Metaconchoecia Howe, 1955
|
publication ID |
https://doi.org/ 10.11646/zootaxa.2857.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/03DC87FD-EC1F-FFC6-6FDD-F8DDA36DFDD1 |
|
treatment provided by |
Felipe |
|
scientific name |
Metaconchoecia Howe, 1955 |
| status |
|
Genus Metaconchoecia Howe, 1955 View in CoL
Synonymy.
1890 Conchoecia roduntata —Müller, p. 275.
1894 Conchoecia rotundata —Müller, p. 229.
1906a Conchoecia rotundata group—Müller, p. 79 (part).
1909 Conchoecia rotundata —Fowler, p 249.
1920 Rotundata group—Skogsberg, p. 648 (part).
1949 Metaconchoecia —Granata & Caporiacco, p. 15 (name unavailable; no type species designated).
1955 Metaconchoecia, Howe , p. 118.
1968 Conchoecia rotundata group—Deevey, p. 50 (part).
1973 Metaconchoecia —Poulsen p. 70 (part) (nomen nudum).
1978a Conchoecia rotundata Group—Deevey, p. 53 (part).
1980 Conchoecia rotundata group—Deevey & Brooks, p. 85 (part).
1981 Conchoecia skogsbergi species complex—Gooday, p. 140, 141 (part), 159 (part), 160.
1986 Metaconchoecia Granata & Caporiacco —Kempf, p. 498 (part).
1992 Metaconchoecia —Kock, p. 68. Predated by Howe 1955.
1995 Metaconchoecia Kock —Kempf, p.149 (part).
1999 Metaconchoecia — Angel Figs 9.58 View FIGURE 9 –76 (part).
2003 Metaconchoecia —Chavtur, p 2.
Nomenclatural issues. The proper authority for this genus has remained a problem for many years. In their original use of the name Granata and Caporiacco (1949) failed to designate a type species, hence technically rendering the generic name unavailable. The next use of the generic name was by Howe (1955) when he drew up a list of ostracod genera for a handbook for geologists. His entry for Metaconchoecia (p.118) reads as follows:
‘ METACONCHOECIA [Genotype not stated but genus set up for M. macromma , M. pusilla , M. glandulosa , M. kyrtophora , M. nasotuberculata , M. rotundata , M. isocheira , all of which were originally described by G.W. Müller as Conchoecia . Of these M. rotundata is discussed in detail and may be considered as their genotype] Granata and Caporiacco 1949, p.12, 15, 22 [ Halocypridae by G&C] Recent, Atlantic.’
Kempf (1986) in his Index and Bibliography of Marine Ostracoda listed the genus, giving M. rotundata ( Müller 1890) as the type species, but without other comment. We note that Müller (1890) in his original description of Conchoecia rotundata gives the name as Conchoecia roduntata in the text and in the caption for his figures 41–43, but Conchoecia rotundata in the caption for his figure 44. In subsequent publications Müller consistently uses ‘ rotundata’, so we regard the spelling ‘ roduntata’ as a lapsus calami under Article 32.5 of the International Code of Zoological Nomenclature.
Kock (1992) when he published the first adequate description of the genus Metaconchoecia , designated Conchoecia fowleri ( Gooday 1981) as the type species. Kock’s designation was clearly pre-empted by Howe’s (1955), moreover his designation of M. fowleri ( Gooday 1981) would seem inappropriate as this species was unknown when Müller (1906a) first established his ‘ rotundata ’ group. It is clear from his name for the group that Müller regarded Conchoecia rotundata Müller 1890 as the typical form. Kempf (1995) subsequently not only accepted Kock (1992) as the authority for the genus, but also automatically accepted Kock’s designation of M. fowleri as the type species. Herein, we regard Howe’s (1955) designation of M. rotundata as the type species as being in full accord with the rules of nomenclature, so that Howe 1955 is the appropriate authority for the genus. The situation is even more complicated because Müller (1890) first described the species (females 1.15 mm long) from samples, which had been collected at two localities in the Pacific at 13°N 120°W and 1°S 100°W. He (1894) later described two sizes of Conchoecia rotundata from the Mediterranean—a short form which has a length of <1 mm and a long form. So the probability is that neither of the two forms from the Mediterranean is conspecific with the original concept of C. rotundata , and until material from the type locality in the tropical Pacific is examined uncertainty will prevail.
Type species. Conchoecia rotundata Müller 1890 View in CoL .
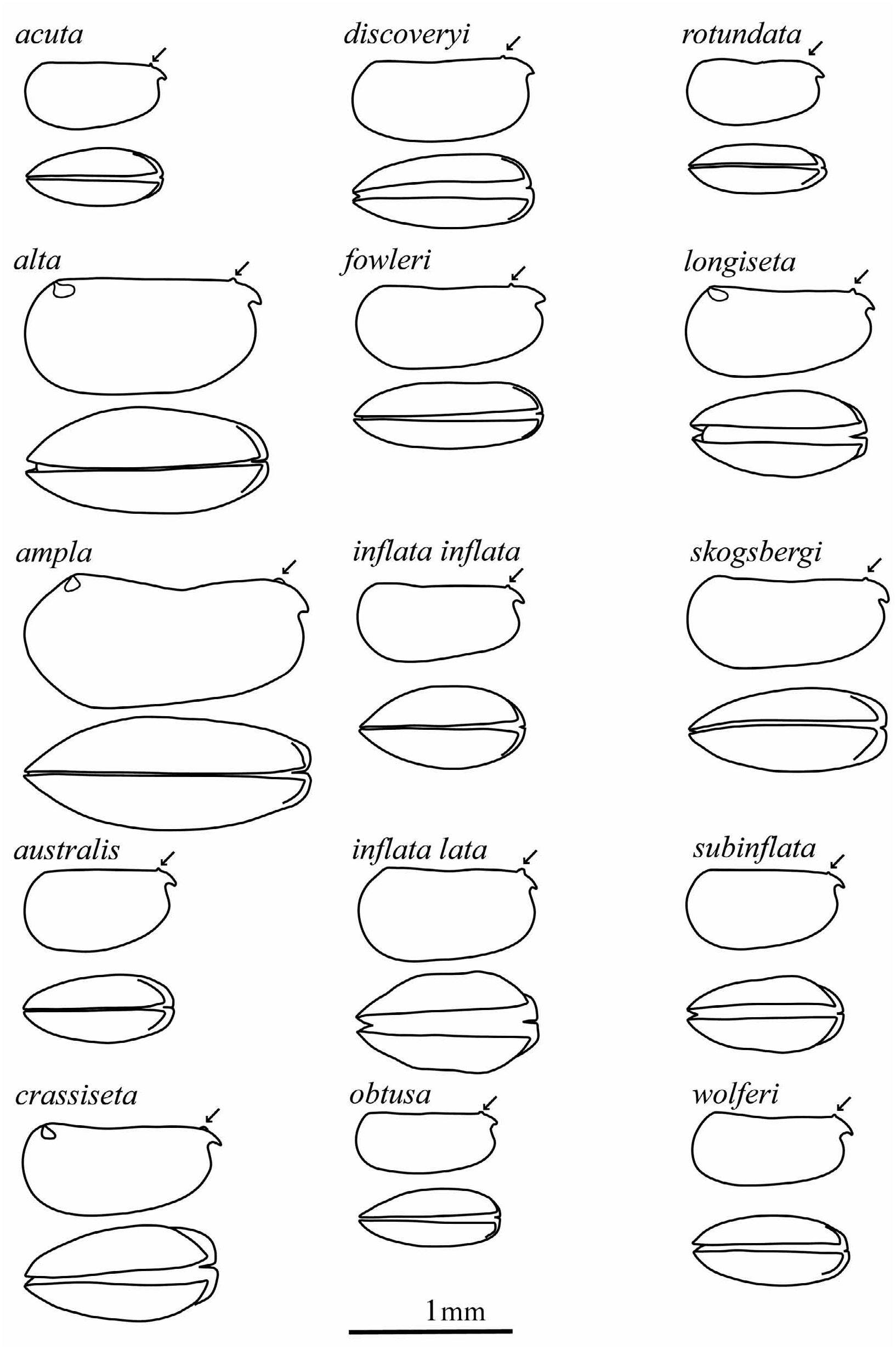
Composition. Genus includes 14 described species, 2 described subspecies listed by original binomen (see Table 2 for later combinations) and 5 putative species or subspecies.
Conchoecia acuta Gooday, 1981 .
Metaconchoecia sp.1 aff. C. acuta ( Gooday, 1981) (= “Broad Form” of Gooday 1981).
Metaconchoecia sp.2 aff. C. acuta ( Gooday, 1981) (= “Narrow Form” of Gooday 1981).
Metaconchoecia alta Chavtur, 2003 View in CoL .
Metaconchoecia ampla Chavtur, 2003 View in CoL .
Conchoecia australis Gooday, 1981 .
Metaconchoecia crassiseta Chavtur, 2003 View in CoL .
Conchoecia discoveryi Gooday, 1981 .
Conchoecia fowleri Gooday, 1981 .
Conchoecia fowleri Form A of Gooday 1981.
Conchoecia inflata Gooday, 1981 .
Metaconchoecia inflata lata Chavtur, 2003 View in CoL .
Metaconchoecia longiseta Chavtur, 2003 View in CoL .
Conchoecia obtusa Gooday, 1981 .
Conchoecia rotundata Müller, 1890 View in CoL .
Conchoecia skogsbergi Iles, 1953 .
Conchoecia sp. aff. C. skogsbergi ( Iles, 1953) (= Conchoecia skogsbergi sensu Angel 1968 ).
Conchoecia subinflata Gooday, 1981 .
Metaconchoecia View in CoL sp. nov. 1 sensu Chavtur 1992.
Metaconchoecia View in CoL sp. nov. 6 sensu Chavtur 1992.
Conchoecia wolferi Gooday, 1981 .
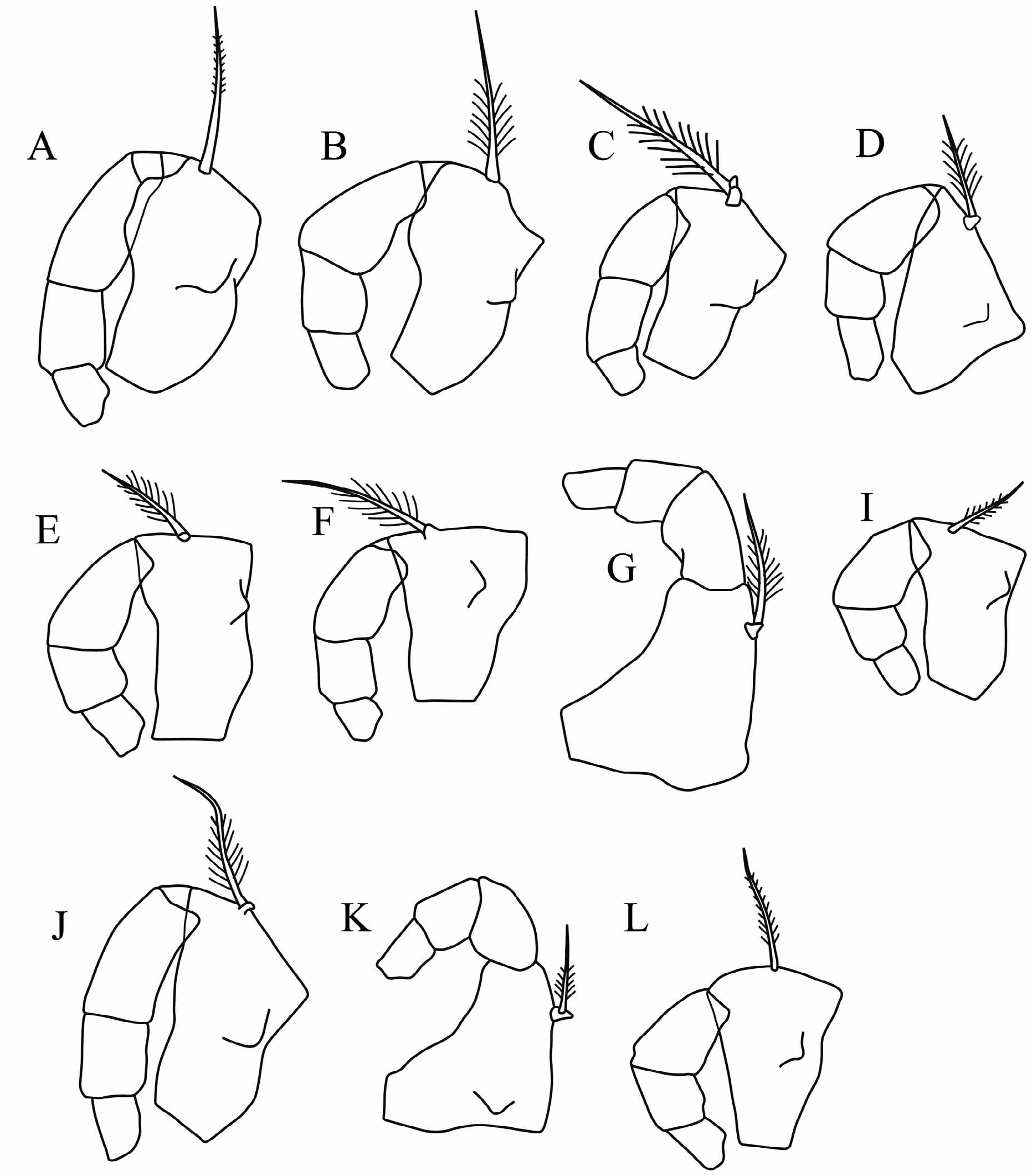
The changes in the classification of these species are summarized in Table 2. The carapace outlines of females (lateral and ventral) are illustrated in Figure 3 View FIGURE 3 , all drawn to the same scale.
Differential diagnosis. Metaconchoecia species are small to medium length (0.71–1.75 mm) halocyprids that are cylindrical to globose in shape (length:height ratio ranging from 35–56%). The LAG opens on the dorsal margin 7–17%CL behind the tip of the rostrum. The RAG opens <7%CL behind the posterior end of the hinge, and almost level with the dorsal margin. In males the capitulum of the frontal organ has a concavity near its base on its dorsal margin, and the A1 e-seta armature consists of 9–19 pairs of slim spines that in some species become alternate proximally. The female frontal organ is fused into a single structure with the capitulum region shaped like a scalpel.
Redescription. Males. Carapace. Length range 0.71–1.73 mm. The carapace is relatively elongate. The maximum height of 42–56%CL is located posterior to mid-length, so in lateral aspect the carapace tapers anteriorly. In specimens that have not splayed during preservation the breadth of the carapace is always slightly less than the height (29–54%CL). In most species in ventral aspect the carapace curves smoothly in a single arc from the posterior margin to the rostrum ( Fig. 3 View FIGURE 3 ), and the rostra are always clearly visible. The LAG generally opens 7–10%CL posterior to the tip of the rostrum (i.e. posterior to both the hindmost margin of the rostral incisure and the anterior end of the carapace hinge), but in M. discoveryi it opens further back at 14–17%CL (i.e. slightly closer to the rostral tip than reported by Gooday (1981)). The RAG opens close to the posterior dorsal corner, <1.0–6.5% CL below the dorsal margin. The rostrum is relatively small and short, the distance from the tip of the rostrum to the anterior end of the carapace hinge is 5–11.5%CL, and from the tip of the rostrum to the inner face of the incisure it is 3–7%CL. All the species lack surface ornamentation.
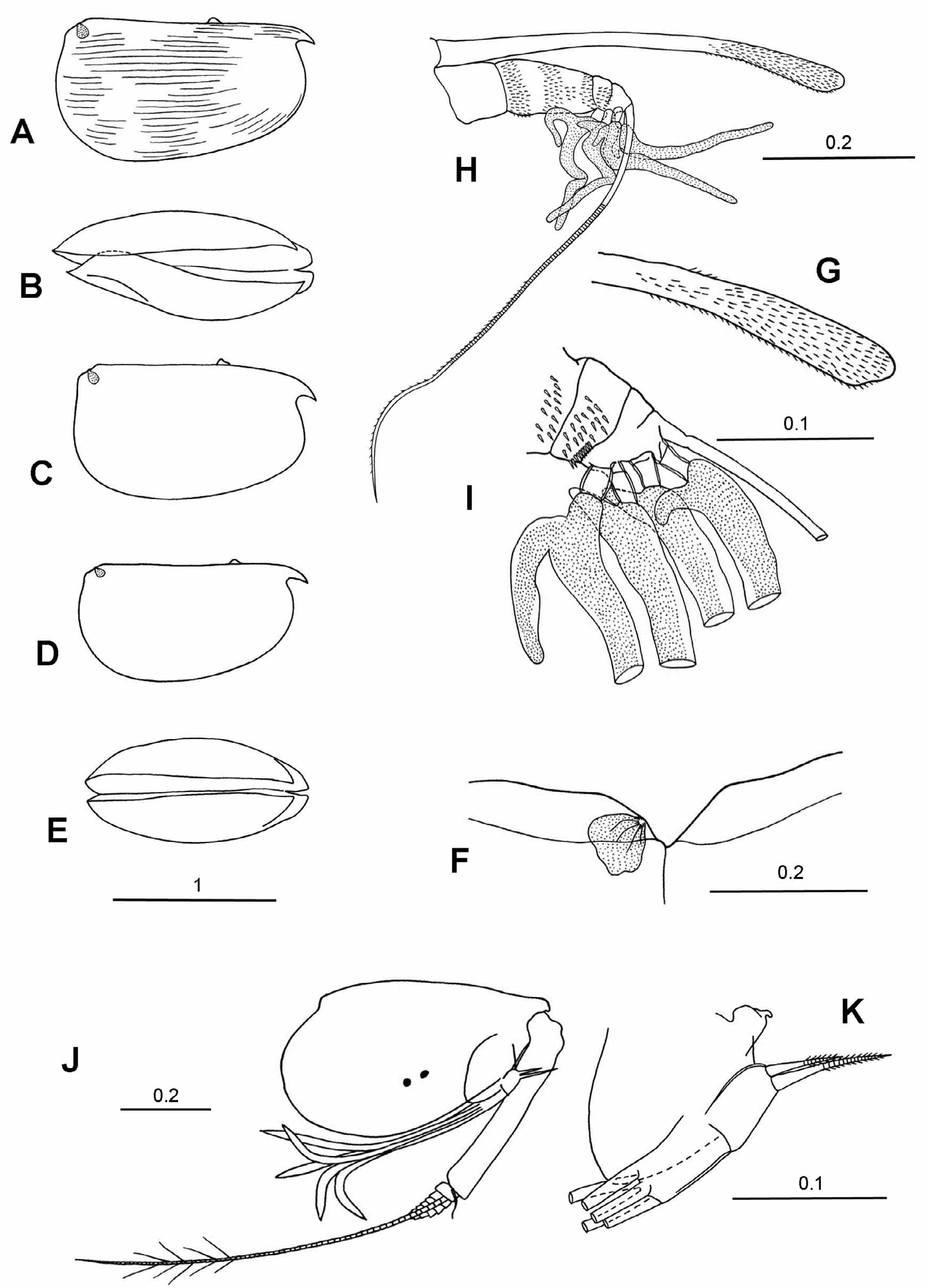
Frontal organ. The shaft is slightly shorter or similar in length of the A1. The capitulum is short, broad, distally tapered and rounded. It extends beyond the end of the A1. The shape of the capitulum varies, but in all species there is a concavity near its base on its dorsal side, which is opposite to a deep convex protuberance on the ventral edge.
First antenna. Its length is 26–42%CL and 3–3.5 times longer than the capitulum of the frontal organ. The aseta is S–shaped, swollen at its base, and lacks supplementary filaments. It is relatively long (14–39%CL, mean 26%) and in some species extends beyond the base of the limb. The c-seta is very short (2.5–6.5%CL), and is shorter than the combined length of the third, fourth and fifth segments. The e-seta armature consists of 7 to 17 pairs of spines, but in five of the species the spines are arranged in pairs distally but become alternate proximally. The spines are generally long, slim and point basally, but in a few species they are short and stout. The e-seta is the longest of the setae on the limb (38–67%CL and 1.4–2 times the length of the limb). The b- and d-seta are subequal and are only slightly shorter than the e-seta.
Second antenna. The protopodite is large (41–53%CL), and the first exopodite segment is usually quite thick and relatively long, 58–72% (83% in M. ampla ) of the protopodite. The total length of the exopodite is 19–28%CL. Some species have a short terminal seta on the first exopodite segment that curves around the base of the second segment. This seta has not previously been noted in the literature, so its significance and systematic value can not be assessed at present. The swimming setae are usually slightly longer than the protopodite. On the endopodite the longest seta (g) is almost as long (77 to 97%) as the protopodite and is 34–50%CL. The f-seta is shorter (27– 44%CL), and the sensory h-, i- and j-setae are 10–19%CL. The f- and g-setae may either be cylindrical or terminally slightly flattened; they are always pointed. The h-seta is always simple and undivided. The c- and d-setae differ in length; they vary from being short and slim to being moderately long (i.e. as long as the segment that carries them). The e-seta also varies in size, but is always shorter than the segment on which it is inserted. The hook appendages on both the left and right endopodite are curved and terminally rounded. The hook on the left limb is always smaller and straighter than the one on the right limb.
Labrum. The upper lip or labrum in common with all members of the tribe has a narrow deep cleft. It is fringed with short, stubby filaments.
Mandible. The exopodite ( Boxshall 1998) is present on the dorsal surface of the protopodite either as a small process on which is inserted a long, slim, pappose seta ( Fig. 2G View FIGURE 2 ) or there is just the seta and no process ( Fig. 2A View FIGURE 2 ). Subterminally on the dorsal surface of the first endopodite segment there is a moderately long seta that is usually armed with long setules. On the inner, ventral surface there are two setae, one of which is long and extends well beyond the end of the limb, and the other is shorter and just reaches to the end of the limb. The terminal segment carries seven setae, two of which are claw-like and spinose; the longest seta ranges from 77–107% the length of the endopodite and 17–23% CL. The coxale is long and about 80% the length of the endopodite.
Maxilla. The basale does not carry a seta. The exopodite has a complex structure and was described in detail by Skogsberg (1920). The endopodite is two segmented. The first segment has four setae on its anterior face, three setae on the posterior face, and a single lateral seta on the outer face. Terminally the second segment has three thick-walled hook setae, which are subtended by a pair of inner thin-walled setae.
Fifth limb. The epipodial appendage carries three groups of four plumose setae in all the species examined. The setation of the limb shows minor interspecific differences but these may result from variation in how well the specimens are preserved, dissected and mounted. As discussed in Materials and Methods the segment which carries a long dorsal seta is considered to be the basale of a biramous limb and the long seta to be a vestige of the exopodite. Thus the main component of the limb is a 2 segmented endopodite and is not an exopodite as suggested by Iles (1953) and Skogsberg (1920). The terminal segment carries three setae of which the central seta is always the longest of the three (6.8–8.7%CL). The dorsal seta is only a little shorter (75–97%), and the ventral seta is the shortest, and is more variable in length (37–70% of the central seta).
Sixth limb. The epipodial appendage carries three groups of plumose setae, the dorsal group consisting of 6 or 7 setae, the central group usually 5 setae (4 in two species), and the ventral group 5 setae. The basale (note this is referred to as the first exopodite segment in publications prior to Kornicker 2003) generally has 3 or 4 ventral setae, and often a long lateral seta with long setules, but no dorsal setae (i.e. no vestige of an exopodite). The setae on the endopodite segments are always very short, except for the three terminal setae on the third segment. These are all similar in length and distally carry long secondary filaments. These setae are 36–50%CL and 100–150% the length of the limb.
Copulatory appendage. The length of the appendage is 27–35%CL. In the majority of species it is straight, but is curved in M. australis . The distal half is usually slightly broader than the proximal half. Terminally the appendage is either rounded or bluntly pointed. There are 2 to 7 oblique muscle bands.
Females. Carapace. Lengths range from 0.71–1.75 mm. The carapace is slightly more elongate than in males. The maximum height of 42–55%CL is posterior to mid-length, so that the carapace tapers anteriorly. The maximum breadth is at, or just anterior to mid-length. The LAG opens 9.3–16.5%CL posterior to the tip of the rostrum, and the RAG opens 0–4.2%CL behind the posterior end of the hinge, in much the same positions as in males. The rostra are small and short (LC2 = 5–10.2%CL) and the incisures are shallow (LC3 = 2–8.8%CL). As in the males the lateral flanks curve smoothly from the posterior margin to the rostra in the majority of species. The tips of the rostra are always just visible in ventral aspect. There is no surface sculpture.
Frontal organ. The capitulum and shaft are fused in all species and are difficult to differentiate. The shaft is 19.5–26.5%CL and the capitulum 9.5–12.5%CL. The total overall length of the frontal organ is 31–38%CL. The shaft is always much longer than the limb of the first antenna. The capitulum is scalpel-like and slightly downcurved. Its proximal end is expanded and then it usually tapers to a pointed tip.
First antenna. The segments are always fused and the total length of the limb is 11.5–20.5%CL. The e-seta is 31–38%CL (i.e. 1.5–3x the length of the limb and 1.5–2.5x the lengths of the sensory a- to d-setae). The e-seta has thin, long spines along its trailing edge beyond the ends of the sensory setae. In common with most other species in the tribe, there is no dorsal seta on the second segment, except in two North Pacific species, Metaconchoecia alta and M. longiseta .
Second antenna. The protopodite is 40–49%CL. The exopodite is relatively long (i.e. 64–82% the length of the protopodite) and thick. In some species, the base of the first segment is noticeably enlarged. The terminal setae on the endopodite are thin, parallel-sided and subequal in length, with tips that are either rounded or pointed. They are 52–85% the length of the exopodite, 40–55% the length of the protopodite and 1.6–2.6x the length of the endopodite.
Labrum, Mandible and Maxilla are similar in structure to those of males.
Fifth limb. The epipodial appendage has three groups of 4 + 4 + 4 setae. The basale (considered to be the first endopodite in Skogsberg 1920 and Iles 1953) carries a group of three setae at mid-length and a single subterminal seta. Laterally there are two setae and also a long seta with long setules, which can appear to be inserted dorsally depending on how the limb is orientated. Dorsally there is a long subterminal seta that extends beyond the end of the limb. The first endopodite segment carries a pair of ventral setae and a single dorsal seta; all three extend beyond the end of the segment. The third segment carries three terminal setae with the central seta being the longest and close to 7.5%CL. The dorsal and ventral setae are respectively 75–90% and 37–60% the length of the central seta.
Sixth limb. The epipodial appendage also has three groups of (6 or 7) + 5 + 5 setae. The dorsal group consists of six long setae, which are supplemented with an additional short seta in some specimens. The basale carries 3 or 4 ventral setae. Laterally there is a long seta with long setules in about half of the species, but the presence or absence of this seta is too uncertain to be of value for identification. There is no dorsal seta. The first endopodite segment has only a very short ventral seta. The second segment carries a dorsal seta and a ventral seta inserted at mid-length; both are very short in all species. The terminal segment has three unequal setae. The central seta is always the longest and ranges in length from 9–13%CL and 62–85% of the limb. The dorsal seta is always longer than the ventral seta.
Distribution. The genus has been reported from all oceans, from epipelagic to abyssopelagic depths (surface to 5000m). There are relatively few records from> 1000m, but this is probably a reflection of the paucity of samples collected from bathypelagic and abyssopelagic depths. In the Atlantic Ocean the genus occurs in all areas and peripheral seas that have been investigated, including the Sargasso, Caribbean and Mediterranean Seas. Its previously reported bathymetric range is from the surface to 3600m ( Müller 1890, 1894, 1906a; Vávra 1906; Fowler 1909; Granata & Caporiacco 1949; Iles 1953; Angel 1968a,b, 1969a,b, 1977, 1979, 1981b, 1983a; Deevey 1968, 1970, 1971, 1978b; Angel & Fasham 1975; Deevey & Brooks 1980; Gooday 1981; Gonzales & Breman 1982; Ellis 1985; Angel et al. 2007). However, most of the early pre-1970 records were collected with open nets and so the true depths of occurrence are uncertain. During a recent Polarstern cruise in the tropical and subtropical Eastern Atlantic specimens were caught in nets towed at depths of 4000–5000m (unpublished data). In the Indian Ocean it has been reported from all latitudes from the Arabian Sea and the Bay of Bengal in the north to the Southern Ocean, at depths between 0–3423m ( Müller 1906a, 1908; James 1975; George & Nair 1980; Hanai et al. 1980; Gooday 1981), however it is absent from midwater depths where there is oxygen-depletion (Graves, personal communication). It has been found in all areas of the Pacific Ocean investigated, from the Aleutian Islands to the Southern Ocean, from a wide depth range from surface to 7000m ( Müller 1906b; Rudjakov 1962; Poulsen 1973; Chavtur 1977a, b, c, 1992; Deevey 1978a, 1982a, 1983; Martens 1979; Hanai et al. 1980; Gooday 1981; Chen et al. 1983; Chen & Lin 1995). In addition, species have been reported from the Bering Sea and the Sea of Okhotsk ( Chavtur & Shornikov 1974; Chavtur 1976, 1977b, c, 1992). In the Southern Ocean species are common in all sectors as far south as 78ºS occurring within the depth range 0–5190 m ( Müller 1906c, 1908; Skogsberg 1920; Hillman 1967, 1968, 1969; Deevey 1974, 1978a, 1982a, 1983; Hopkins 1985; Hopkins & Torres 1989; Naldi et al. 1992; Benassi et al. 1992; Kock 1992; Blachowiak-Samolyk 1999, Blachowiak-Samolyk & Zmijewska 1995, 1997; Blachowiak-Samolyk & Angel 2003, 2007; Chavtur & Kruk 2003). In the Arctic Ocean it has been recorded from an Ice Island station in the Central Arctic at 85ºN, 91ºW but from an unknown depth ( Leung 1972, 1973, 1975). It has be recorded from 66ºN, 2ºE in the Norwegian Sea at a depth of 100–600m ( Angel 1968a), but it has not been found in the Atlantic inflow water off Svalblad, despite intensive sampling (Blachowiak-Samolyk, personal communication), nor was it present in samples collected in the Canada Deep at 79°N to the north of the Bering strait (ARCOD samples, unpublished).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |