Dipsas sazimai, Fernandes, Daniel S., Marques, Otavio A. V. & Argôlo, Antônio J. S., 2010
|
publication ID |
https://doi.org/ 10.5281/zenodo.276504 |
|
DOI |
https://doi.org/10.5281/zenodo.6198076 |
|
persistent identifier |
https://treatment.plazi.org/id/038F1A1C-FFBF-DB2A-FF14-55F1FCECFD54 |
|
treatment provided by |
Plazi |
|
scientific name |
Dipsas sazimai |
| status |
sp. nov. |
Dipsas sazimai , new species
( Figs. 1–5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 )
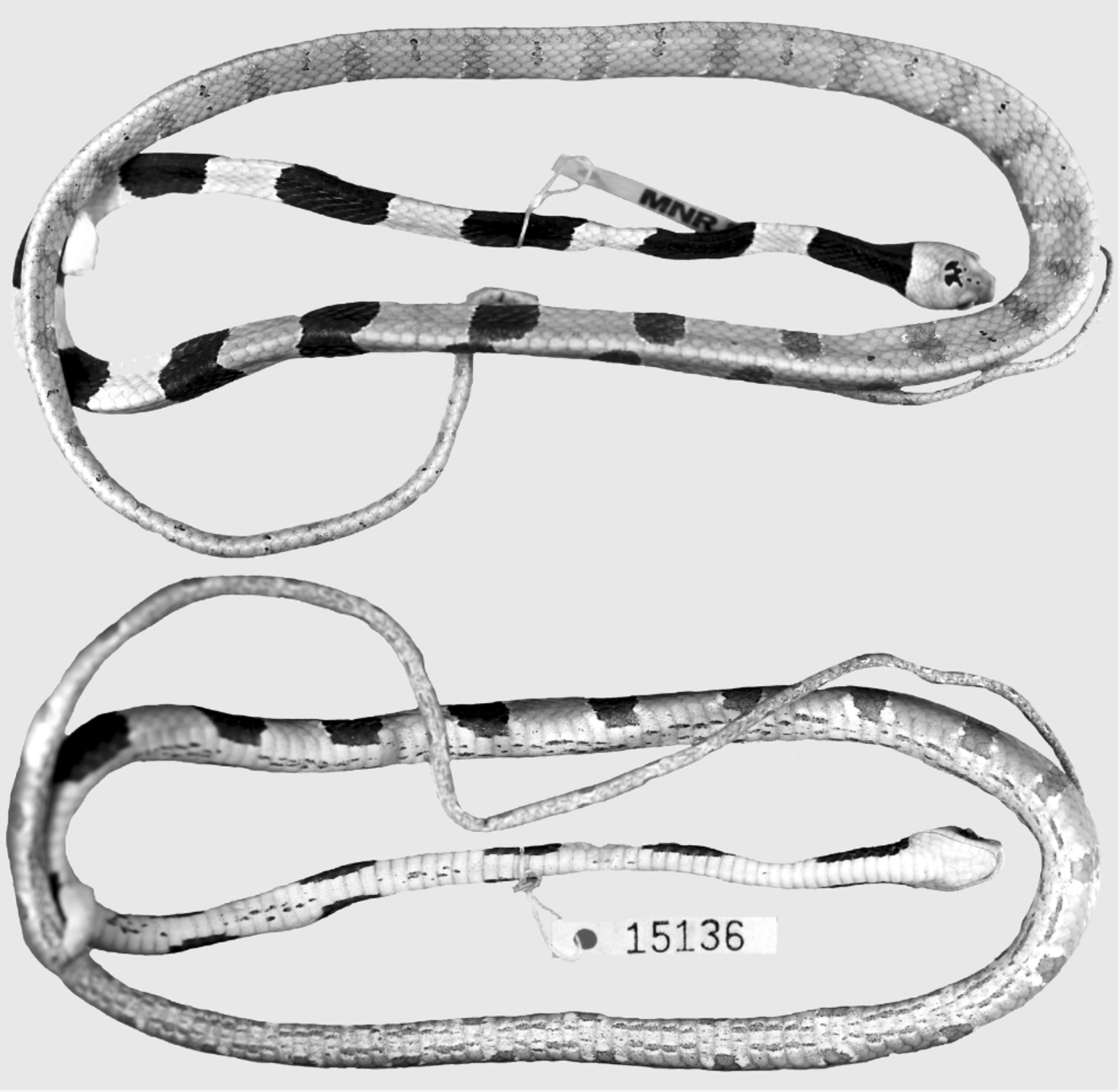
Holotype: MNRJ 15136, adult male, Brazil, state of Rio de Janeiro, municipality of Casimiro de Abreu (22º 28’S, 42º 12’W, ca. 80 m), collected by C. C. Siqueira and W. C. Kiefer on 30 October 2005.
Paratypes: MZUESC 8199, young female, Brazil, state of Alagoas, Fazenda Bananeira, municipality of Murici (09º 18’S, 35o 57’W, ca. 550 m), collected by Marco A. Freitas, February–March 2010; MZUFBA 1800, adult male, Brazil, state of Bahia, Parque Estadual das Sete Passagens, municipality of Miguel Calmon (11º 26’S, 40o 36’W, ca. 530 m), no collector data, February 2006; MZUESC 6134, adult male, Brazil, state of Bahia, Sítio de Maria das Neves, municipality of Camacan (15º 23’S, 39º 33’W, ca. 450 m), collected by Maria das Neves, January–June 2007; MZUESC 7848, adult female, Brazil, state of Bahia, Serra Bonita, municipality of Camacan (15º 23’S, 39º 33’W, ca. 700 m), collected by Iuri Dias, 16 November 2009; MZUESC 8466, adult male, Brazil, Bahia State, Serra Bonita, municipality of Camacan (15º 23’S, 39º 33’W, ca. 400 m), collected by Iuri Dias, 0 5 June 2010; MZUESC 7988, adult male, Brazil, state of Bahia, municipality of Jequié (13º 57’S, 39º 59’W, ca. 650 m), collected by Juliana Rodrigues, 0 5 January 2010; MNRJ 19275, adult female, Brazil, state of Espírito Santo, Estação Biológica Santa Lúcia, municipality of Santa Teresa (19º 56’S, 40º 36’W, ca. 650 m), collected by A. Giupponi, T. Souza, and M. Milleri, 11–12 May 2005; IBSP 77835, adult female, Brazil, state of Espírito Santo, Fazenda Bandarra, municipality of Mimoso do Sul (21º 04’S, 41º 22’W, ca. 270 m), collected by J. L. Gasparini, 16 December 2007; IBSP 69143, adult male, Brazil, state of São Paulo, Picinguaba, municipality of Ubatuba (23o 23’S, 44o 50’W, ca. 40 m), collected by P. A. Hartmann, 0 1 March 2004.
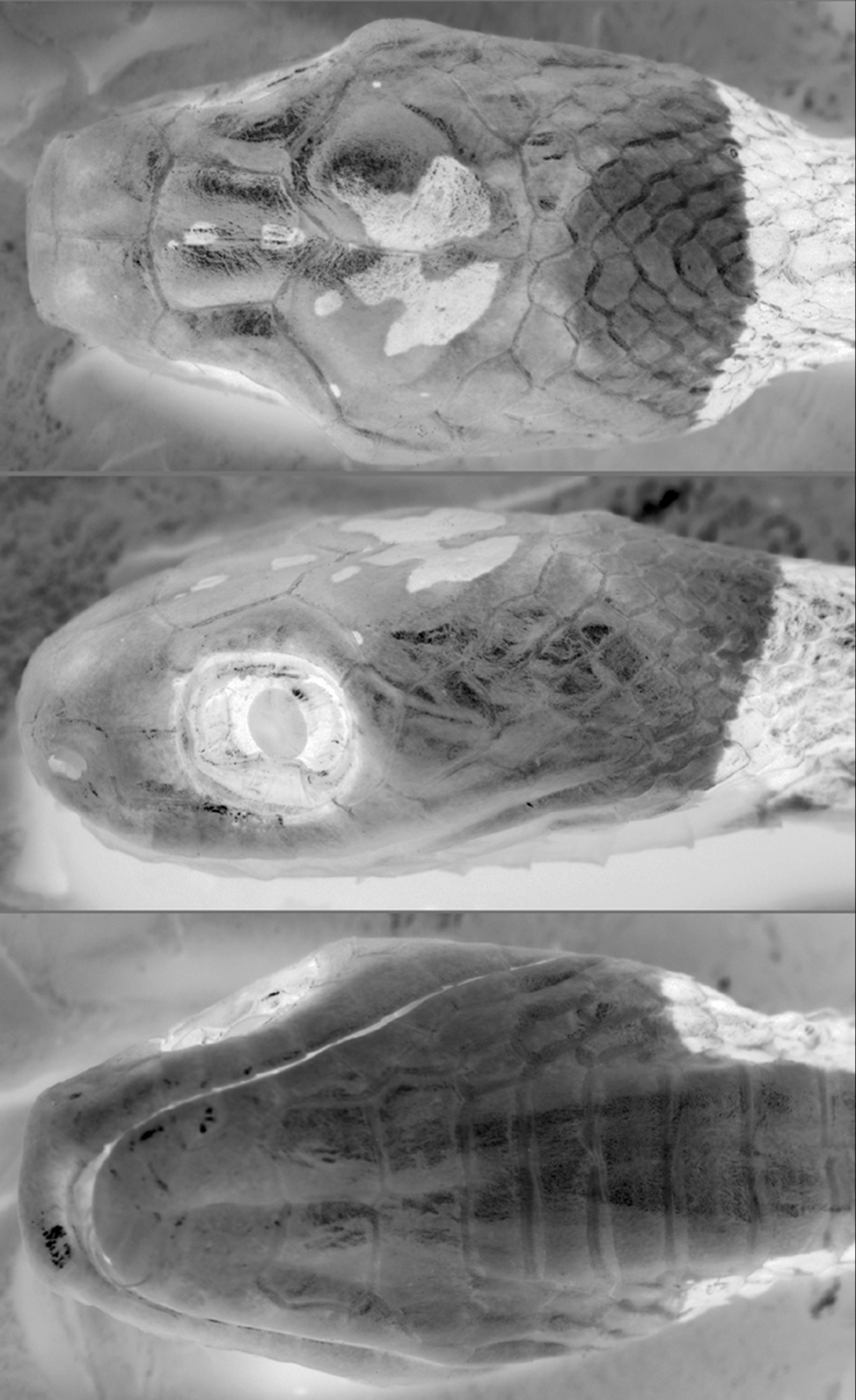
Diagnosis ( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ). Distinguished from all congeners by the following combination of characters: (1) 15- 15-15 dorsals; (2) temporals not entering orbit; (3) loreal enters orbit; (4) prefrontals generally enter orbit; (5) one pair of infralabials in contact behind symphysial; (6) infralabials contact second pair of chinshields; (7) 187–209 ventral scales in males, 193–202 in females; (8) 107–129 subcaudal scales in males, 107–116 in females; (9) 17–21 maxillary teeth; (10) anterior portion of body with rounded dorsal blotches generally wider than interblotches; (11) median and mostly posterior portion of body with blotches higher than long and narrower than interblotches; (12) posterior body blotches lighter than anterior blotches; (13) posterior blotches with conspicuous white edge in paraventral region; (14) tiny and vertically oriented streaks in the interblotches from the posterior half of body; (15) labial scales not heavily pigmented; (16) head mostly immaculate; (17) the first blotch not reaching the rictus.
Description of the holotype ( Figs. 1–2 View FIGURE 1 View FIGURE 2 ). Adult male, SVL 498 mm; TL 245 mm (49% SVL); head length 13.9 mm (3% SVL) from tip of snout to corner of the mouth; head width 7.3mm (53% head length) taken at broadest point; interocular distance 6.2 mm; snout-orbit distance 3.8 mm (1.6 times interocular distance); head broadly distinct from body; rostral 3.2 mm wide, broader than high, sub-triangular in frontal view, slightly visible from above; internasals 2.0 mm wide, broader than long; internasal suture slightly sinistral with respect to prefrontal suture; prefrontals 2.6 mm wide, broader than long, enter the orbit; supraocular 3.5 mm long, longer than broad; frontal 3.8 mm long, longer than broad, with a pentagonal shape in dorsal view; parietals 5.0 mm long, about as long as broad; nasal entire; loreal 1.9 mm long, slightly longer than high, enters the orbit; eye diameter 3.1 mm; pupil semi-elliptical; no preoculars; on the right side, between supraocular and prefrontal, the later entering the orbit, there is an azygous; 1/2 postoculars; upper postocular higher than lower; temporals 1+3; upper posterior temporal elongate, about twice as long as high; 9/8 supralabials, 4–6/3–5 contacting orbit; symphysial 1.9 mm wide, about two times broader than long, separated from chinshields by the first infralabial on the left side and two infralabials on the right side; only the first pair of infralabials in contact behind symphysial; 10 infralabials, 1–5/1– 6 contacting chinshields; three pairs of chinshields, first pair longer than broad, second pair slightly broader than long; the third pair of chinshields is present but the sulcus is incomplete; 1 preventral separating chinshields from first ventral; dorsal scales in 15-15-15 rows, smooth, without apical pits; 209 ventrals; 129 divided subcaudals; 17 maxillary teeth; cloacal plate entire.
Color in preservative of holotype ( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ) Dorsal ground color of head uniformly brown except for two small dark-brown, white bordered spots on the frontal and a pair of dark-brown, white bordered spots on the parietals connected to each other medially, with divergent anterior and posterior edges; supralabials light-brown; infralabials and gular region uniformly creamish; dorsal ground colour of body beige; first blotch 10 vertebral scales long, extending to the level of the 10th ventral scale, with a thin white border; dorsum of body with 27/26 blotches; anterior third of body with well defined, dark-brown rounded blotches with a thin white border; anterior blotches wider (7–8 scales long) than interblotches (4–5 scales long); median and posterior portion of body with well defined, brown and narrower blotches with a white edge more conspicuous in the paraventral region; blotches narrower (1.5–3 scales long) than interblotches (4–5 scales long), especially in the posterior portion where blotches are 1.5–2 and interblotches are 4–5 scales long; blotches contacting the opposite one in the vertebral region, only in the first half of body; blotches along the body extending through the paraventral region; tiny and vertically oriented (less than 1 scale long and 4–5 dorsal scales high) brown to dark-brown streaks appear from the second half of body in the interblotches, at the level of paravertebral region; ground color of anterior part of belly creamish, posteriorly becoming light-brown, with irregular streaks of different sizes along the venter; tail with 25/23 blotches with the same pattern as that on posterior portion of body and with some streaks in the interblotches.
Hemipenis of holotype ( Fig. 4 View FIGURE 4 ). Everted organ extends to the level of the seventh subcaudal, single with a bulbous shape, unicapitate; capitulum completely encircles the organ on the sulcate side and occupies more than the distal half of the hemipenial body; on the asulcate side, capitulum occupies less than the distal half of the hemipenial body and a capitular crotch is present; capitulum covered with papillate calyces; sulcus spermaticus divides approximately on the basal region of capitulum; branches have centrolineal orientation terminating on distal region of the organ; on the asulcate side the free capitular flap partially overlaps the most distal row of spines; five asulcate spine rows, most proximal ones with curved spines; asulcate patch not conspicuous and basal hooks with distinct asymmetry; sulcate side with two rows of spines; basal naked pocket present next to the lateral hook terminating at the level of spine rows; basal portion of hemipenial body with spinules, mostly on the sulcate side.
Variation. Largest male the holotype. Largest female IBSP 77835, SVL 375 mm, TL 165 mm. Ventrals 187– 209 (197.5±7.1; n =6) in males, and 193–202 (196.8±3.9; n =4) in females; subcaudals 107–129 (119.7±8.3; n =6) in males, and 107–116 (111.7±4.5; n =3) in females; specimen MNRJ 19275 shows prefrontals that do not enter the orbit due to the presence of a preocular above the loreal; no preoculars (n =8), 1 preocular above the loreal (n =1), 1 preocular below the loreal (n =1); postoculars 1 (n =2 sides), 2 (n =12 sides) or 3 (n =4 sides); temporal formula 1+2 (n =4 sides), 1+3 (n =4 sides), 2+2 (n =1 side), 2+3 (n =4 sides), 1+2+2 (n =1 side) or 1+2+3 (n =2 sides), 2+2+3 (n=1 side), 3+3+3 (n =1 side); supralabials 8 (n =5 sides), 9 (n =12 sides) or 10 (n =1 side); supralabials touching the orbit 3–5th (n =4 sides), 3–6th (n =2 sides), 4–5th (n =1 side), 4–6th (n =9 sides), 4–7th (n =1 side) or 5–6th (n =1 side); infralabials 8 (n =1 side), 9 (n =5 sides), 10 (n =9 sides) or 11 (n =3 sides), up to fourth (n =1 side), fifth (n =9 sides) or sixth (n =8 sides) touching chinshields; two (n =4) or three (n =5) pairs of chinshields with complete sulci; number of maxillary teeth 17–21 (18.4±1.5; n =8); first blotch 7.5–11.5 vertebral scales long (9.6±1.2; n =10); number of dorsal blotches along the body 20–30 (25.9±3.0; n =10); number of caudal blotches 16–25 (20.4±3.3; n =6); size of anterior dorsal blotches 5–8 scales long (6.6±1.0; n =10); size of anterior interblotches 2.5–7 scales long (4.4±1.2; n =10); size of midbody dorsal blotches 1–4 scales long (2.7±1.0; n =10); size of midbody dorsal interblotches 4–6 (5.2±0.8; n =10); size of posterior dorsal blotches 0.5–2.5 scales long (1.6±0.7; n =10); size of posterior dorsal interblotches 4–6 (5.0±0.8; n =10); retracted hemipenis extends 8–10 subcaudals scales (8.8±1.0; n =4). The remaining characters are invariable with respect to the holotype.
Etymology. The specific epithet honors Ivan Sazima for his relevant contributions to herpetology in Brazil and his immense contributions to the knowledge of the Brazilian vertebrate fauna.
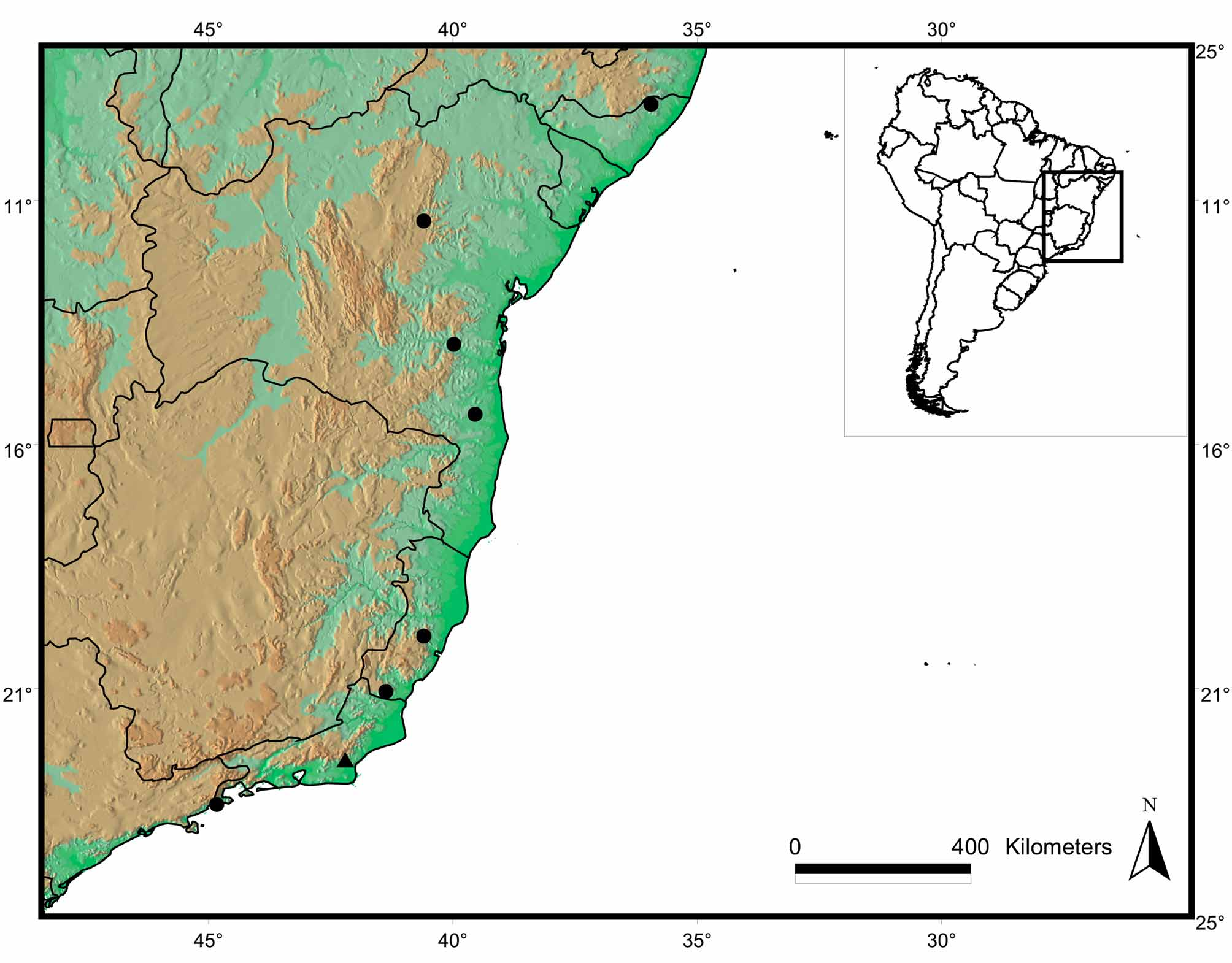
Distribution ( Fig. 6 View FIGURE 6 ). The new species is known from the state of Alagoas (09º 18’S) to the north of São Paulo (23o 23’S). It occurs in lowland areas (0–270 m) near the coast at higher latitudes (above 20o S, in the states of São Paulo, Rio de Janeiro and southern Espírito Santo). In lower latitudes (below 20o S, in the states of Espírito Santo, Bahia and Alagoas), this snake was found in elevations up to 700 m. In the northern Atlantic Forest (Bahia), it occurs on isolated and distinct mountainous complexes, such as Chapada Diamantina and upland regions near to coast ( SEI, 2003).
Natural history and conservation. One individual (IBSP 69143) had remains of a slug in the stomach ( Hartmann et al., 2009). Three individuals were found on vegetation (1.5–2.0 m above ground) at night: two specimens (IBSP 69143 and MZUESC 7848) were moving whereas another (IBSP 77835) was coiled among vines. Another individual was found run over, which suggests that it was crawling on the ground (cf. Hartmann et al., 2009). The semi-arboreal habits are characteristic within the Dipsas genus (Marques et al., 2004). These snakes are usually found resting on vegetation but can forage for snails and slugs on the ground ( Sazima, 1989). The new species inhabits dense umbrophilous forests within the Atlantic Forest domain (every Dipsas species inhabits forests). The sampling data suggest D. sazimai is the rarest species of Dipsas in the Atlantic Forest domain. We found only ten preserved specimens of D. sazimai in herpetological collections in contrast with higher number of other Dipsas from the Atlantic Forest region ( D. albifrons , n=285; D. bucephala , n=170; D. catesbyi , n=262; Dipsas i. indica , n=82; D. i. petersi, n=162; D. neivai (sensu Porto & Fernandes, 1996) , n=506; D. alternans , n=96). The Atlantic Forest of Brazil has been identified as one of the five global biodiversity hotspots ( Myers et al., 2000). Deforestation has reduced the Atlantic Forest to 12% of its original coverage ( Ribeiro et al., 2009). The remaining forest is severely perturbed or fragmented particularly in the northern portion as well as in coastal plain in southern areas where D. sazimai was recorded ( Foury, 1972; Gonzaga et al., 1995; Ribeiro et al., 2009). In Atlantic Forest, the populations of arboreal snakes are more susceptible to perturbation or loss of their habitat than terrestrial taxa (Marques & Sazima, 2004). Thus, the new species due to its natural history traits, low abundance, and loss of habitat may be considered potentially threatened.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.