsal tubercle ( Fig. 4
View FIGURE 4
). Metatarsal fold absent. Preaxial edge of tarsus smooth, with no fringe. Basal webbing present between toes III and IV. Less conspicuous basal webbing is present between toes II and III. Relative lengths of toes: I <II <V <III <IV. Disc of Toe I weakly expanded, width 1.2 times the width of adjacent phalanx. Discs of Toes II, III, IV and V moderately expanded, width of discs 1.5, 1.5, 1.7 and 1.4 times the width of adjacent phalanx, respectively ( Fig. 4
View FIGURE 4
).
Skin on dorsum is moderately granular, with relatively low granules scattered from the urostyle region to about the level of tympana (granules are evident by aggregations of melanophores on their tips). Skin is smooth ventrally and laterally. Dermal flap absent above cloaca (Fig 1A, B).
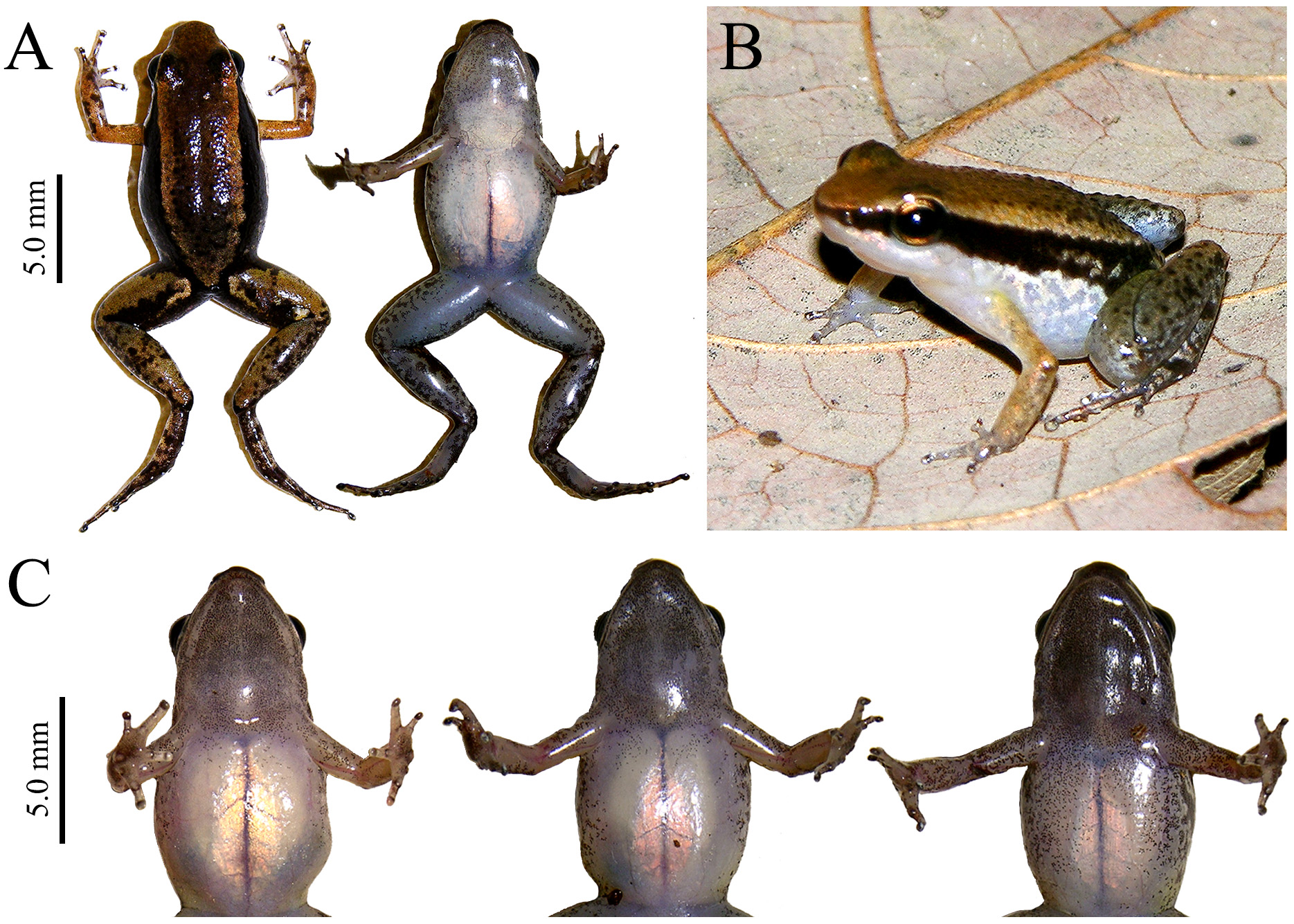
Color in alcohol of holotype. Dorsal surface of body uniformly tan brown, darker only above the orbits and on tips of larger skin granules (Fig 1A). A pale dorsolateral stripe is present, characterized by a narrow line (~ 0.8 mm wide at mid-abdomen level) adjacent to the darker lateral surface of body where density of melanophores is reduced; inner edge of pale dorsolateral stripe well marked, not diffuse (Fig. 1A). Lateral surface of body characterized by a solid dark brown stripe, extending from tip of snout to groin. Pale ventrolateral line present, cream colored, extending from snout to groin, diffuse anteriorly, wider and more conspicuous from arm-insertion level to groin. Irregular projections of ventrolateral line form a marbling pattern on the ventrolateral region towards the abdomen, on light brown background ( Fig. 2A
View FIGURE 2
). Throat, gular, and anterior pectoral regions light brown, with evenly scattered melanophores. Posterior pectoral region, abdomen and ventral surface of thigh uniformly cream (Fig. 1B).Tongue is cream-colored.
Arms cream to pale brown in dorsal view, melanophores concentrated in small and irregular light brown blotches on upper arm, forearm and hand. Tip of fingers light brown. Paired scutes on finger discs cream. Upper arm and forearm cream to translucent in ventral view, continuous with color pattern of abdomen. Outer lateral edge of forearm tan brown. Carpal and metarcarpal regions tan brown in ventral view (Fig. 1A, B; Fig. 2A
View FIGURE 2
).
Area immediately around vent solid dark brown, flanked by an unpigmented, C-shaped transverse band, corresponding to the pale paracloacal mark. Thigh light brown in dorsal view, with a few dark brown irregular spots. Inner and outer dorsolateral surfaces of thigh dark brown. Dorsal surface of shank same color as thigh, with irregular dark brown blotches, more frequent on the outer dorsolateral edge. Outer dorsal surface of tarsal region lighter than overall pattern of legs, with scattered dark brown blotches. Toes light brown, with irregularly distributed melanophores. Ventral surface of shank and tarsal region predominantly cream to translucent. Dark brown marbling appears along inner and outer ventrolateral edges and on knee. Ventral surface of metatarsal regions uniformly dark brown. Toes and toe discs light brown in ventral view (Fig. 1A, B; Fig. 2A
View FIGURE 2
).
Variation in type-series. Variation in morphometric measurements is presented in Table 1. Specimens in typeseries resemble the overall external morphology of the holotype (Fig. 1C, D; Fig. 2B
View FIGURE 2
; Fig. 5
View FIGURE 5
). Females are slightly larger than males in average, but SVL of largest male and smallest female paratypes overlap. Head proportions and relative length of tibia also equivalent between sexes, head slightly wider than long in male and female specimens, and tibia length about half the SVL (average HL/SVL = 0.30 among male paratypes and among female paratypes; average HW/SVL = 0.35 among male paratypes, 0.34 among female paratypes; average TL/SVL = 0.52 in both sexes). Preaxial swelling of Finger III variable among specimens in the type-series: not swollen in the two female ( Fig. 3B
View FIGURE 3
) and one male paratype. Finger III swollen in the remaining male paratypes. Finger III swelling is strong in preserved males with more evident vocal slits; weak in specimens with less conspicuous vocal slits. Width of pale dorsolateral stripe variable among type specimens ( Fig. 5
View FIGURE 5
), ranging from 0.59 to 0.95 mm. Pigmentation on throat variable among male and female specimens, general throat color varying from predominantly cream (with melanophores densely scattered only on chin) to uniformly light brown (melanophores densely scattered on most of the throat surface).
Color in life. Dorsal surface of body tan brown, with scattered darker brown spots on tips of skin granules that are more evident from mid to posterior dorsum. Pale dorsolateral stripe evident, bright tan brown ( Figs. 6
View FIGURE 6
, 7
View FIGURE 7
) Lateral surface of body surrounded by a dark brown stripe from tip of snout to groin. A few faint spots may be present post-laterally on the dark brown stripe about the inguinal region, but never forming a pale oblique lateral line. Iridescent white ventrolateral stripe present along the lower margin of dark brown flanks, from tip of snout to groin, often interrupted, frequently at the level of arm insertion and below the eye. Pale iridescent marbling, same color as of ventrolateral stripe, present below ventrolateral stripe, towards the abdomen, over unpigmented background ( Figs. 6
View FIGURE 6
, 7
View FIGURE 7
). Color of throat can vary from light gray to dark gray in male and female individuals ( Fig.7
View FIGURE 7
). Color of throat is sexually dimorphic only posteriorly among live specimens, where the parietal peritoneum projects anteriorly past chest level in females and is visible through skin, rendering a white color, instead of the light to dark gray posterior throat in males ( Fig. 7A
View FIGURE 7
). Pectoral region and abdomen white to translucent, with scattered brown pigmentation appearing only ventrolaterally in some specimens ( Fig. 7C
View FIGURE 7
). Vocal sac of males light gray to gray when inflated.
Dorsal surface of upper and forearm predominantly light tan brown, with golden flecks, light gray to translucent only around arm-body insertion. A few dark brown scattered blotches may be present laterally on upper arm and forearm. Upper arm light gray to translucent, same color as chest in ventral view. Forearm, carpal and metacarpal regions gray to dark brown in ventral view. Fingers gray in ventral and dorsal views, darker in ventral view. Paired scutes on finger discs iridescent white.
Surfaces immediately adjacent to vent solid dark brown, flanked by a light cream paracloacal mark. Distal edge of paracloacal mark merging shortly with darker brown shades on thigh. Dorsal surface of thigh grayish brown, with irregular dark brown blotches extending from lateral surfaces. Dorsal surface of shank same color as thigh, with a variable number of dark brown blotches. Ventral surface of thigh and shank generally light gray to translucent, darker than abdomen ( Fig. 7A
View FIGURE 7
), with dark brown marbling along inner and outer edges. Tarsal region light gray in ventral view, brown with dark brown flecks in dorsal view. Toes with gray and dark brown patterning. Paired scutes on toe discs are iridescent white.
Call
description and comparisons. Calls of
Allobates bacurau
are constituted by the emission of short notes arranged in trills ( Fig. 8A
View FIGURE 8
). The average duration of note trills among all individuals recorded was 9.72 ± 1.19 (6.92–11.07) seconds. The average rate of note emission was 7.21 ± 0.64 (6.70–8.75) notes/s, and each note trill contained an average of 69 ± 6 (60–81) notes. Average note duration was 0.040 ± 0.006 (0.029–0.045) seconds. Notes have an ascending frequency modulation ( Fig. 8B
View FIGURE 8
) Average peak frequency was 6.12 ± 0.15 (5.89–6.34) kHz. Lower frequency of notes was around 5.49 ± 0.14 (5.26–5.76) kHz on average and average upper frequency reached 6.74 ± 0.18 (6.53–7.06) kHz. Average duration of silent intervals between notes was 0.101 ± 0.015 (0.071– 0.129) seconds. The fundamental frequency was observed between 2.75–3.55 kHz, only in sonograms of recordings with very low background noise (including the holotype ´s—Fig. 8).
Advertisement calls of
Allobates algorei
,
A. caeruleodactylus
,
A. goianus
,
A. magnussoni
,
A. masniger
,
A. nidicola
and
A. subfolionidificans
are constituted by a single note, emitted continuously between regular (
A. goianus
,
A. magnussoni
,
A. nidicola
,
A. masniger
) or irregular (
A. algorei
,
A. caeruleodactylus
,
A. subfolionidificans
) inter-note silent intervals, not arranged in discrete note trills ( Lima & Caldwell 2001; Caldwell & Lima 2003; Lima et al. 2007; 2014; Barrio-Amorós & Santos 2009; Bastos et al. 2011; Kaefer et al. 2012). Some
Allobates
species alternate between two types of advertisement calls, emitting notes continuously or arranged in bouts or trills. However, note trills emitted by these species are shorter in length and formed by a smaller number of notes when compared to trills emitted by
A. bacurau
:
Allobates brunneus
(1.70– 4.20 s, 6–11 notes),
A. marchesianus
(3.39– 4.40 s, 21–24 notes),
A. sumtuosus
(1.95– 5.8 s, 15–35 notes) and
A. tapajos
(2.46– 3.37 s, 10–14 notes) ( Caldwell et al. 2002; Simões et al. 2013a; Lima et al. 2009; 2015).
Advertisement calls of some
Allobates
species are constituted exclusively by note trills. However, when compared to those of
A. bacurau
, note trills are shorter and formed by a smaller number of notes in
A. amissibilis
(0.02– 7.97 s, 1–19 notes),
A. crombiei
(1.91– 4.53 s, 25–59 notes),
A. grillissimilis
(0.12– 0.30 s, 3–15 notes),
A. paleovarzensis
(0.72– 3.02 s, 3–21 notes) ( Lima et al. 2010; 2012; Kok et al. 2013; Simões et al. 2013b).
Advertisement calls of
A. flaviventris
and
A. granti
are emitted as trills of note-pairs or couplets, not trills of single notes ( Kok et al. 2006; Kok & Ernst 2007; Melo-Sampaio et al. 2014; Lima et al. 2014).
Considering the overlap of advertisement calls in spectral space,
A. goianus
,
A. magnussoni
,
A. masniger
,
A. nidicola
and
A. paleovarzensis
emit calls at a frequency bandwidth lower than 5.3 kHz, below the lower frequency threshold of
A. bacurau
( Caldwell & Lima 2003; Lima et al. 2010; 2014; Bastos et al. 2011; Kaefer et al. 2012).
Importantly, the advertisement calls of
A. bacurau
can be sonographically distinguished from calls of all cryptically colored
Allobates
species distributed in the east bank of the middle and lower Madeira River ( Fig. 9
View FIGURE 9
).
Genetic distances and evolutionary relationships. Based on a phylogenetic analysis of a 481 bp fragment of the 16S rRNA mitochondrial gene, the six
Allobates bacurau
paratypes form a well-supported clade, monophyletic in relation to all species of cryptically-colored
Allobates
occurring in Brazil and in other regions of cis-Andean South America for which similar sequence samples were available ( Fig. 10
View FIGURE 10
). Sequences proceeding from topotypic specimens of
A. sumtuosus
form the sister clade to
A. bacurau
, but their evolutionary relationship is weakly supported (i.e. the sister clade may also contain sequences of
A. sumtuosus
occurring in Manaus, out of
A. sumtuosus
type locality). Average uncorrected pairwise and Kimura-2-Parameter (K2P) genetic distances between
A. bacurau
and
A. sumtuosus
are 4% ( Table 2).
Average genetic distances between
A. bacurau
and the remaining samples analyzed are never lower than 5%. Average uncorrected pairwise genetic distances estimated between
A. bacurau
and other cryptically colored
Allobates
distributed on the east bank of the middle and lower Madeira River (
A. caeruleodactylus
,
A. grillisimilis
,
A. masniger
) are no lower than 9% (10% considering K2P genetic distances). Two additional species are distributed on the east bank along the upper river course,
A. gasconi
and
A. flaviventris
, but minimum average pairwise genetic distance estimated between
A. bacurau
and the available specimens sampled is 7% (8% considering K2P distances between
A. bacurau
and
A. gasconi
)( Table 2).
Geographic distribution.
Allobates bacurau
is known only from the type locality, in the the Municipality of Manicoré, on the right bank of the Madeira River, Amazonas State ( Fig. 11
View FIGURE 11
). This region is bounded north by the Aripuanã and south by the Ji-Paraná River. Both are large blackwater tributaries of the Madeira River, and have been frequently considered important geographic barriers to vertebrate species ranges and genetic clusters (see Discussion).
I sampled one location on the immediately opposite bank of the Madeira River (Village of Democracia, 05°48'21" S, 61°26'43" W—Fig. 11) for diurnal frogs for four days prior to fieldwork in Manicoré, but no
A. bacurau
specimens were found. Many locations on the eastern bank of the Madeira River, upstream and downstream of Manicoré, have also been systematically sampled for aromobatid frogs in the recent years (e.g. Kaefer et al. 2013; Simões et al. 2013b; Dias-Terceiro et al. 2015) and the new species has never been detected in any of them.
At the type locality,
A. bacurau
is syntopic with at least two larger congeneric species: the aposematically colored
A. femoralis
, and the cryptically colored
A. masniger
. Three additional
Allobates
species occur north of the Aripuanã River, in forested sites near the cities of Novo Aripuanã, Borba and Nova Olinda do Norte ( Fig. 11
View FIGURE 11
):
A. caeruleodactylus
,
A. grillisimilis
and a third, undescribed species, which can be easily distinguished from all mentioned species by the bright yellow vocal sac of males ( Fig. 12
View FIGURE 12
).