Lygodactylus madagascariensis (Boettger, 1881)
|
publication ID |
https://doi.org/ 10.13140/rg.2.2.11871.87201 |
|
persistent identifier |
https://treatment.plazi.org/id/039D87D5-0F37-FFD6-FF0D-2DFDED8AFBE1 |
|
treatment provided by |
Felipe |
|
scientific name |
Lygodactylus madagascariensis |
| status |
|
Lygodactylus madagascariensis group
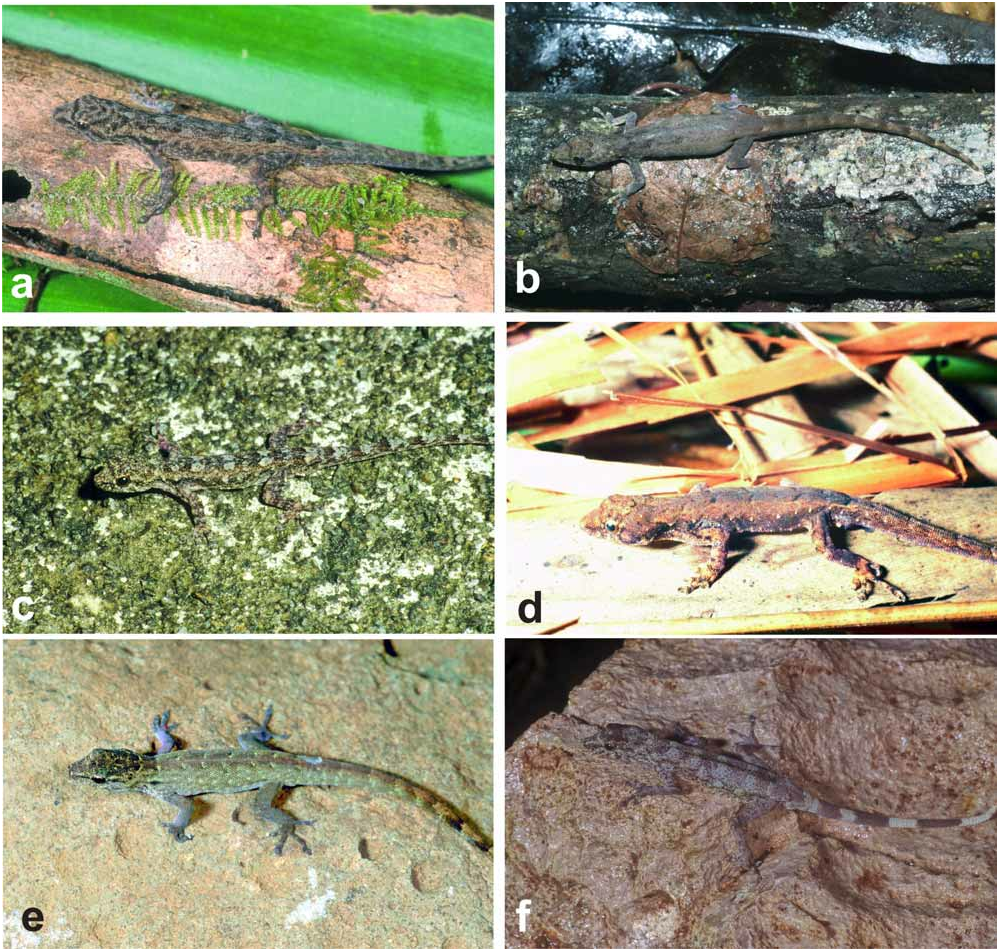
Contains L. expectatus , L. guibei , L. madagascariensis (with subspecies L. m. petteri), L. miops , and L. rarus . This is a well defined species group that previously ( Pasteur 1964) has been described as subgenus Domerguella and in a preliminary molecular study ( Puente et al. 2005b) was confirmed to be monophyletic. The group is characterized by an undivided mental scale with usually two postmentals (unique among Malagasy Lygodactylus ), by the absence of a claw on the first finger, by the presence of postanal sacs and 5–7 preanal pores in males. Hemipenial structures are characteristic as well, bilobed but the two lobes being rather short, and lacking fields and serrated ridges with pointed papillae which in other species groups make up longitudinal serrated ridges. The tail is without whorls. Typically, the digits on fore- and hindlimbs are elongated in their distal part that bears the claw, as compared to most other Malagasy Lygodactylus . There is no dorsal colour or pattern that would be diagnostic for the whole group (see Fig. 6 View FIGURE 6 ), but some species have characteristic patterns, such as the distinct broad crossbands on the tail of L. rarus and a pair of blackish spots on the neck, typical for L. expectatus .
Lygodactylus expectatus Pasteur & Blanc, 1967
( Fig. 7 View FIGURE 7 )
Lygodactylus expectatus Pasteur & Blanc, 1967 .— Name-bearing type: male holotype MNHN 1990.1 About MNHN (original number BP 640), “(7 pores préanaux) à queue partiellement régénérée et à corps de 31 millimètres” according to the original description.— Type locality: “Karst d’Ambilobe (Ankarana), à une douzaine de kilomètres au NNW de cette localité“, according to the original description.— Other types: according to the original description, five specimens were examined but explicitely only two of these were designated as paratypes, namely MNHN 1990.2 About MNHN – 3 About MNHN (BP 641, female, and BP 642, young female, according to original description).— Etymology: From Latin expectatus = expected. As explained in the original description, G. Pasteur and C.P. Blanc were expecting to find a new species, closely related to L. madagascariensis , in the karstic regions of Ambilobe.
Diagnosis. An apparent endemic of limestone karst areas of northern Madagascar, rather small-sized compared to related species. It can clearly be assigned to the L. madagascariensis group by sharing the characters listed in the group definition above. It differs from all species in the L. madagascariensis group by its dorsolateral scales which are enlarged relative to the dorsal and lateral scales (not distinctly enlarged in the other species), and by the presence of two dark spots in the region of the neck (not distinct in the other species). L. expectatus also appears to attain smaller maximum sizes (adult SVL 27.0– 29.7 mm vs. a maximum size larger than 30 mm in all other species in the group). Furthermore distinguished from L. miops and especially L. guibei by the absence (vs. presence) of dorsolateral tubercles and spiny tubercles at the tail base, and from L. rarus by the absence of distinct broad crossbands on tail.
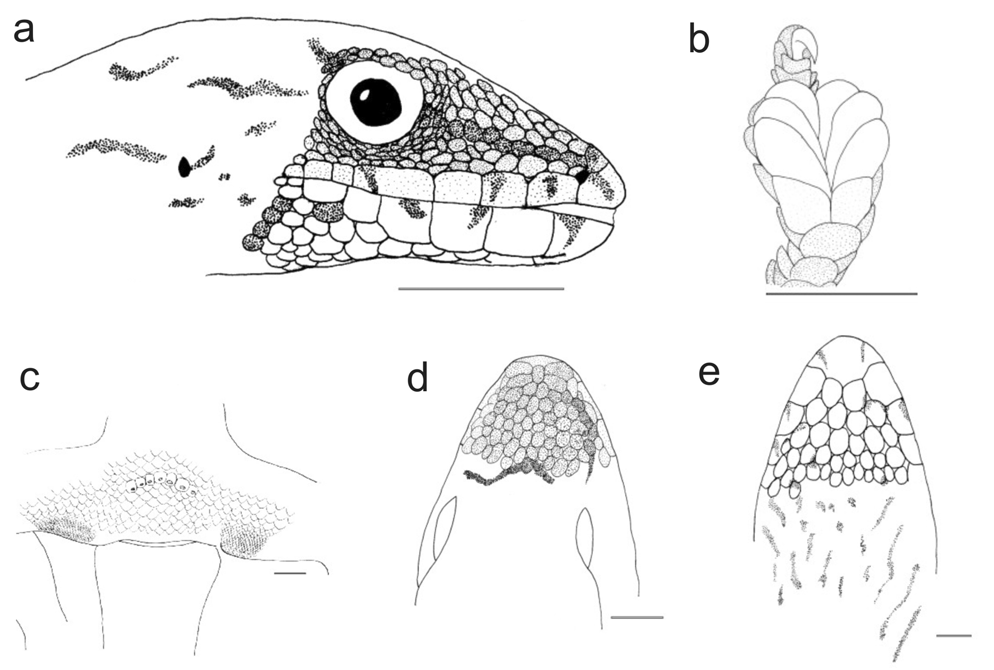
Description. (1) An average-sized Lygodactylus , adult SVL 27.0– 29.7 mm (27.0± 1.07 mm, n=6); (2) TAL 25.3–29.7 mm (28.9 ± 5.16 mm, n=2); (3) granular dorsal scales; (4, 5) first finger present, without a claw; (6) three pairs of subdigital lamellae on the fourth toe (n=9); (7,8) mental scale undivided, its posterior projection in contact with first infralabial scale; (9, 10) two symmetrical postmental scales (n=8); (11) four postpostmental scales (n=8); (12) 5–6 infralabial scales (5.38±0.51, n=8); (13) 5–7 supralabial scales (6.00±0.53, n=8); (14) 1–2 internasal scales (1.13±0.35, n=8); (15) 6–7 preanal pores (6.33±0.57, n=3); (16) tail without whorls but with 6–8 rings of slightly different colour giving the impression of whorls; (17, 18) dorsolateral tubercles absent; (19, 20) beige-brownish dorsal colour, but without a clearly defined pattern; usually two dark spots with a white border on both sides of the neck; (21) light ventral colour; (22) 113–145 dorsal scales along the body (n=2); (23) 57–63 dorsal scales around the body (n=2) ( Figs. 6e View FIGURE 6 , 7 View FIGURE 7 ).
Material examined. MNHN 1933.168 About MNHN (Ankarana, cave entrance) ; MNHN 1990.1 About MNHN – 3 About MNHN (holotype and paratypes, G. Pasteur and C.P. Blanc, 11 Nov. 1966, karst d'Ambilobe [Ankarana]) , UADBA 6059 View Materials (Ankarana) , ZSM 282 View Materials /2004, 284/2004 (F. Glaw, M. Puente & R. Randrianiaina, 26 Feb. 2004, Ankarana , near Point de Vue Petit Tsingy) .
Distribution. According to the specimens examined in this study, Lygodactylus expectatus is only known from the type locality, namely the Tsingy limestone formations of Ankarana, in the Ambilobe region. It remains to be assessed if within this area, the species is localized or widespread. A further locality mentioned in the literature is the Diego-Suárez region ( Pasteur & Blanc 1967), but it is uncertain whether this refers to Ankarana (located south of Antsiranana = Diego Suarez), or to another karstic area in this region.
Habitat. According to Pasteur & Blanc (1967), L. expectatus occurs in the shelter of vegetation in rocky areas. We found this species at Ankarana during the day on trees around the karstic rocks, at ca. 1–2 m from the ground (M. Puente & F. Glaw, personal observation).
Lygodactylus guibei Pasteur, 1964
( Fig. 8 View FIGURE 8 )
Lygodactylus guibei Pasteur, 1964 .— Name-bearing type: the holotype has the provisional number BGP 198 and is a male “à 7 pores et queue partiellement coupée (corps: 30 mm)”, according to the original description (not examined by us); holotype number is MNHN A.60 according to Krüger (2001) which, however, must refer to another provisional number.— Type locality: “Périnet (Est)” (=Andasibe), according to the original description.— Other types: according to the original description, there were two paratypes; only one of these could be located and examined, the male MNHN 1933.156.— Etymology: dedicated to J. Guibé.
Diagnosis. An apparently rather localized forest-dwelling species from mid-altitudes in the Northern Central East of Madagascar. It can clearly be assigned to the L. madagascariensis group by sharing the characters listed in the group definition above. L. guibei is distinguished from L. madagascariensis , L. expectatus , and L. rarus by the presence of distinct dorsolateral tubercles and lateral spiny tubercles on the base of the tail (vs. absence); from L. expectatus by non-enlarged dorsolateral scales (vs. enlarged), from L. rarus by the absence of broad and distinct crossbands on tail (vs. presence), and from L. miops by a stronger expression of dorsolateral tubercles and spiny tubercles on tail base, and by usually four postpostmentals (vs. usually five). Furthermore, the head of L. guibei appears to be rather massive compared to other species (including L. miops ), but this character needs to be validated by further studies.
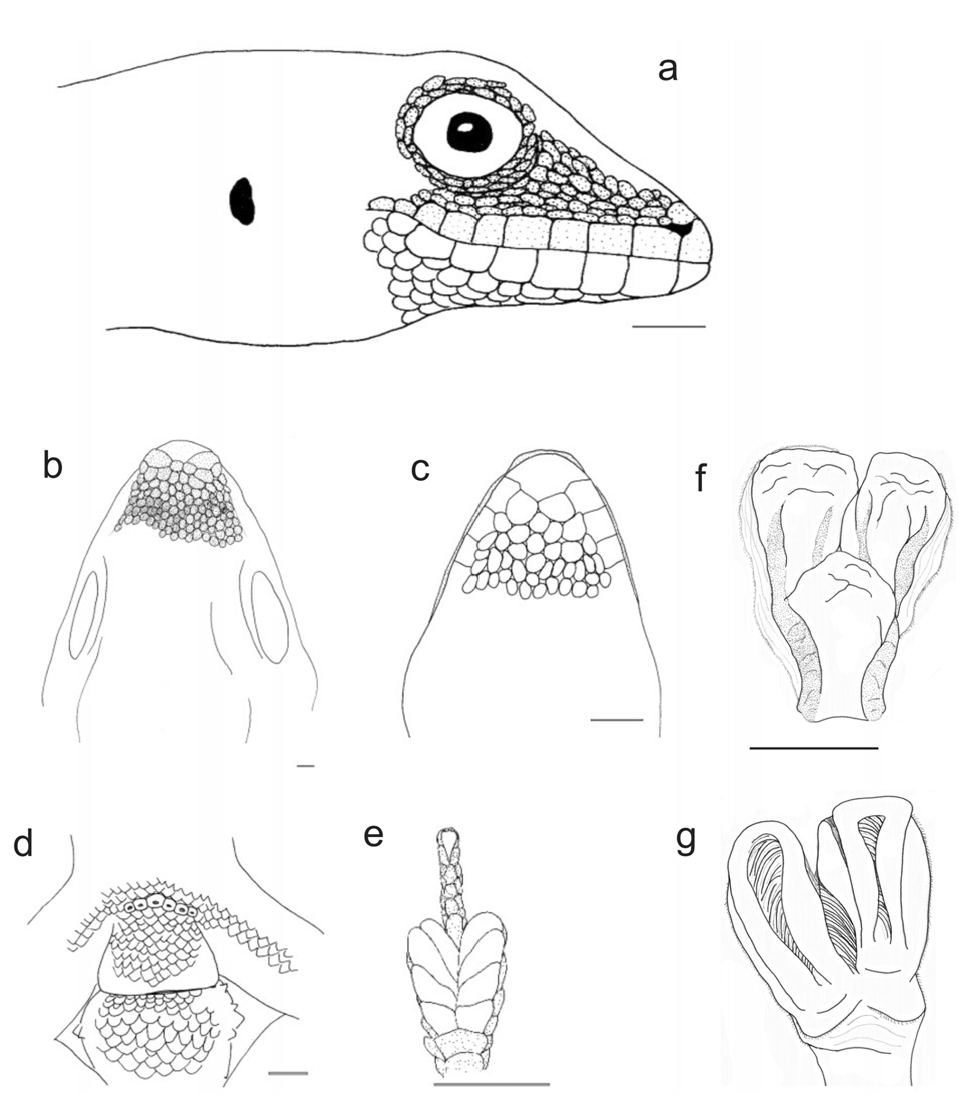
Description. (1) A moderately large Lygodactylus, SVL of adults 28.6–38.3 mm (33.2± 3.2 mm, n=15); (2) TAL 31.3–46.5 mm (40.0± 4.1 mm, n=10); (3) granular dorsal scales; (4, 5) first finger present, without a claw; (6) usually three, sometimes four pairs of subdigital lamellae on the fourth toe (3.13±0.35, n=15); (7,8) mental scale undivided, its posterior projection without distinct contact to infralabial scales; (9, 10) 2–3 postmental scales (2.13±0.35, n=15), usually asymmetrical; (11) up to six but usually four postpostmental scales (4.20±0.56, n=15); (12) 5–7 infralabial scales, (5.80±0.56, n=15); (13) 6–8 supralabial scales (6.60±0.63, n=15); (14) 1–2 internasal scales (1.73±0.45, n=15); (15) 6–7 preanal pores (6.25±0.50, n=4); (16) tail without whorls but with 10–14 rings of slightly different colour giving the impression of whorls; (17, 18) usually with dorsolateral tubercles, each containing one spiny scale; postanal sacs prominent with one big spiny scale on each sac; (19, 20) dorsally beige-brownish without a clearly defined pattern; (21) ventrally light; (22) 143–186 dorsal scales along the body (168.00±22.33, n=3); (23) 80–86 dorsal scales around the body (n=2); (24) 94 ventral scales (n=1) ( Figs. 6d View FIGURE 6 , 8 View FIGURE 8 ).
Material examined. MNHN 1933.156 About MNHN (paratype, Moramanga ) , MNHN 1990.1853 About MNHN – 1855 About MNHN (C. Domergue, Nov. 1965, Andasibe ) , MNHN 1990.3567 About MNHN (18 Nov. 1986, Ambohitantely ) , MNHN 1990.3568 About MNHN (28 Feb. 1987, Ambohitantely ) , MNHN 1993.60 About MNHN (C. Domergue, 31 Oct. 1960, Andasibe ) , ZMA 19631 (F. Glaw & M. Vences, Feb. 2003, Vohidrazana ) ZFMK 17711 About ZFMK ( Andasibe ) , ZFMK 51873 About ZFMK (Wasserthal, Nov. 1989, Andasibe ) , ZFMK 53945 About ZFMK (F. Glaw & J. Müller, Jan. 1992, Andasibe ) , ZFMK 61534 About ZFMK (J. Krüger, 15–16 Sep. 1994, Andasibe ) , ZFMK 61536–61537 About ZFMK ( Andasibe ) , ZFMK 62553 About ZFMK (K. Liebel, Andasibe ) ; ZMA 19631 ( Andasibe ) , ZSM 5117 View Materials /2005 (F. Glaw, R. Randrianiaina & R. Dolch , 8 Feb. 2005, Andasibe) .
Distribution. According to the specimens examined in this study, Lygodactylus guibei is known from the following localities: (1) Andasibe (type locality), (2) Ambohitantely, (3) Moramanga, and (4) Vohidrazana. No further localities are mentioned in the literature.
Remark. Andasibe (including the nearby sites Vohidrazana and Moramanga) and the isolated locality Ambohitantely are geographically at a distance of about 150 km from each other. We could not identify any relevant morphological or meristic difference among specimens from these two areas. However, considering that haplotypes of several widespread species such as Trachylepis gravenhorstii and Phelsuma lineata differ distinctly between these two areas, it will be necessary to obtain genetic data for conclusive information on the differentiation of L. guibei from the two populations.
Habitat. According to Pasteur (1964) L. guibei is arboreal. We found this species in rainforest in trees at ca. 1–3 m from the ground during the day; in each tree, only one adult male was found together with several females and juveniles (M. Puente, M. Thomas & R. Randrianiaina, personal observation, 2003) .
Lygodactylus madagascariensis ( Boettger, 1881)
( Fig. 9 View FIGURE 9 )
Scalabotes madagascariensis Boettger, 1881 .— Name-bearing type: male lectotype SMF 8937 (designated by Mertens 1967), collected by A. Stumpff. Krüger (2001) considered SMF 8937 as holotype.— Type locality: Nosy Be; “hab. in insula Nossi-Bé rarus”, according to the original description.— Other types: One paralectoptype (the description was based on two specimens "(2 spec.)" according to the original description).— Etymology: name derived from its general provenance, Madagascar.
Lygodactylus madagascariensis petteri Pasteur & Blanc, 1967 .— Name-bearing type: holotype MNHN 1990.4 About MNHN , female of 36 mm SVL.— Type locality: “Montagne d’Ambre, forêt ancienne-Roussettes” according to the original description.— Other types: two paratypes; MNHN 1990.5 About MNHN , male; and MNHN 1893.194 About MNHN .— Etymology: dedicated to Jean-Jacques Petter.
Diagnosis. This species is the most common forest-dwelling Lygodactylus in the Sambirano region in the far northwestern part of Madagascar, and can clearly be assigned to the L. madagascariensis group by sharing the characters listed in the group definition above. It can reach rather large sizes of 35 mm SVL (37 mm in the subspecies petteri). From L. miops which is common in eastern rainforests (reaching the North East region), L. madagascariensis differs by lacking enlarged tubercles at the base of the tail as well as enlarged dorsolateral tubercles, characters that are often visible in L. miops , and by having four postpostmentals scales (usually five in L. miops ). L. guibei has very distinct dorsolateral tubercles and spiny tubercles at the base of the tail (absent in L. madagascariensis ). L. expectatus often has two dark spots on the neck (absent in L. madagascariensis ), and is characterized by the size of the dorsolateral scales which are larger than the vertebral and lateral ones (not distinctly enlarged in L. madagascariensis ). L. rarus has a typical colour pattern with a few distinct light-dark crossbands on the tail.
Description. Referring to specimens excluding those from the Montagne d'Ambre region that are assigned to L. m. petteri (see below). (1, 2) A moderately large Lygodactylus , adult SVL 26.8–36.8 mm (30.60± 1.99 mm, n=17); TAL 26.6–39.0 mm (33.37 ± 3.92 mm, n=11); (3) granular dorsal scales; (4, 5) first finger present, without claw; (6) usually three pairs of subdigital lamellae on the fourth toe (3.35±0.49, n=17); (7, 8) mental scale undivided, contact between first infralabial scale and posterior projection of mental sometimes existing but not distinct; (9, 10) two postmental scales, asymmetrical or symmetrical; (11) usually four postpostmental scales; (12) 5–8 infralabial scales (6.47±0.71, n=17); (13) 5–8 supralabial scales (6.71±0.84, n=17); (14) 1–3 internasal scales (2.12±0.69, n=17); (15) males with 6–7 preanal pores (6.60±0.54, n=5); (16) tail without whorls but with 6–13 rings of slightly different colour giving the impression of whorls; (17, 18) dorsolateral tubercles absent; (19, 20) dorsal colour beige-brownish but without a clearly defined pattern, sometimes slightly striated; (21) ventrally uniformly whitish, sometimes with indistinct dark spots on the throat; (22) 179–212 dorsal scales along the body (195.17±12.56, n=6); (23) 73–99 dorsal scales around the body (88.89±8.10, n=9); (24) 96–117 ventral scales (104.33±6.74, 96, n=8) ( Figs. 6a, 6b View FIGURE 6 , 9 View FIGURE 9 ).
Hemipenial structure. Based on ZSM 783/2001, adult male from Tsaratanana (but see remark below regarding the possible taxonomic distinctness of this specimen). Hemipenis with a total length of ca. 2 mm; pedicel short (ca. 0.5 mm). The truncus bears small but deep calyces, giving the truncus a papillate appearance. These calyces are absent around the sulcus spermaticus. Fields and serrated ridges with pointed papillae absent. Sulcus spermaticus without well developed sulcal lips and with two channels starting on the pedicel, leading each to a terminal position of one of the apex lobes, respectively. Apex bilobed, formed by two broad lobes of about 1.5 mm length each ( Fig. 9 View FIGURE 9 ).
Material examined. BMNH 1988.9 – 16 (C.J. Raxworthy, 12 Jan.–11 Feb. 1988, Manongarivo Special Reserve ), MRSN R1892 (F. Andreone, 1997, Ambolokopatrika ), MRSN R1909–1910 (F. Andreone, Feb. 1999, Nosy Be ), MRSN R1919 (F. Andreone, Feb. 2001, Tsaratanana ), MRSN R1920 (F. Andreone, Feb. 2001, Tsaratanana ) , SMF 8937 About SMF (lectotype, A. Stumpff, 1881, Nosy Be ) , ZFMK 48241 About ZFMK (Nosy Be, Lokobe ) , ZMA 19370 (M. Vences & F. Glaw, Jan. 2003, Manongarivo ) , ZMA 19581 (M. Vences & F. Glaw, Feb. 2003, Manongarivo ) , ZSM 332 View Materials /2003 (M. Vences & F. Glaw, Feb. 2003, Manongarivo ) , ZSM 782 View Materials /2001 (F. Andreone, J.E. Randrianirina & M. Vences, 2 Feb. 2001, Andampy campsite, Tsaratanana) , ZSM 783 View Materials /2001 (F. Andreone, J.E. Randrianirina & M. Vences, 4–9 Feb. 2001, Antsahamanara campsite, Tsaratanana) , ZSM 813 View Materials / 2003 (M. Vences & F. Glaw, 31 Jan. 2003, Camp Norbert , Manongarivo) , ZSM 832 View Materials /2003 (M. Vences & F. Glaw, 1 Feb. 2003, Manongarivo ) .
Further material (assigned to L. m. petteri; see Variation below): MNHN 1990.4 About MNHN (holotype, J.J. Petter [don. C.A. Domergue], 10 Nov. 1965, Montagne d'Ambre), MNHN 1990.5 About MNHN (paratype, J.J. Petter [don. C.A. Domergue], 10 Nov. 1965, Montagne d'Ambre), MNHN 1893.194 About MNHN ( Pasteur & Blanc, 1967, Mararaomby, Montagne d'Ambre), ZSM 914 View Materials /2003 (F. Glaw, R. D. Randrianiaina & A. Razafimanantsoa, 17–19 Feb. 2003, Montagne d’Ambre,), ZSM 270 View Materials /2004 (F. Glaw, M. Puente, R. Randrianiaina & A. Razafimanantsoa, 20 Feb. 2004, Montagne d’Ambre) .
Distribution. According to the specimens examined in this study, Lygodactylus madagascariensis is known from the following localities: (1) Nosy Be (type locality), (2) Ambolokopatrika, (3) Manongarivo, (4) Tsaratanana. A further probable locality is (5) the Tsingy de Bemaraha (see remark below). Furthermore, the subspecies L. m. petteri occurs at (6) Montagne d'Ambre in northernmost Madagascar.
Angel (1942) mentions further localities which almost certainly all refer to other species and are not considered here: "Andrahomana; Antanimora; Amboasary; Ivohibe; Karianga; Ifandana; Moramanga, grotte de l'Ankarana, district d'Ambilobe; Suberbieville; Ambongo; Ravin d’Ianzamaly; Tsivono; Tuléar; Vallée de l’Onilahy; Nossi Trozona; forêts de l'Ikongo, Mandritsara."
Habitat. At Manongarivo, we found specimens on trees in disturbed low-altitude rainforest, usually close to rivers ca. 1–1.5 m from the ground (F. Glaw and M. Vences, personal observation, February 2003).
Variation. Based on a number of morphological characters (body size, size of scales, coloration), Pasteur & Blanc (1967) considered the population of L. madagascariensis from Montagne d'Ambre in far northern Madagascar as a distinct subspecies, L. m. petteri. In a later study, molecular divergences were observed as compared to specimens from Manongarivo ( Puente et al. 2005b) but a molecular comparison with specimens from the type locality of L. madagascariensis (Nosy Be) is so far missing.
Morphologically, petteri appears to reach larger body sizes (in our sample, up to a maximum of 36.8 mm vs. 34.5 mm in madagascariensis , mean 35.1 vs. 30.6 mm), and to possibly have fewer ventral scales along the body (73 vs. 96–117). A comprehensive revision of L. madagascariensis is needed to assess the status of the taxon petteri. We here give a separate description based on the material available to us: (1, 2) Adult SVL 33.2–36.8 mm (35.1± 1.52 mm, n=4); (2) TAL 39 mm (n=1); (3) granular dorsal scales; (4, 5) first finger present, without claw; (6) usually three pairs of subdigital lamellae on the fourth toe (3.43±0.53, n=7); (7, 8) mental scale undivided, contact between first infralabial scale and posterior projection of mental sometimes existing but not distinct; (9, 10) two postmental scales, usually of symmetrical shape; (11) four postpostmental scales; (12) 6–7 infralabial scales (6.50±0.54, n=6); (13) 6–8 supralabial scales (6.50±0.54, n=6); (14) 1–2 internasal scales (mean±SD 1.33±0.51, n=6); (15) males with seven preanal pores; (16) tail without whorls but with rings of slightly different colour giving the impression of whorls; (17, 18) dorsolateral tubercles absent; (19, 20) dorsal colour beige-brownish but without a clearly defined pattern, sometimes slightly striated; (21) ventrally uniformly whitish, sometimes with indistinct dark spots on the throat; (22) 190 dorsal scales along the body (n=1); (23) 97 scales around the body (n=1); (24) 73 ventral scales (n=1).
Remarks. The specimen ZSM 783/2001 (Tsaratanana), morphologically similar to L. madagascariensis , seems to be a different species according to its strong molecular differentiation ( Puente et al. 2005b). This specimen is quite similar morphologically to another specimen also collected at Tsaratanana, ZSM 782/2001, which is placed within Lygodactylus madagascariensis in the molecular analysis ( Puente et al. 2005b), but erroneously appears as ZSM 781/ 2001 in Table 1 of that publication.
One specimen clearly assignable to the L. madagascariensis group (ZSM 77/2006) was collected in 2006 by one of us (FG) in Tsingy de Bemaraha National Park in the West region of Madagascar. This is the only reliable record of this whole group from the arid west of Madagascar, but the Bemaraha region is known to harbour faunal elements typical for northern and eastern Madagascar in its relictual humid forest ( Köhler et al. 2007, Glaw et al. 2007, Bora et al. submitted). Since this specimen was collected after the analyses for the present study were completed, we here do not present its detailed morphological data but assign it to L. madagascariensis (rather than to L. miops ) based on the absence of dorsolateral tubercles.
A further specimen from the West is ZFMK 51239 (Ankarafantsika). Although unlikely, this specimen could be related to the L. madagascariensis group. Our own collection in Ankarafantsika (DRV & MV in 2001) only yielded L. tolampyae which is extremely common at this site. The specimen is a female of 29.4 mm SVL, with a broken tail and granular dorsal scales. It has a claw on the first finger of one hand, and three bisymmetrical postmental scales (typically two postmentals and no claw in the L. madagascariensis group). Its mental scale is divided on one side (undivided in the L. madagascariensis group). However, the medial contact between first infralabial and posterior part of mental scale is not distinct, while it is very distinct in L. tolampyae , the other Lygodactylus known from Ankarafantsika. Further characters: five postpostmental scales; five infralabial scales; six supralabial scales; one internasal scale; dorsolateral tubercles absent; dorsal colour brown-beige, with an indistinct pattern of longitudinal stripes; ventral colour light. Thus only the mental scale that on one side is undivided indicates a possible affinity to the L. madagascariensis group, but assigning the specimen to any other Lygodactylus species is not readily possible.
Lygodactylus miops Günther, 1891
( Fig. 10 View FIGURE 10 )
Lygodactylus miops Günther, 1891 .— Name-bearing type: holotype, BMNH 1946.8.22.55, female, 31.8 mm SVL.— Type locality: “Senbendrana", Madagascar according to the original description, (probably referring to Sahembendrana; see Blommers-Schlösser & Blanc 1991).— Other types: none according to original description.— Etymology: no data from the original description.
Microscalabotes spinulifer Boettger, 1913 .— Name-bearing type: lectotype (designated by Mertens 1967), SMF 8931 (F. Sikora, Moramanga).— Other types: 2 or 3 paralectotypes (in the original description Boettger (1913) mentioned 4 females, but provided measurements only for 3 specimens).— Type locality: “Im Walde bei Moramanga, O. Mad., am Fusse des Ostplateaus" according to the original description.— Etymology: likely referring to the longitudinal rows of dorsolateral tubercles that can give an impression of small spines.
Lygodactylus septemtuberculatus Angel, 1942 .— Name-bearing type: female holotype, MNHN 1893.63 About MNHN .— Type locality: “provenant de l’Est de Madagascar: forêt de Moramanga” according to the original description.— Other types: no further types following the original description.— Etymology: the name derives from the prominent scales (tubercles) on the flanks of the species, according to the original description.
Diagnosis. A common forest-dwelling species in the South East, Southern Central East, Northern Central East, and North East of Madagascar. It can clearly be assigned to the L. madagascariensis group by sharing the characters listed in the group definition above. L. miops differs from other species of the Lygodactylus madagascariensis group, by the number of typically five (vs. four) postpostmental scales. Further distinguished from L. madagascariensis , L. expectatus , and L. rarus by the presence of dorsolateral tubercles and lateral spines on the base of the tail (vs. absence); from L. expectatus by non-enlarged dorsolateral scales (vs. enlarged), from L. rarus by the absence of broad and distinct crossbands on tail (vs. presence), and from L. guibei by smaller dorsolateral tubercles and smaller spiny tubercles on tail base (vs. larger and more distinct tubercles).
Description. (1) A moderately sized species, adult SVL 20.5–32.6 mm (28.0± 2.56 mm, n=40); (2) TAL 17.4–36.7 mm (28.0± 4.70 mm, n=23); (3) dorsal scales granular; (4, 5) first finger present, without bearing a claw; (6) three pairs of subdigital lamellae on the fourth toe; (7,8) mental scale undivided and its posterior projection sometimes in contact with first infralabial scale; (9, 10) two postmental scales, sometimes three (2.10±0.30, n=41), typically asymmetrical; (11) 4–6, typically five postpostmentals (4.85±0.57, n=41); (12) 5–8 infralabial scales (6.34±0.76, n=41); (13) 5–8 supralabial scales (mean±SD 6.90±0.70, n=41); (14) 1–3 internasal scales (2.35±0.70, n=41); (15) 5–7 preanal pores (6.30±0.67, n=10); (16) tail without whorls but with rings of slightly different colour giving the impression of whorls; (17,18) usually with 4–5 rather distinct dorsolateral tubercles which can consist of two scales, one larger than the other and spiny, or of 4–6 scales; two spiny scales can be present on the base of the tail, but these are less distinct than in L. guibei ; (19, 20) dorsally beige-brownish, without a clearly defined pattern; (21) ventrally light coloured; (22) 134–235 dorsal scales along the body (208.96±20.17, n=23); (23) 70–112 dorsal scales around the body (90.64±9.82, n=28); (24) 73–113 ventral scales (95.82±10.05, n=28) ( Figs. 6c View FIGURE 6 , 10 View FIGURE 10 ).
Hemipenial structure. Based on MRSN R1893, adult male from Ambolokopatrika. Hemipenis bilobed, with a total length of ca. 3 mm. Short pedicel (ca. 1 mm). The truncus bears small but deep calyces, resulting in a papillate appearance. No calyces along the sulcus spermaticus. Fields and serrated ridges with pointed papillae absent. Sulcus spermaticus without well developed sulcal lips and with two channels starting on the pedicel, leading each to a terminal position of one of the apex lobes, respectively. Apex formed by two broad lobes, each slightly longer than 2 mm ( Fig. 10 View FIGURE 10 ).
Material examined. BMNH 1948.8 . 2255 (holotype of Lygodactylus miops ), BMNH 1946.8 .2255 (Majastre, Senbendrana), BMNH 1998.65 (C.J. Raxworthy, 25 Aug. 1986, Anandrivola forest , NE Madagascar) , MNHN 1893.63 About MNHN (holotype of L. septemtuberculatus, Angel, Forêt de Moramanga ) , MNHN 1921.251 About MNHN – 252 About MNHN (collector and locality unknown) , MNHN 1930.269 About MNHN ( Karianga ) , MNHN 1935.122 About MNHN (Bezabona, Tolagnaro region ) , MNHN 1938.195 About MNHN (South Madagascar) , MNHN 1990.1839 About MNHN (C. Domergue, locality unknown) , MNHN 1990.1857 About MNHN (environments of Maroantsetra, 1963) , MNHN 1990.1875 About MNHN (C.P. Blanc, 28 Nov. 1963, Mananara ) , MRSN R1888 – R1891 and MRSN R1893 – R1894 (F. Andreone, 1997, Ambolokopatrika ) , MRSN R1897 – R1898 (F. Andreone, Jun. 1996, Besariaka ) , MRSN R1899 – R1904 (F. Andreone, Dec. 1998, Masoala ) , MRSN R1905 – R1906 (F. Andreone, Dec. 1999, Masoala ) , MRSN R1907 – R1908 (F. Andreone, Jan. 1999, Moramanga ) , MRSN R1923 (F. Andreone, Jun–Jul. 1995, Tolongoina ) , MRSN R1924 – R1925 (F. Andreone, 1997, Ambolokopatrika ) , MRSN R1150 (F. Andreone, Nov. 1994, Andohahela ) , MRSN R1179.1 – 2 (F. Andreone, May 1994, Ifanadiana ) , MRSN R1195.1 – 2 (F. Andreone, Apr. 1994, Andohahela ) , SMF 8931 About SMF (lectotype of Microscalabotes spinulifer ) , ZFMK 17713 About ZFMK (H. Meier, Jan. 1976, Kianjavato ) , ZFMK 50591 About ZFMK (F.W. Henkel and others, May 1989, Ranomafana ) , ZFMK 52304 About ZFMK (F. W. Henkel & W. Schmidt, Lac Arnouche , Toamasina) , ZMA 19678–19679 View Materials (field numbers LLS 182 and 223, B. van Opzeeland, Mananara ) , ZSM 731 View Materials / 2003–733/2003 (F. Glaw, M. Puente, L. Raharivololoniaina, M. Thomas & D. R. Vieites, 22 Jan. 2003, Ranomafana region )
Distribution. According to the specimens examined in this study, Lygodactylus miops is known from the following localities: (1) Sahembendrana (type locality), (2) Ambolokopatrika, (3) Anandrivola, (4) Andohahela, (5) Besariaka, (6) Bezabona (Tolagnaro region), (7) Ifanadiana, (8) Karianga, (9) Kianjavato, (10) Lac Arnouche, (11) Mananara, (12) Maroantsetra, (13) Masoala, (14) Moramanga, (15) Ranomafana, (16) Tolongoina. Also known from (17) Nahampoana (FG, personal observation). Further localities mentioned in the literature are Analamazaotra and Ambila ( Angel, 1942).
Habitat. According to Angel (1942), L. miops occurs in forest. In the Ranomafana area, specimens were found active during the day on tree trunks in rainforest (MP and DRV, personal observation in 2003) and at night, sleeping on leaves at a perch height of about 1.5 m (MV, personal observation in 2004). At Nahampoana, specimens were active during the day on mossy rocks in rainforest (FG, personal observation).
Variation. The localities of L. miops span a north-south band along almost the whole of the eastern coast of Madagascar. To detect possible geographic variation we compared specimens from the more northern, the central, and more southern localities. 21 specimens examined from the northern localities (Maroantsetra, Mananara, Ambolokopatrika, Besariaka, Masoala) reach slightly larger sizes ( SVL 22.8–32.6 mm, mean 28.0 mm) compared to the three specimens from a central locality (Moramanga) ( SVL 25.3 –29.0 mm, mean 26.9 mm) and the 13 specimens from more southern localities (Ifanadiana, Andohahela, Kianjavato, Ranomafana, Bezabona-Tolagnaro, Tolongoina) ( SVL 13.3–31.3 mm, mean 26.6 mm). Most northern specimens have a contact between posterior part of mental and first infralabial scale which is absent in most of the southern specimens; northern and central specimens have 1–3 internasal scales while southern specimens have 2–3 internasals, with none of the nine southern specimens examined having a single internasal. The specimens from the north-eastern sites Ambolokopatrika and Besariaka (NE Madagascar) show further morphological differentation: three of the five males examined have seven preanal pores instead 5–6 in southern localities .
One further specimen, MNHN 1990.1838 (C. Domergue, Madagascar, precise locality unknown) was assigned to L. miops in the MNHN catalogue but would represent the only individual belonging to the L. madagascariensis group with three postmental scales, and the only L. miops with five supralabial scales. Its identity must be considered as uncertain. Female; 26.6 mm SVL; 19.5 mm TAL; granular dorsal scales; first finger without claw; three pairs of lamellae, undivided mental scale; faint contact between infralabial and mental scale; three bisymmetrical post mental scales; five postpostmental scales; five infralabial scales; six supralabial scales; two internasal scales; postanal sacs apparently present; eight faint, coloured rings on tail but no whorls; four dorsolateral tubercles with one spiny scale; dorsal colour brown without a clear pattern; ventral colour light with spots.
Lygodactylus rarus Pasteur & Blanc, 1973
( Fig. 11 View FIGURE 11 )
Lygodactylus (Domerguella) rarus Pasteur & Blanc, 1973 .— Name-bearing type: female holotype, MNHN 1990.6 About MNHN , SVL 23 mm — Type locality: “falaise orientale du karst d’Ambilobe (extrémité nord-est du Massif de l’Ankarana )”, according to the original description.— Other types: none according to original description.— Etymology: derived from Latin rarus (rare, unusual).
Diagnosis. A rather large-sized endemic of limestone karst areas of northern Madagascar, characterized by a long-legged, long-tailed and slender appearance. It can clearly be assigned to the L. madagascariensis group by sharing the characters listed in the group definition above. It differs from all species in the L. madagascariensis group by the presence (vs. absence) of broad crossbands in the tail, of alternate light grey/ dark grey colour. Furthermore distinguished from L. miops and especially L. guibei by the absence (vs. presence) of dorsolateral tubercles and spiny tubercles at the tail base. Further distinguished from the sympatric L. expectatus by its non-enlarged dorsolateral scales (vs. enlarged), absence of dark spots on the neck (vs. presence), and larger size (adult SVL 31.6–36.5 mm vs. 27.0– 29.7 mm).
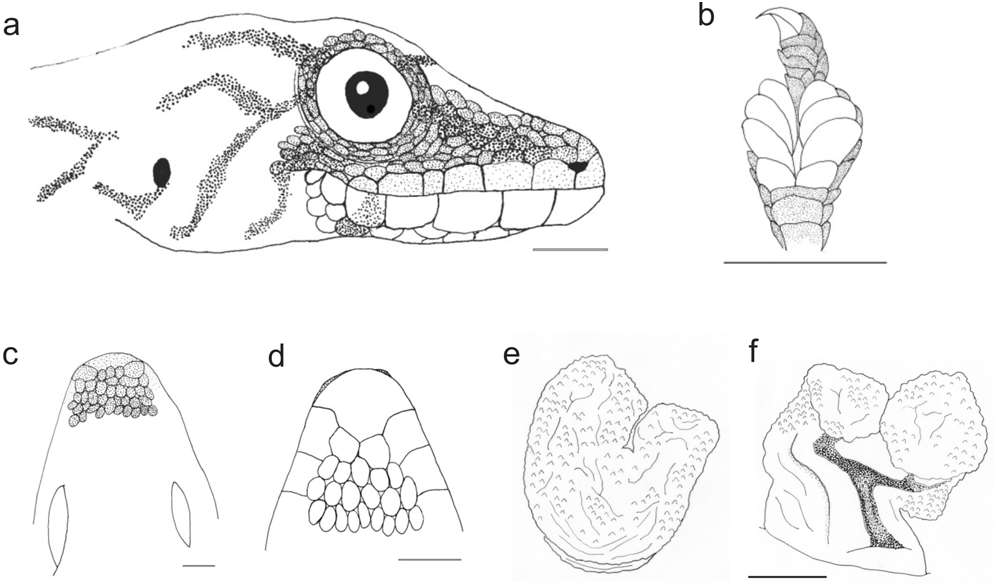
Description. (1, 2) A large-sized Lygodactylus with a long tail, SVL 31.6–36.5 mm (34.0± 2.45 mm, n=3); (2) TAL 31.5–43.7 mm (37.9± 6.12 mm, n=3); (3) granular dorsal scales, (4, 5) first finger present, without bearing a claw; (6) 3–4 pairs of subdigital lamellae on the fourth toe (3.20±0.44, n=5); (7,8) mental scale undivided, its posterior projection not in contact with the first infralabial scale; (9, 10) two symmetrical postmental scales; (11) 4–5 postpostmental scales (4.20±0.44, 4, n=5); (12) 5–6 infralabial scales (5.80±0.44, n=5); (13) 6–8 supralabial scales (6.80±0.83, n=5); (14) 1–3 internasal scales (2.00±1.00, n=5); (15) males with seven preanal pores (n=2); (16) tail without whorls but with broad crossbands of alternating white/dark grey colour; (17, 18) dorsolateral tubercles and spiny tubercles at tail base absent; (19, 20) dorsally beigebrownish with a central row of dark grey markings that are broader than long and extend over most of the dorsum, usually without a clearly defined pattern; often with some transversal striations on the limbs; (21) ventral colour light; (22) 167–181 dorsal scales along the body (n=2); (23) 73–87 dorsal scales around the body (n=2); (24) 99–116 ventral scales (n=2) ( Figs. 6f View FIGURE 6 , 11 View FIGURE 11 ).
Hemipenial structure. Based on ZSM 913/2003, adult male from Ankarana. Hemipenis with a total length of ca. 1.5–2 mm; pedicel short (ca. 0.75 mm). Truncus with small but deep calyces, giving a papillate appearance. No calyces along the sulcus spermaticus. Fields and serrated ridges with pointed papillae absent. Sulcus spermaticus without well developed sulcal lips and with two channels starting on the pedicel, leading each to a terminal position of one of the apex lobes, respectively. Apex bilobed, each lobe about 1 mm in length. ( Fig. 11 View FIGURE 11 ).
Material examined. MNHN 1990.6 About MNHN (holotype, north-eastern extreme of Ankarana Massif ) , MNHN 1990.1887 About MNHN – 1888 About MNHN (C.P. Blanc, 4 Nov. 1966, Mangindrano ) , ZSM 860 View Materials /2003 and ZSM 913 View Materials /2003 (F. Glaw, R. D. Randrianiaina & A. Razafimanantsoa, 11–15 Feb. 2003, Ankarana,) .
Distribution. According to the specimens examined in this study, Lygodactylus rarus is known from the following localities: (1) Ankarana Massif (type locality), (2) Mangindrano (Tsaratanana). No further localities are mentioned in the literature.
Habitat. We observed the species in the karstic limestone massif of Ankarana, the type locality. Specimens were active during the day, on big karstic rocks in an area with reduced vegetation.
| R |
Departamento de Geologia, Universidad de Chile |
| ZMA |
Universiteit van Amsterdam, Zoologisch Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Lygodactylus madagascariensis
| Puente, Marta, Glaw, Frank, Vieites, David R. & Vences, Miguel 2009 |
L. rarus
| Pasteur & Blanc 1973 |
L. rarus
| Pasteur & Blanc 1973 |
L. rarus
| Pasteur & Blanc 1973 |
L. expectatus
| Pasteur & Blanc 1967 |
L. expectatus
| Pasteur & Blanc 1967 |
L. expectatus
| Pasteur & Blanc 1967 |
Lygodactylus expectatus
| Pasteur & Blanc 1967 |
Lygodactylus expectatus
| Pasteur & Blanc 1967 |
L. miops
| Gunther 1891 |
L. miops
| Gunther 1891 |