Tetracampos ciliotheca Wedl, 1861
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3309.1.1 |
|
DOI |
https://doi.org/10.5281/zenodo.6174511 |
|
persistent identifier |
https://treatment.plazi.org/id/038A9703-0A29-FFB4-FF7D-7731069DF984 |
|
treatment provided by |
Plazi |
|
scientific name |
Tetracampos ciliotheca Wedl, 1861 |
| status |
|
Tetracampos ciliotheca Wedl, 1861
( Figs. 28–32 View FIGURES 23 – 32 , 41–52 View FIGURES 41 – 48 View FIGURES 49 – 52 )
Syns: Clestobothrium clarias Woodland, 1925 ; Polyonchobothrium cylindraceum forma major Janicki, 1926; P. cylindraceum forma minor Janicki, 1926; Polyonchobothrium fulgidum Meggitt, 1930 ; Polyonchobothrium clarias ( Woodland, 1925) Meggitt, 1930 ; Polyonchobothrium ciliotheca ( Wedl, 1861) Dollfus, 1934 ; Polyoncobothrium ciliotheca ( Wedl, 1861) Yamaguti, 1959 ; Polyoncobothrium clarias ( Woodland, 1925) Yamaguti, 1959 .
Type host: Clarias anguillaris (Linnaeus) ( Siluriformes : Clariidae ).
Other definitive hosts: Clarias gariepinus (Burchell) , Clarias liocephalus Boulenger , Clarias werneri Boulenger.
Life cycle: Khalil & Thurston (1973) observed hatching of eggs in 10 minutes after their transfer to tap water. Liberated coracidia had embryophore 36–42 μm long by 30–35 μm wide, cilia 18 μm long and embryonic hooks 1 μm in length ( Diab 2007). Freshwater copepods serve as the first intermediate hosts, in which procercoids developed within 20–26 days. Developed procercoids (252–610 μm long) were infective for small fish, such as tilapias ( Oreochromis niloticus ). Experimentally infected tilapias were exposed to C. gariepinus , in which adult worms were found ( Diab 2007; Ramadan 2007). Small fish that harbour immature cestodes in natural conditions, such as schilbeid and mochokid catfish ( Schilbe uranoscopus , Synodontis membranacea and S. zambezensis ) and tilapias ( Oreochromis niloticus , Sarotherodon galilaeus ) ( Douellou 1992; Owolabi 2008; Eissa et al. 2011a, b), may play a role of paratenic hosts.
Type locality: Egypt, Nile River.
Distribution: Lower Guinea – Gabon; Gambia basin – Senegal; Turkana basin – Kenya (all parts of the Lake Turkana); Limpopo basin – South Africa; Upper Guinea – Sierra Leone (Moa River); Niger basin – Mali, Nigeria; Nile basin – Egypt, Ethiopia, the Sudan, Tanzania, Uganda; Volta basin – Ghana; Zambezi basin – Zimbabwe, Malawi. Besides Africa, T. ciliotheca has been reported also from Asia – Israel and Turkey, probably as a consequence of introduction with host – see Remarks ( Paperna 1964; Soylu & Emre 2005; present study).
Prevalence and intensity of infection: Usually high, with values between 52% and 100% in most studies from Egypt, Nigeria and South Africa ( Aderounmu & Adeniyi 1972; Shotter 1980; Faisal et al. 1989; Anosike et al. 1992; Barson & Avenant-Oldewage 2006). In the present study the overall prevalence was 5–17% in the Sudan, 26% in Ethiopia and 33% in Kenya ( Appendix 1).
Type material: Not known to exist. To enable taxonomic comparative studies in the future, the specimen from Clarias sp. from Blue Nile, Sennar Dam, the Sudan (field No. Sud 438) is designated as neotype and is deposited in IPCAS (No. C-466).
Material studied: Type material: Clestobothrium clarias Woodland, 1925 ex C. anguillaris ( BMNH 1965.2.24.29–35) ; Polyonchobothrium fulgidum Meggitt, 1930 ex C. anguillaris ( BMNH 1932.5.31.801–806) ; Polyonchobothrium interruptus – nomen nudum ( USNPC 74291–2) ; vouchers: P. cylindraceum ex C. anguillaris from Mali, Diafarabe ( MNHNP C79) ; P. clarias ex C. anguillaris from Senegal, Guerina ( RMCA 34773) and Ghana ( BMNH 1976.4.12.155–161) ; ex C. gariepinus from Nigeria, Lekki Lagoon and Kainji Dam ( BMNH 2004.2.18.38, 1970.8.24.37) ; from Tanzania, Lake Victoria, Mwanza Gulf ( MHNG 33983 ) , Zimbabwe, Save-Runde River Floodplain ( BMNH 2006.9.1.6) and Sierra Leone ( BMNH 1965.2.24.59–6) ; ex Heterobranchus bidorsalis from Senegal, Guerina ( RMCA 34723) ; ex Schilbe uranoscopus from unknown locality, collected by McClelland (RVC C1108) ; T. ciliotheca ex Clarias sp. from Egypt, Luxor , collected by A. de Chambrier ( MHNG 31547 ; 17.iv.2001) ; ex C. gariepinus from South Africa, Rietvlei Dam, collected by M. Barson and from Turkey, Antalya ( IPCAS C–466) ; new material: tens of T. ciliotheca ex 2/18 C. anguillaris from the Sudan, Kostí and Sennar Dam; 12 worms ex 3/23 C. anguillaris from Senegal, Niokolo-Koba National Park, Gambia River collected by B. Koubková (2004; Sen 52, 53, 121) ; 84/322 C. gariepinus from Ethiopia, Lake Tana and Great Rift Lakes (Awasa, Langano and Ziway) , 14/43 C. gariepinus from Kenya, Lake Turkana and 5/30 C. gariepinus from the Sudan, Al Kawa, Khartoum, Er Roseires Dam, Sennar Dam ; 4/88 Clarias sp. from the Sudan, Khartoum, Lake Nubia (Asuan Dam), Sennar Dam; one C. gariepinus from Lake Malawi, collected by S. Hendrix (SSH96-09-M-1) . The new material is deposited in BMNH (Nos. 2012.3.20.16–25), IPCAS (No. C-466), MHNG (Nos. 55309, 55337, 55338, 62879, 62904, 63006–63328), USNPC (Nos. 105395–105400, 105404–105408) and ZMB (Nos. 7517–7523).
Published records: Wedl (1861); Woodland (1925); Janicki (1926); Meggitt (1930); Tadros (1968); Khalil (1969, 1973); Aderounmu & Adeniyi (1972); Khalil & Thurston (1973); Amin (1978); Tadros et al. (1979); Shotter (1980); Wabuke-Bunoti (1980); Onwuliri & Mgbemena (1987); Faisal et al. (1989); Mashego & Saayman (1989); Imam & El-Askalany (1990); Imam et al. (1991a, b); Anosike et al. (1992); Douellou (1992); Al-Bassel (2003); El-Garhy (2003); Rizkalla et al. (2003); Hamanda & Abdrabouh (2004); Oniye et al. (2004); Akinsanya & Otubanjo (2006); Barson & Avenant-Oldewage (2006); Olofintoye (2006); Diab (2007); Ayanda (2008, 2009a, b); Barson et al. (2008); Mwita & Nkwengulila (2008); Moyo et al. (2009); Bichi & Yelwa (2010); Madanire-Moyo & Barson (2010); Madanire-Moyo et al. (2010); Eissa et al. (2011a, b).
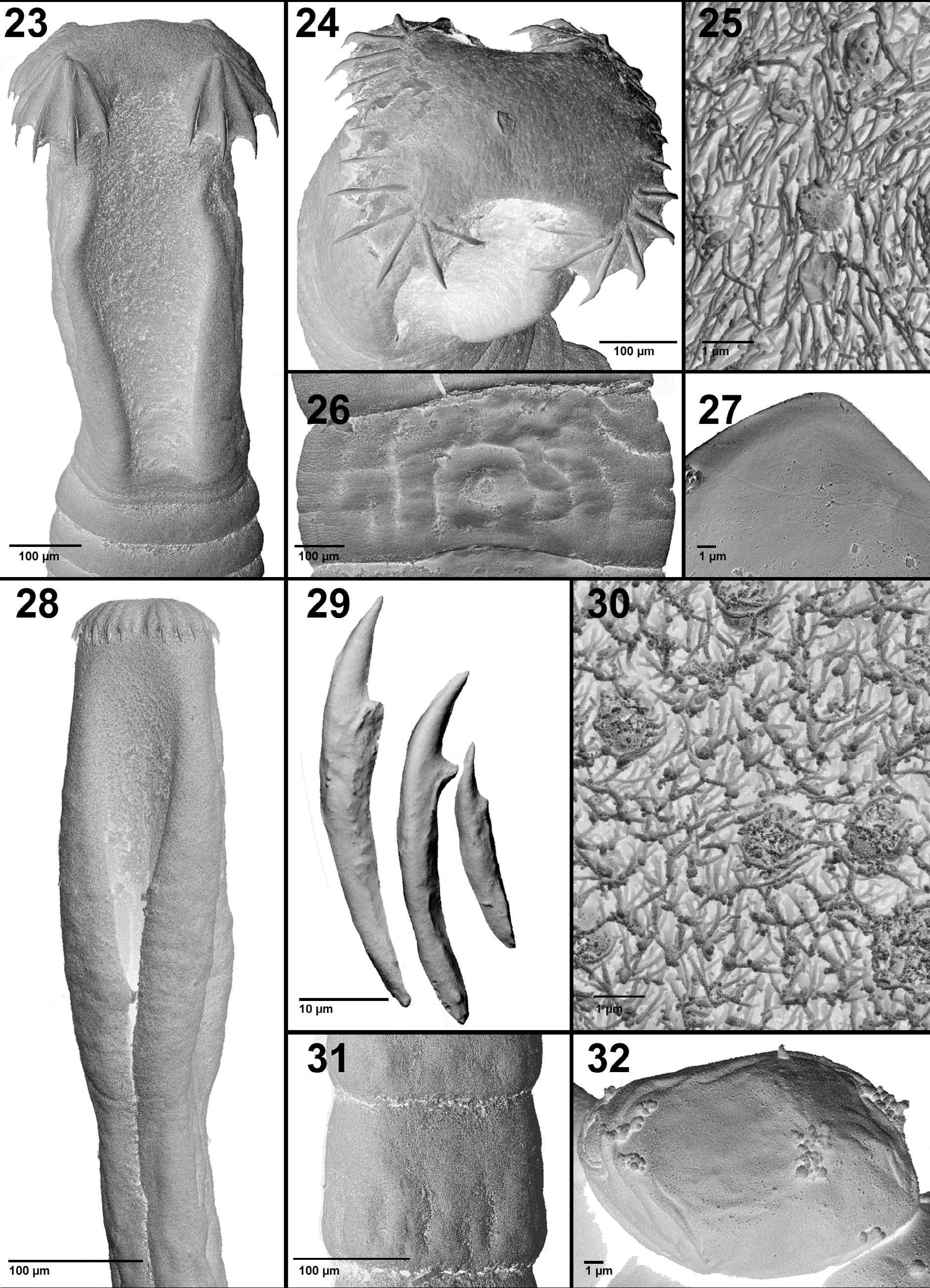
Re-description (based on 25 complete worms from Ethiopia, Kenya and the Sudan): Bothriocephalidea , Bothriocephalidae . Strobila small, oval or almost spherical in cross section, up to 30 mm long; maximum width 475. External and internal segmentation present; segments wider than long, acraspedote ( Figs. 31 View FIGURES 23 – 32 , 41, 44 View FIGURES 41 – 48 ).
Two pairs of osmoregulatory canals; dorsal canals narrow; ventral canals wide, connected by transverse anastomoses. Inner longitudinal musculature well developed, muscle fibres diffused ( Fig. 46 View FIGURES 41 – 48 ). Surface of strobila covered with capilliform filitriches.
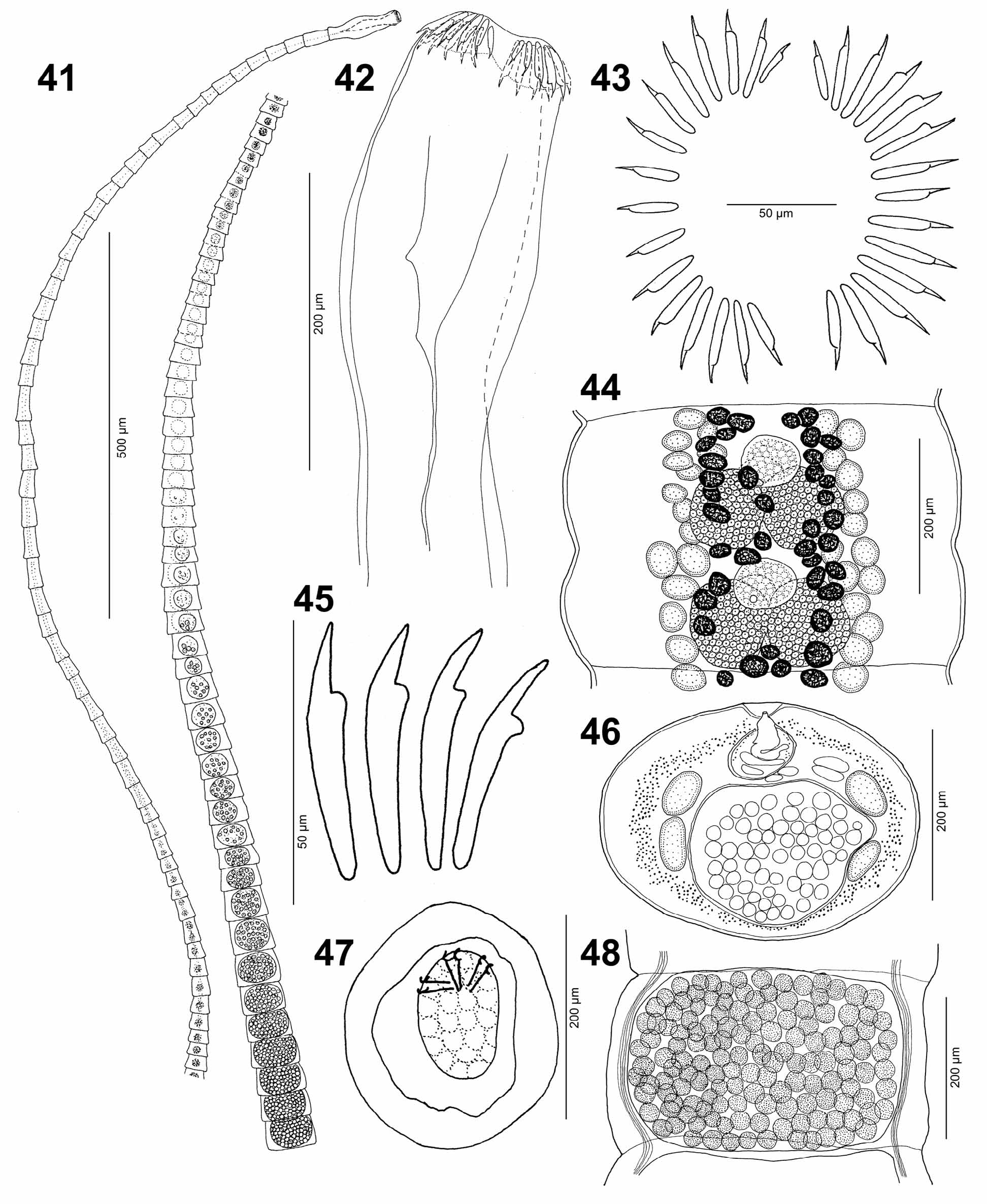
Scolex elongate to ovoid, 285–510 (396 ± 62) long by 115–245 (165 ± 42) wide (n = 20) ( Figs. 28 View FIGURES 23 – 32 , 42 View FIGURES 41 – 48 ). Apical disc weakly developed, 104–290 (156 ± 63) wide and 35–120 (97 ± 24) high (n = 20), armed with 25–35 (29 ± 2; n = 18) small hooks ( Amin 1978 reported as many as 41 hooks) 12–51 (37 ± 7; n = 537) long, arranged in two lateral semicircles separated from each other on dorsal and ventral side. Hooks variable in size in each semicircle, with largest hook 40–51 (46 ± 3; n = 20) in each corner of apical dic ( Figs. 29 View FIGURES 23 – 32 , 43, 45 View FIGURES 41 – 48 ). Bothria elongate, shallow, 200– 410 (308 ± 56) long by 57–120 (79 ± 22) wide (n = 20) ( Figs. 28 View FIGURES 23 – 32 , 42 View FIGURES 41 – 48 ). Surface of scolex covered with capilliform filitriches and numerous tumuliform globular structures (diameter around 1) ( Fig. 30 View FIGURES 23 – 32 ). Neck absent, first segments appearing immediately posterior to scolex ( Fig. 41 View FIGURES 41 – 48 ).
Immature segments 80–235 (144 ± 39) long by 84–261 (167 ± 57) wide; length/width ratio 0.41–2.58: 1 (n = 38) ( Fig. 41 View FIGURES 41 – 48 ). Mature segments wider than long by, 90–400 (182 ± 68) long by 135–480 (255 ± 96) wide; length/ width ratio 0.3–1.0: 1 (n = 41) ( Fig. 41 View FIGURES 41 – 48 ). Gravid segments wider than long, 178–488 (198 ± 69) long by 180–455 (316 ± 71) wide; length/width ratio 0.5–1.2: 1 (n = 35) ( Figs. 31 View FIGURES 23 – 32 , 41, 44, 48 View FIGURES 41 – 48 ).
Testes medullary, spherical, 5–15 (10 ± 3; n = 21) in number per segment, 21–48 (33 ± 7; n = 60) in diameter, forming 2 narrow longitudinal bands (4–9 testes per band), confluent between segments, absent medially and near lateral margins ( Fig. 44 View FIGURES 41 – 48 ). Cirrus-sac large, thin-walled (thickness of sac wall up to 4), oval, 32–66 (48 ± 9) long by 28–68 (45 ± 10) wide (length/width ratio 0.77–1.73: 1) (n = 15), equatorial (39–59% of length of mature segment; n = 10) ( Fig. 46 View FIGURES 41 – 48 ). Internal seminal vesicle absent; cirrus unarmed, opening into genital atrium. Vas deferens forms numerous loops lateral to cirrus-sac; internal sperm ducts strongly coiled. Genital pore dorsal, median, pre-equatorial.
Ovary symmetrical, forming two spherical lobes, 31–91 (59 ± 18) long by 76–183 (113 ± 29) wide (n = 14) ( Fig. 44 View FIGURES 41 – 48 ). Vagina a straight, thin-walled tube, 6–16 (11 ± 4; n = 9) in diameter, opens posterior to cirrus-sac into genital atrium; vaginal sphincter absent. Vitelline follicles few, small, spherical, 12–40 (19 ± 8; n = 28) in diameter, medullary, distributed among testes, visible only in some mature and gravid proglottides ( Fig. 44 View FIGURES 41 – 48 ).
Uterine duct winding, short, filled with eggs ( Fig. 41 View FIGURES 41 – 48 ). Uterus thin-walled, median, spherical, enlarged in gravid segments, occupying 57–80% of segment surface ( Figs. 41, 48 View FIGURES 41 – 48 ). Uterine pore thick-walled, opens in centre of uterus. Eggs widely oval to spherical, 28–72 (46 ± 9) long by 27–51 (40 ± 6) wide (n = 46), with external hyaline membrane and internal granular layer surrounding fully formed oncospheres, 17–45 (27 ± 8) long by 17–31 (23 ± 4) wide (n = 41) in terminal segments; eggs enlarging during their development in uterus ( Figs. 32 View FIGURES 23 – 32 , 47 View FIGURES 41 – 48 ).
Remarks: Taxonomic history of bothriocephalideans parasitic in clariid catfish in Africa is complicated because apparently conspecific tapeworms were reported under different species names and were placed in several genera. Most commonly, they were identified as Polyonchobothrium clarias ( Woodland, 1925) , but this species is a junior synonym of Tetracampos ciliotheca (see Kuchta et al. 2008b). Wedl (1861) described T. ciliotheca from cestodes parasitic in Heterobranchus anguillaris (= Clarias anguillaris ) from Egypt. Since the original description was incomplete, most subsequent authors considered T. ciliotheca as a nomen nudum or placed it in the order Proteocephalidea or even Tetraphyllidea , because its eggs possess a transparent, hyaline external envelope ( Southwell 1925; Janicki 1926). Kuchta et al. (2008a, b) resurrected the genus with T. ciliotheca as its type and only species because it differs from other bothriocephalideans in egg morphology, the possession of an unflattened strobila, almost round in cross section, and medullary position of vitelline follicles. The latter characteristic is also present in two other bothriocephalidean cestodes, Ptychobothrium Lönnberg, 1889 and Taphrobothrium Lühe, 1899 , but they parasitize marine teleosts and their morphology is otherwise markedly different (see Kuchta et al. 2008b).
Tetracampos ciliotheca is a common parasite of clariid catfish and it is widely distributed throughout Africa, with most published reports from Egypt, Nigeria, South Africa and the Sudan (see above). The cestode has also been reported from Israel ( Paperna 1964 – as P. clarias ) and Turkey [ Soylu & Emre 2005 – as Polyonchobothrium magnum (Zmeev, 1936) ; present study], apparently as a consequence of import of African species of Clarias to these countries. Records of T. ciliotheca in other catfish, such as Heterobranchus bidorsalis from Senegal (present study; Khalil 1973; RMCA 34723), Bagrus bayad from Egypt ( Imam et al. 1991a) and Chrysichthys auratus from the Sudan (present study), may represent incidental infections or these fish may serve as postcyclic or accidental hosts.
Omar M. Amin deposited tapeworms found in C. anguillaris from Egypt under the name Polyonchobothrium interruptus (USNPC 74291–2), but that species has never been formally described and thus represents nomen nudum. In 1978 Amin himself identified these tapeworms as Polyonchobothrium clarias (= T. ciliotheca ).
Host-parasite relationships of T. ciliotheca and its fish host have been studied by several authors (most of them referred to this species as Polyonchobothrium clarias – see above). The tapeworms penetrates deeply into the mucosa of the intestinal wall and may cause mechanical injury by the attachment of the apical crown of hooks on the scolex ( Tadros 1979; Akinsanya & Otubanjo 2006; present study Figs. 49–52 View FIGURES 49 – 52 ). Adults of T. ciliotheca were also found in the gall bladder ( Amin 1978; Shotter 1980; Faisal et al. 1989; Barson et al. 2008), where tapeworms may cause formation of nodular outgrowths in the mucosa ( Wabuke-Bunoti 1980). Faisal et al. (1989) reported complete penetration of T. ciliotheca tapeworms through the intestinal wall, with their attachment in the liver, spleen and ovary. In fish with perforated intestine, the intestinal contents filled the peritoneal cavity ( Wabuke-Bunoti 1980). Despite the high number of examined hosts and observed cestodes, we never found T. ciliotheca tapeworms in extraintestinal localization.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |