Elmomorphus brevicornis Sharp, 1888
|
publication ID |
https://doi.org/ 10.5852/ejt.2021.758.1427 |
|
publication LSID |
lsid:zoobank.org:pub:D063C3C3-76CD-450A-AC01-0035CFA51379 |
|
DOI |
https://doi.org/10.5281/zenodo.5095032 |
|
persistent identifier |
https://treatment.plazi.org/id/6960BF58-FF99-FFD9-5618-4C0BFC52A09A |
|
treatment provided by |
Felipe |
|
scientific name |
Elmomorphus brevicornis Sharp, 1888 |
| status |
|
Elmomorphus brevicornis Sharp, 1888
Figs 4A–C View Fig , 5A View Fig , 6A, C, E View Fig , 7A–D View Fig , 8A–B, E–F View Fig , 9A–B, D View Fig , 10A View Fig
Elmomorphus brevicornis Sharp, 1888: 243 (original description, type locality).
Helichus View in CoL “ 862 spec.?” Lewis 1879: 12 (first indication).
Elmomorphus brevicornis – Zaitzev 1910: 19 (catalogue). — Kôno 1934: 125 (catalogue). — Bollow 1940: 59–62, figs 271–276 (description, Japanese records). — Chûjô & Satô 1964: 193. — Delève 1968: 151 (differential diagnosis). — Satô 1981: 53. — Jäch 1984: 314 (checklist). — Kodada & Jäch 2006: 442 (catalogue). — Hayashi & Shimada 2006: 129 (Japanese records, in Japanese). — Yoshioka 2007: 236, 242, fig. 17a (Japanese records, in Japanese); 2008: 224 (Japanese records, in Japanese). — Hayashi 2011: 93. — Kamezawa & Nomura 2013: 29 (Japanese records, in Japanese). — Jung & Bae 2014: 2–7, figs 2a–c, 3–5 (description, identification key, Korean records). — Kodada & Jäch 2016: 606 (catalogue). — Yoshitomi & Haga 2018: 341–342 (identification key). — Nakajima et al. 2020: 177, 322 (diagnosis, identification key, photographs of living specimens).
non Elmomorphus brevicornis – Devi et al. 2016: 371–373 (misidentification, invalid lectotype
designation)].
Type locality
Kobe, Hyōgo Prefecture, southern Honshu, Japan.
Material examined
Syntypes
JAPAN • 1♂ ( Fig.4C View Fig );“Kobe 18.V.71 [handwritten]|Japan.G Lewis1910—320.[printed]| Elmomorphus brevicornis [handwritten] | Elmomorphus brevicornis . [handwritten] | Elmomorphus brevicornis Sharp det. H. Bollow 1934 [handwritten and printed]”; NHMUK • 1 ♀ ( Fig. 4A–B View Fig ); “ Elmomorphus brevicornis Type D.S. [David Sharp] Jokio [sic]. Japan Lewis. [handwritten] | Japan. G.Lewis [printed] | Sharp Coll. 1905-313. [printed] | Type [printed, round label with red frame]”; NHMUK.
Additional material
JAPAN • 1 ♀; “ Shimakatsu Mie-Pref. 16.XI.1958 Coll. Shozo Ishida | Elmomorphus brevicornis Sharp Det. M. SATO 1971 ”; NHMW • 1 ♂; “ Nagara-River Gifu-City 26.VII.1951 Coll. Zen. Naruse ”; NHMW • 1 ♂; “ Kurokawa , N-Echigo 2.IX.1960 Col. K. Baba ”; NHMW • 1 ♂; “ Kabuchi-gawa Gifu Pref. 18.VIII.1967 M. Sato leg.”; NHMW • 10 ♂♂, 6 ♀♀; “ Japan, Shimane Pref.: Matsue: Yakumo 26.–30. iii.2019 M. Hayashi ”; JKCB • 5 ♂♂, 3 ♀♀; “ Japan, Shimane Pref.: Izumo, Nishitani 30.iii.2019 M. Hayashi ”; JKCB • 1 ♀; “ NE-JAPAN Miyagi Pr. , Mts. Abukuma, 6.6. Uchikawa riv., leg. Ohmomo 1987 | Elmomorphus brevicornis Sharp ”; NHMW .
SOUTH KOREA • 1 ♂, 1 spec. sex not examined; “ South Korea, Jeollabuk-do, Buan-gun, Byeonsanmyeon , Junggye-ri , Jiko Falls 15.VI.2011, leg. S.W.Jung ”; NHMW .
Note
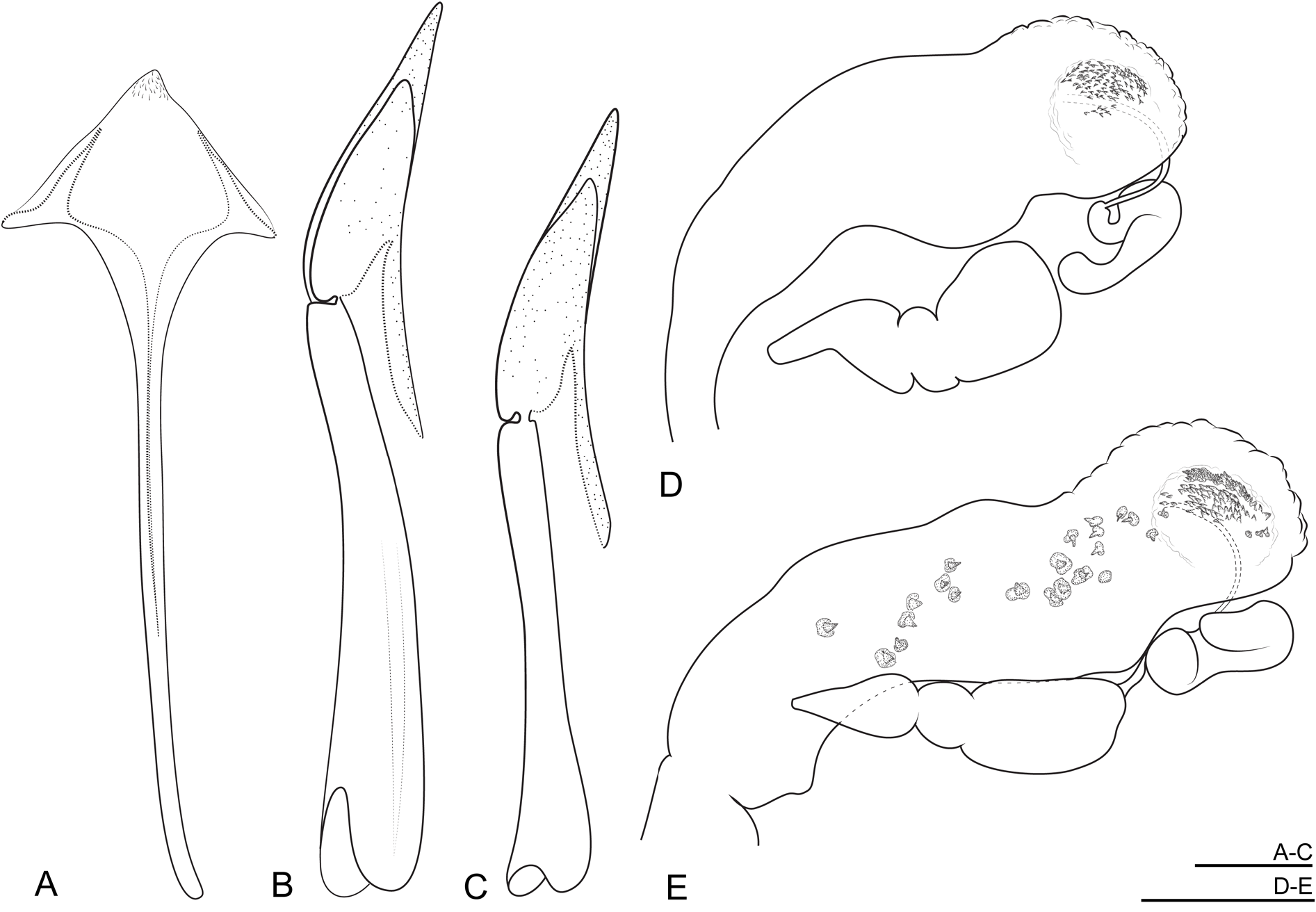
The original description was based on two specimens collected by G. Lewis. Both specimens are still preserved in the collection of David Sharp in the NHMUK. The female specimen is glued on a larger rectangular card with the following handwritten text: “ Elmomorphus brevicornis Type D.S. Jokio. Japan Lewis.”. This style of labelling and label text is typical of David Sharp’s type material. The male specimen and the dissected aedeagus are glued on one card, while its detached appendages are glued on a second card, pinned below the specimen. The two syntypes are conspecific. However, while the type locality mentioned in the original description (“Kobé”) is written on the original label of the male specimen ( Fig. 4C View Fig ), the name “Jokio” [Sharp’s spelling of Tokyo] is found on the label of the female specimen ( Fig. 4A View Fig ). Since no other species is known from Honshu and since taxonomic confusion is improbable, a lectotype designation is considered unnecessary (see ICZN 1999: Recommendation 74G, https://www.iczn.org/the-code/declaration-44-amendment-of-article-74-7-3/).
In the abstract of their most remarkable article, Devi et al. (2016: 371) wrote that “A lectotype is designated for this species [ Elmomorphus brevicornis ]”. However, the term “ lectotype ” is not used anywhere else in their article, and it does not contain sufficient information to ensure recognition of the specimen designated. Therefore, the requirements of the International Code of Zoological Nomenclature ( ICZN 1999: Art. 74.7) are not fulfilled.
Diagnosis
Elmomorphus brevicornis is a medium-sized (TL 2.59–3.51 mm), elongated oval, dorsally moderately convex species, with the highest point at the anterior third of the elytral length (lateral view). Body outline discontinuous between pronotum and elytra; yellowish plastron microscales cover the entire surface of the cranium, lateral portions of the pronotum and the entire dorsal surface of the elytra, so most of the dorsal surface appears to be matt ( Fig. 5A View Fig ). Each elytron has nine rows of large round punctures arranged in shallow striae. Legs conspicuously long and robust, only moderately shorter than the combined length of pronotum and elytra.
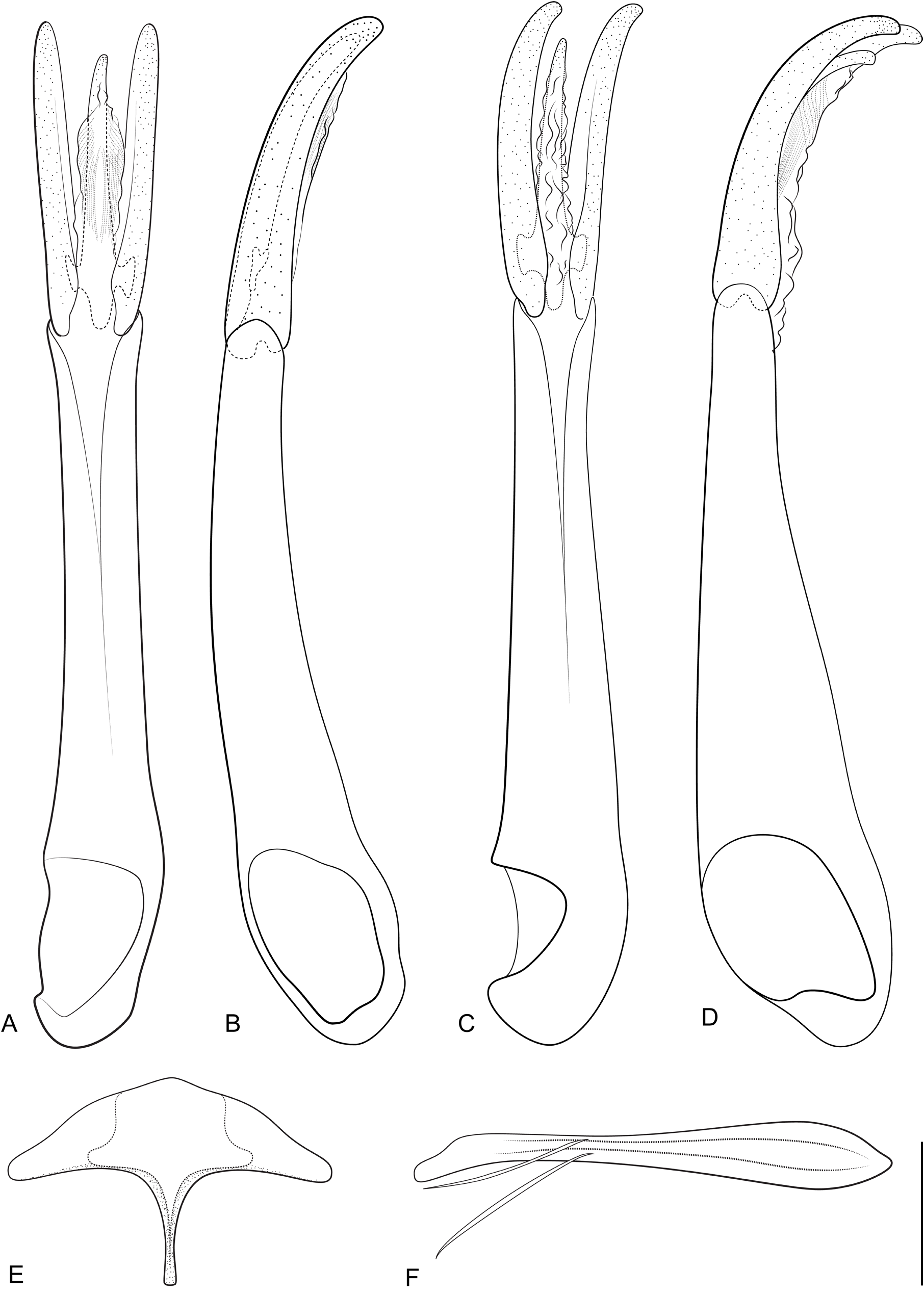
Elmomorphus brevicornis closely resembles E. amamiensis in external morphology, and it can be distinguished by the following characteristics: (1) elytral sides in E. brevicornis subparallel in the anterior half, while in E. amamiensis they are arcuate and more convergent ( Fig. 5B View Fig ), thus the outline appears to be generally broader and less oval in E. brevicornis ; (2) sides of pronotum usually more rounded in E. brevicornis , while they are almost straight in E. amamiensis ; (3) males of E. brevicornis with several longer setae in a transverse row on the labrum, and with similar setae in two pairs of clusters on each prosternal process and on the admedian portions of metaventrite, row and clusters of setae rather inconspicuous ( Fig. 6A, C, E View Fig ); similar, longer setae also near the apex of the fifth ventrite ( Fig. 7D View Fig ); in the male of E. amamiensis the setal row and all setal clusters are well developed, conspicuous ( Figs 6B, D, F View Fig , 7E View Fig ); (4) parameres weakly curved ventrad in E. brevicornis ( Fig. 8B View Fig ), but strongly curved in E. amamiensis ( Fig. 8D View Fig ); (5) bursa copulatrix of the two species distally with one cluster of microspines on each side ( Fig. 9D View Fig ), while in E. amamiensis , besides these microspines there are several additional larger sclerites ( Fig. 9E View Fig ). Except for differences in the morphological characters, the two species differ by 22.7–23.9% in the partial mtDNA encoding for cytochrome c oxidase subunit I.
Measurements (mm)
TL: ♂♂ 2.89–3.38 (3.10 ± 0.13, n = 14), ♀♀ 3.15–3.51 (3.34 ± 0.12, n = 7); PL: ♂♂ 0.65–0.85 (0.78 ± 0.05, n = 14), ♀♀ 0.75–0.90 (0.83 ± 0.05, n = 7); PW: ♂♂ 1.20–1.43 (1.33 ± 0.06, n = 14), ♀♀ 1.30–1.47 (1.40 ± 0.06, n = 7); EL: ♂♂ 2.22–2.57 (2.42 ± 0.09, n = 14), ♀♀ 2.50–2.86 (2.66 ± 0.11, n = 7); EW: ♂♂ 1.44–1.66 (1.54 ± 0.05, n = 14), ♀♀ 1.51–1.77 (1.65 ± 0.09, n = 7); MsTL: ♂♂ 0.82– 0.94 (0.88 ± 0.03, n = 14), ♀♀ 0.87–0.97 (0.91 ± 0.03, n = 7); MtTL: ♂♂ 0.91–1.09 (1.02 ± 0.05, n = 14), ♀♀ 0.97–1.12 (1.06 ± 0.05, n = 7); PhL: 0.73–0.89 (0.83 ± 0.04, n = 11); PrL: 0.32–0.39 (0.37 ± 0.02, n = 11).
Redescription
BODY. Elongated oval, widest behind mid-length of elytra, moderately convex dorsally, with the highest point at anterior third of elytral length (lateral view).Colouration black, except reddish-brown mouthparts, antennal clubs, tarsi, claws, trochanters, and ventral portions of femora. Tibiae and remainder of femoral surface dark brown to black. Dorsal pubescence consists mainly of short, thin decumbent yellowish setae arising from small punctures. Plastron microscales ( Fig. 7B View Fig ) on the entire cranium, on pronotum in two lateral bands, covering ca 0.2 of pronotal width on each side, and on the entire surface of elytra. Ventral surface with dense, thin plastron hair-like setae ( Fig. 7C View Fig ) except for prosternal process and median part of metaventrite.
HEAD. Dorsally entirely covered with plastron scales and with round, setiferous punctures; puncture diameter ca 0.75× diameter of an eye facet, and their distance varies between 0.50–1.00× of a facet diameter. Labrum transverse, with anterior margin straight; anterolateral angles rounded; exposed portion microreticulate, with small setiferous punctures; two types of hair-like setae present, short decumbent setae on most of the surface, and several longer semi-erect setae in a hardly discernible transverse row near mid-length ( Fig. 6A View Fig ), setae nearly half as long as ID and more obvious in males (in older material long setae often abraded). Clypeus with anterior margin straight and with a row of the fine short hairs. Eyes oval, protruding and large, interfacetal setae short; ID: ♂♂ 0.38–0.43 mm (0.41 ± 0.02, n = 14), ♀♀ 0.42–0.48 mm (0.44 ± 0.02, n = 7); APD/ID: ♂♂ 1.93–2.09 (2.01 ± 0.04, n = 14), ♀♀ 1.89–2.04 (1.96 ± 0.05, n = 7). Antennae short, reaching slightly behind middle of eyes, 10-segmented; densely covered by plastron; each club segment with a conspicuous peg-like sensillum on anterior face and numerous hair-like, peg-like and branched sensilla; scape and pedicel small and subequal in length, not enlarged.
THORAX. Pronotum transverse, widest at base, strongly convex, PW/PL: ♂♂ 1.60–1.85 (1.72 ± 0.07, n = 14), ♀♀ 1.60–1.76 (1.68 ± 0.05, n = 7); plastron in lateral band on each side along entire pronotal length, mesally reaching level of third elytral row; disc smooth, shiny, with large round setiferous punctures, punctures nearly as large as facets, separated by 0.50–1.00× of a facet diameter; punctures on plastron area smaller, similar to those on head. Anterior angles strongly deflexed, protruding and acute, third as long as interocular distance; pronotal sides convergent anteriad, distinctly arcuate near middle, moderately explanate along entire length. Hypomeron widest behind mid-length. Prosternal process wide and short, lateral margins divergent and moderately arcuate; posterior margin widely rounded; lateral portion raised, wide and in male with group of longer setae ( Fig. 6C View Fig ) forming a hardly discernible setal cluster (these setae only moderately longer than other hair-like ventral setae and often abraded); median keel moderately convex, ca ⅓ as wide as width of prosternal process. Scutellum longer than wide, smooth, with small, sparse punctures. Metaventral process with lateral sides strongly raised, anterior margin not raised. Metaventral disc flat in female, finely depressed in male; longitudinal step-like elevation along sides delimits lateral area with plastron from smooth central one, surface irregularly and moderately sparsely punctate and in some specimens finely irregularly wrinkled; discrimen well impressed, distinct; a small, hardly discernible setal cluster present in front of metakatepisternal suture in males on each side of metaventral disc ( Fig. 6E View Fig ). Elytra oblong, widest behind middle; moderately convex dorsally, in dorsal view with the highest point at anterior third; sides subparallel in anterior half, more strongly arcuate in posterior half; EL/EW: ♂♂ 1.53–1.69 (1.57 ± 0.04, n = 14), ♀♀ 1.53–1.66 (1.61 ± 0.04, n = 7); surface entirely covered by plastron scales and with numerous minute punctures distinctly smaller than a facet diameter, punctures separated by about 0.50–1.00× a facet diameter. Strial punctures large and deep on elytral disc and subequal in size with facet size, laterad and posteriad progressively smaller. All tibiae straight, to slightly bent near mid-length; protibia ca 1.25× as long as protarsus; PrTL/PL: ♂♂ 1.01–1.20 (1.10 ± 0.05, n = 14), ♀♀ 1.00–1.08 (1.05 ± 0.03, n = 7). Terminal tarsomere in male foreleg ca 1.20× as long as all preceding tarsomeres combined; male foreclaws strongly curved, not widened or thickened, about half of terminal tarsomere length, both similar to each other ( Fig. 7A View Fig ) and similar to female foreclaws.
ABDOMEN. All ventrites completely covered with plastron; ventrites 1–5 with a length ratio about 1.00: 0.76: 0.60: 0.40: 1.26 in male, and 1.00: 0.90: 0.73: 0.60: 1.60 in female; intercoxal process wide, about twice as wide as long, subtriangular, sides moderately arcuate; admedian keels feebly raised, reaching posterior margin of ventrite. Male ventrite 5 near anterior margin declivous and then shortly flattened, laterad and posteriad evenly deflexed; apex with distinct triangular excision ( Fig. 7D View Fig ); several longer semi-erect setae on each side of excision in hardly discernible clusters. Female ventrite 5 evenly convex and not flattened anteriorly, with minute longitudinal smooth keel at apex and few longer setae alongside keel; apex rounded. Sternite VIII moderately sclerotized laterally, membranous medially, in males with short strut on anterior margin ( Fig. 8E View Fig ), in females with strut almost as long as abdomen ( Fig. 9A View Fig ). Male sternite IX narrow, with sclerotized anterior strut ( Fig. 8F View Fig ). Aedeagus: phallobase long, narrow, expanded proximally ( Fig. 8A View Fig ), PhL/PrL: 2.14–2.36 (2.24 ± 0.07, n = 11); parameres slightly curved ventrad along apical half, nearly straight, with apices narrowly rounded ( Fig. 8B View Fig ); apex of penis slightly expanded and rounded in lateral aspect, not reaching apex of parameres; ventral sclerotized fibula absent. Ovipositor heavily sclerotized; right coxite ca 1.40× as long as left one; paraproct ca 1.80× as long as right coxite ( Fig. 9B View Fig ). Bursa copulatrix with a cluster of numerous minute spines arranged on each side of distal portions ( Fig. 9D View Fig ). Spermatheca tubular; accessory gland large, semi-tubular.
Sexual dimorphism
Females are, on average longer and broader than males. For an unambiguous distinction between males and females, the apex of the fifth ventrite should be examined: it is distinctly excised in males ( Fig. 7D View Fig ), while it is rounded, with a short, smooth keel in females. The presence of several longer semi-erect setae on the labrum, prosternal process, metaventral disc and on the apex of the fifth ventrite in males is hard to observe even in well-preserved specimens, and even under a high-quality microscope at a magnification of about 100×.
Distribution
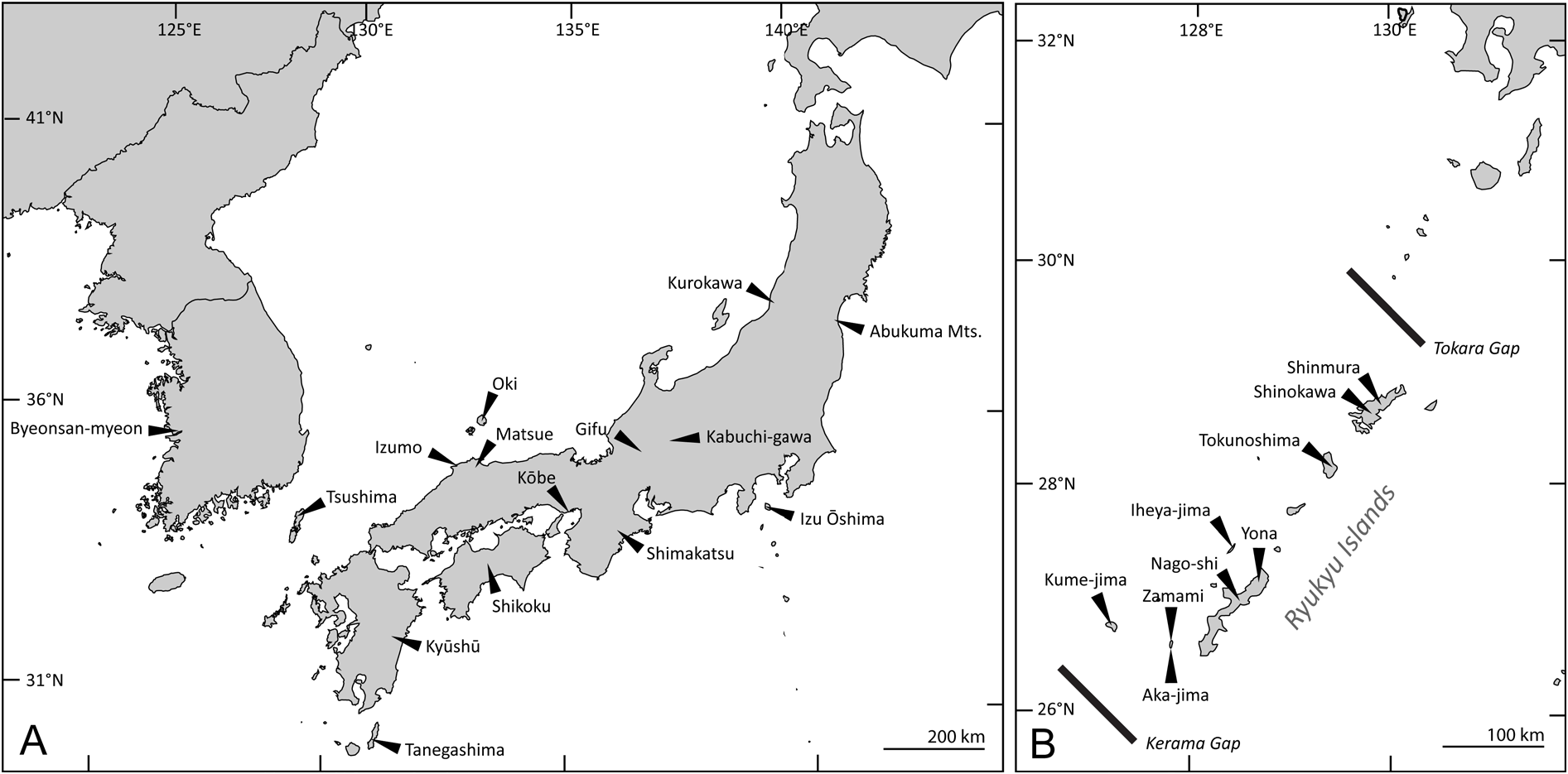
Japan: Honshu, Izu-Ôshima, Oki, Shikoku, Kyushu, Tsushima, and Tanegashima ( Nakajima et al. 2020); South Korea ( Fig. 10A View Fig ).
Devi et al. (2016) erroneously recorded E. brevicornis from India. Their record was published in the unreviewed “ Journal of Entomology and Zoology Studies ”, a so-called predatory journal (see https://predatoryjournals.com/journals/). The “redescription” provided in this article does not allow any conclusions to be drawn on the identity of the specimens. The “Aedagues” [sic] depicted in their fig. 1c is, in fact, an ovipositor! Judging from their habitus photograph (fig. 1a), these specimens, beyond any doubt, do not belong to E. brevicornis or any other species similar to E. brevicornis .
Immature stages
The larva was described by Hayashi & Kadowaki (2008) and Hayashi (2015). It was found in a river on submerged roots of Salix L. and Carex L. (Hayashi pers. com.).
| NHMW |
Austria, Wien, Naturhistorisches Museum Wien |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Polyphaga |
|
SuperFamily |
Byrrhoidea |
|
Family |
|
|
Genus |
Elmomorphus brevicornis Sharp, 1888
| Kodada, Ján, Selnekovič, Dávid, Jäch, Manfred A., Goffová, Katarína & Vďačný, Peter 2021 |
Elmomorphus brevicornis
| Devi M. B. & Devi O. S. & Wanghengbam L. 2016: 371 |
Elmomorphus brevicornis
| Nakajima J. & Hayashi M. & Ishida K. & Kitano T. & Yoshitomi H. 2020: 177 |
| Yoshitomi H. & Haga K. 2018: 341 |
| Jung S. W. & Bae Y. J. 2014: 2 |
| Kamezawa H. & Nomura S. 2013: 29 |
| Hayashi M. 2011: 93 |
| Yoshioka M. 2007: 236 |
| Kodada J. & Jach M. A. 2006: 442 |
| Hayashi M. & Shimada T. 2006: 129 |
| Jach M. A. 1984: 314 |
| Sato M. 1981: 53 |
| Deleve J. 1968: 151 |
| Chujo M. & Sato M. 1964: 193 |
| Bollow H. 1940: 59 |
| Kono H. 1934: 125 |
| Zaitzev P. 1910: 19 |
Elmomorphus brevicornis
| Sharp D. 1888: 243 |
Helichus
| Lewis G. 1879: 12 |