Parahyaena brunnea (Thunberg, 1820)
|
publication ID |
https://doi.org/10.5281/zenodo.5676766 |
|
DOI |
https://doi.org/10.5281/zenodo.6554231 |
|
persistent identifier |
https://treatment.plazi.org/id/03928788-FFED-FF81-2FA7-FBEBF8DECE95 |
|
treatment provided by |
Conny (2021-10-06 23:57:12, last updated 2024-11-27 13:48:05) |
|
scientific name |
Parahyaena brunnea |
| status |
|
Brown Hyena
Parahyaena brunnea View in CoL
French: Hyene brune / German: Braune Hyane / Spanish: Hiena parda
Taxonomy. Hyaena brunnea Thunberg, 1820 View in CoL ,
South Africa, Western Cape Province, Cape of Good Hope.
Formerly classified as Hyaena brunnea View in CoL , but a recent molecular analysis assigns this species to a separate genus from that of the Striped Hyena. Along with Striped and Spotted Hyenas, the Brown Hyena belongs to the subfamily Hyaeninae View in CoL . Monotypic.
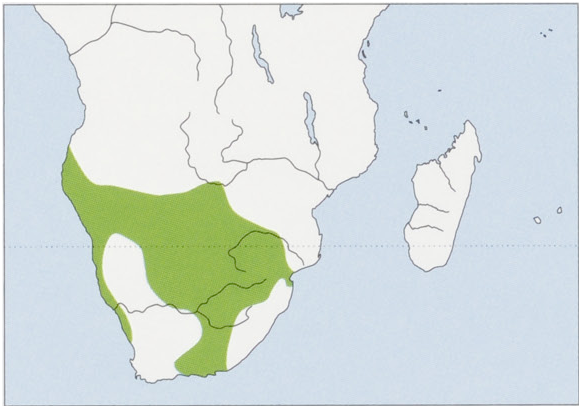
Distribution. Namibia, Botswana, W & S Zimbabwe, S Mozambique, Swaziland, W Lesotho, and South Africa. Records from the SW of Angola are all before 1970. View Figure
Descriptive notes. Head-body 110-136 cm; average 123 cm (males), 117 cm (females), tail 18.7-26. 5 cm, average height at shoulders 79 cm (males) and 74 cm (females); weight (adult) varies somewhat regionally, ranges from 28 to 47-5 kg and averages about 40 kg. Most studies show some sexual size dimorphism, but it is often minimal, with males slightly heavier and longer than females. Has a typical hyena appearance with front legs longer and more robust than the rear legs, a broad head and short muzzle, thick neck and short tail. Like the Striped Hyena it has large pointed ears and course shaggy fur that is longest along the back and on the tail. The general color is dark brown with lighter tawny hair on the neck and shoulders. The legs are banded with dark horizontalstripes and the front feet are large and well-developed for digging. Like the Spotted and Striped Hyenas, the Brown Hyena possesses the bonecrushing third premolar that is unique to this family. In contrast to Spotted Hyenas, there is no masculinization of the female genitalia in Brown Hyenas. Females have two to six pairs ofteats, but only the two most caudal pairs are functional.
Habitat. Brown Hyenas are found in a variety of relatively arid habitats from open desert or semi-desert in the Namib and Kalahari, to dry open scrub and woodland savannah, mopani scrub and tree savannah, as well as the bushveld of the northern Transvaal. They do not need drinking water and inhabit areas where annual rainfall may be even lower than 100 mm, up to about 650 mm.
Food and Feeding. These hyenas forage alone at night and do not cooperate in hunting or in feeding, although group members tolerate each other at large food items. Although not competent hunters, Brown Hyenas are extremely efficient scavengers with an omnivorous diet. They are opportunistic feeders on a range of vertebrates, primarily mammals, the vast majority of which are scavenged, often from the kills of other carnivores. Fruits, insects and reptiles can be important supplementary foods when carcasses are rare. In one population 58 different food items were identified from fecal analysis. Brown Hyenas in the southern Kalahari spent 30% of their feeding time eating carrion, 28% on vegetable matter, 4-5% on small mammals, 1-5% on birds’ eggs, and 29% on unknown items. Only 5-8% of food they were seen eating was killed by the hyenas themselves. In the central Kalahari 35-9% ofall observed feeding bouts were on fresh scavenged kills, 33-9% on old carcasses, 16% on their own kills, and 12:5% on vegetative food sources. Brown Hyenas do not depend on standing water. In the central Kalahari, no free water orrain is typically found for eight months of the year. Although they will drink on a daily basis when water is present, much of the water during dry seasons is obtained from Cucurbitaceae fruits such as the tsama melon, gemsbok cucumber, and Hookeri melon, which can compose significant proportions of the diet in these seasons. Brown Hyenas inhabiting the Namibian coast feed almost exclusively on subadult Brown Fur Seals. The majority of these seals are thought to be scavenged, although Brown Hyenas have been seen hunting seal pups. In fact, hunting efficiency on seal pups during the peak pupping season can be as high as 47%, and an average of almost five seals per day may be killed from a single colony. Small rodents and seabirds make up the rest of this unique diet. Elsewhere hunting attempts by Brown Hyenas are opportunistic and directed at small mammals such as Springbok lambs, spring hares, Bat-eared Foxes, and ground nesting birds. The hunting technique of the Brown Hyena is unspecialized, rarely successful (except in the case of Brown Fur Seal pups), and may include a brief lunge at a surprised prey, a prolonged chase of up 1 km, or an attempt to dig up a burrowed animal. The percent of observed hunting attempts that were successful was 4:7% in the southern Kalahari and 13-7% in the central Kalahari. The hyenas generally feed where they find food, but food from larger carcasses is frequently cached nearby in a clump of grass or under a bush. Considerable time is often spent at carcasses removing limbs for this purpose and one animal may remove and cache up to three legs before any competitors arrive. Sites where food is cached are scent-marked and may be re-visited over multiple days. In a remarkable example of food caching in the southern Kalahari, a Brown Hyena arrived at an abandoned ostrich (Struthio camelus) nest with 26 eggs, which are prized food items. The hyena spent four hours carrying 14 eggs distances of 150-600 m from the nest, some of which it simply dropped in the open. It ate only three eggs during this period. Brown Hyenas also carry food back to cubs at the den. This provisioning of cubs can result in significant bone accumulations at densites. In the southern Kalahari the average distance moved between significant meals was 7-2 km, and the average nightly distance traveled was 31-1 km. During the dry season, nightly movements were longer and were recorded as high as 54-4 km. During the wet season movements were reduced and ranged from 10-20 km. In Namibia, daily distances traveled ranged from 15-47 km. When foraging, Brown Hyenas move at a pace of about 4 km /h, often walking in a zig-zag pattern, probably to maximize their chance of coming across food items. They use smell to locate much of their food, as evidenced by frequent sniffing and moving upwind toward food sources. Their hearing appears to be acute as well and is likely also used in foraging. The Brown Hyena is subordinate at kills to Spotted Hyenas, Lions and African Wild Dogs, although it appears to dominate Leopards in most situations. While they always dominate Cheetahs, they compete heavily with the much smaller Black-backed Jackal, which is often able to steal scraps from hyenas at carcasses. There is some evidence that, where they are sympatric, Brown Hyenas avoid areas frequented by Spotted Hyenas, potentially to avoid direct aggression and competition. For example, dens in the southern Kalahari were rarely found in the prey-rich riverine habitat where most Spotted Hyena dens occurred. Although their presence at livestock carcasses has resulted in much antagonism toward, and persecution of, Brown Hyenas by livestock owners, predation on livestock by these animals appears to be done by a small number of individuals. However, these hyenas, which typically target cow calves and sheep, can account for a large number of kills. Removal of these problem individuals appears effective at halting stock losses.
Activity patterns. Primarily a nocturnal animal, although activity is occasionally observed during the day, particularly on cool, cloudy days during the rainy season. There are typcally two peaks of activity, from 19:30 h to 24:00 h and 2:30 h to 6:00 h, with a rest period in between. Radio-collared adults in the southern Kalahari were active for 42.6% of the 24 h period, and 80.2% of the period between 18:00 h and 06:00 h. In Namibia, three males with satellite collars spent an average of 57-1-72-3% of 24 h active. Brown Hyenas typically rest during the day in a hole, or undera large tree or bush.
Movements, Home range and Social organization. Approximately 65% of Brown Hyenas in a population are members of small social groups called clans, with the remaining individuals living as nomads. Clan size ranges from 4-14 individuals, including cubs, and clans defend large stable territories. In the southern Kalahari far-ranging nomadic males (8% of the adult population) were the only males observed to breed with clan-living females, yet in the central Kalahari, breeding also occurs with resident immigrant males. In the southern Kalahari territories averaged 308 km? (range: 215-461 km?) with never more than 20% overlap between territories. Brown Hyena density there was calculated to be 1-8 hyenas/ 100 km?®. In the central Kalahari territories averaged 170 km?, but varied greatly with annualrainfall, reaching a maximum of 400 km?. In Namibia, where Brown Hyenas depend almost entirely on Brown Fur Seals along the coast, territories of two clans in one study were 31-9 km? and 220 km? In another study the home ranges of three males ranged from 420-1460 km? with the largest home range being an inland location. Density in this latter Namibian study ranged from 1-0-2-9/ 100 km ®. In general, group size appears to be correlated with food abundance and quality within the territory, whereas territory size is influenced by the distribution of food resources. Territories are maintained primarily through scent marking behavior (called “pasting”) and aggression toward intruders. Clan structure appears to vary across regions, but always includes 1-5 breeding females and their subadult offspring. In the central Kalahari, groups often also include at least one adult resident immigrant male. Mean clan size in the southern Kalahari was 3-7 adults and subadults, and total clan size ranged from 4-14. In the central Kalahari, a well-studied clan contained 13 members including cubs. Because adult females and their offspring are the core of a social group, the majority of clan members are related. However, dispersal from and immigration into the clan occurs. Although subadults of both sexes may disperse from their natal clan, males do so more often than females and most males disperse by 36-40 months of age. In two reported cases of female emigration in the central Kalahari, the number of resident adult females was atits zenith (five) and the dispersing females both were targets of severe aggression from other resident females prior to dispersal. In both cases, dispersal appeared to be prompted by conflicts with established adults of the same sex. The central Kalahari and southern Kalahari locales also apparently differ with respect to clan social hierarchies. In the central Kalahari a linear within-sex dominance hierarchy was apparent, and at carcasses with more than one hyena, rank determined priority of access to food. Although immigrant males were dominantto all natal males, the highest ranking male and female appeared to be of equal status. Adult females were typically dominant to natal males of less than 36 months of age. These natal males were tolerated until about 24 months, when aggression gradually increased until their dispersal. However, in one case, a natal male remained in the clan and eventually dominated the clan females. In the southern Kalahari no dominance hierarchy was apparent, with no sex, age-class, or individual consistently winning fights or monopolizing food resources in clans. Differences in the breeding systems and the existence or lack of a hierarchy are thought to be related to significant differences in Brown Hyena density in the two locales. In the central Kalahari, 37-81% of observations involve the association of two or more hyenas. In the southern Kalahari contact between group members appears to be less frequent. Although there is typically aggression between hyenas of the same sex from different groups when they meet, the level of aggression within clans appears to vary between the southern and central Kalahari. In the central Kalahari, neck-biting appears to be used to maintain rank relationships within the clan and is observed with some frequency, while in the southern Kalahari, fighting within the clan is rare, with clan members seldom interacting at all. Here, the only aggression observed is between same-sex members of neighboring clans, and this is extremely infrequent. Interestingly, in the southern Kalahari, where resident males do not breed with clan females, these males show little aggression to nomadic males, who are responsible for mating with groupliving females, suggesting that Brown Hyenas can differentiate between neighboring males and nomadic males. When they meet after being separated, Brown Hyenas from the same group engage in a greeting ceremony in which each animal in turn crouches and presents its extruded anal pouch to the other. This is accompanied by a lowering of the ears and a “grin” (teeth exposed by pulling lips up and corners of the mouth back) by the subordinate animal whenit is greeting a dominant. Greetings can last as long as five minutes. Two additional behaviors that appear to be important in Brown Hyena society are neck-biting and muzzle-wrestling. Neck-biting is a purely agonistic interaction (though cubs may engage in it during play) and is primarily intrasexual. In the southern Kalahari, this behavioris largely restricted to interactions between members of neighboring clans, whereas in the central Kalahari, it can be seen more frequently between clan members and is thought to function in maintenance of a dominance hierarchy. Neck-biting behavioris a ritualized, somewhat elaborate interaction in which dominant and subordinate animals are clear from the start. The submissive animal approaches a standing dominant individual grinning and with its mane and tail raised. Either before or at its approach, the dominant seizes the neck of the subordinate, holding the skin and hair of the neck with its incisors and one or both canines, and vigorously shakes the victim from side to side. This type of interaction typically lasts less than five minutes, and only rarely does the subordinate flee at its conclusion. Muzzle wresting may be observed anywhere in the territory and is exhibited by all clan members. However, adults rarely engage in this behavior with other adults, though they will do so with cubs with some frequency. Most muzzle-wrestling occurs between cubs and subadults. The two participants stand face to face, and attemptto bite each other on the jowls or along the side of face. Their heads pitch rapidly from side to side with mouths open, and they often growl softly throughout. One or both hyenas may be crouched on their carpals, and in some cases one may lie beneath the other. This behavior is clearly less aggressive than neck biting and may often be play, although it can escalate into true aggression. There is no clear loser, and animals typically remain with each other after muzzle-wrestling, which may last from a few minutes to an hour. The most striking visual display of the Brown Hyena is pilo-erection of the long hairs along its neck and back, which is observed in situations calling for either an attack or flight response. Despite its rather elaborate social interactions, Brown Hyenas spend the vast majority of their time alone, and the primary form of communication between hyenas is olfactory. They convey information to conspecifics with latrines, which have accumulations of feces, and grass stalks on which they have deposited a strong-smelling white secretion and a smaller black secretion. Both secretions are deposited during pasting from an extruded anal scent pouch located between the rectum and base ofthe tail. Although all four hyaenid species paste, the deposition of two different secretionsis unique to Brown Hyenas. Whereas the lipid-rich white secretion is discernible to the human nose for well over 30 days, the more watery black secretion appears unscented after a few hours. This black secretion is thought to convey information relating to the time elapsed since it was deposited, and therefore signal that a hyena has recently foraged in the area. It is suggested that this allows other group members to avoid unproductive areas, and minimizes competition between group members for limited resources. The longer-lasting white secretion is thought to function in territory marking and defense. Pasting is done throughout a clan’s territory. Although most pasting occurs in the central part of the territory, where residents spend most of their time, frequency of pasting and over-pasting (deposition on an existing mark) is highest when individuals visit territory boundaries. Very little pasting is done by residents when they are outside of their territory. Pasting during traveling/foraging movements can be quite frequent, with ten individuals averaging a paste every six minutes. However,this is highly variable, with some individuals pasting only once or twice during a night-time observation period. Males and females do not differ in rate of pasting during their travels. At the den, adults and subadults frequently paste soon after arriving and before departing. At least in the southern Kalahari, the perimeter of hyena territories is thought to be too large to make strict border marking possible or effective. Instead, marks are scattered throughout the residents’ territory. This is known as hinterland marking. Given the frequency of pasting, and how widely pastes are deposited across a territory, simulations indicate that in the southern Kalahari, hinterland marking is effective. Intruders would likely encounter resident paste marks very soon after entering a territory. Indeed, individual hyenas are estimated to deposit some 29,000 paste marks in a year. Experiments with translocated pasted grass stalks indicate that hyenas can distinguish between pastes of group and non-group members and that over-pasting is more commonly done on pastings from non-group hyenas. Chemical analysis of white and black paste suggests that the scent of these substances probably varies between individuals, allowing for identification of the paster. In the southern Kalahari, latrines are not as regularly spaced as pastings, and show a clumped distribution, largely around primary foraging areas that occur along the territory border. Latrines are often associated with landmarks such as trees, bushes or roads, and those along the border are visited more frequently than those in the interior. The vocal repertoire of the Brown Hyena is relatively small, as in the Striped Hyena, and consists of eight vocalizations: a yell, a hoot, two whines and four growls, none of which functions as a longdistance communication. Some authors group these into five calls, the squeal/whine, squeak, scream,yell, and growl/grunt. The squeal is a shrill sharp cry emitted by a juvenile or other subordinate while approaching to greet or beg food from a dominant individual. The squeak is a hoarse rasping cry of abject submission associated with carpal crawling. A scream is a high-pitched, cackling shriek given by a hyena whose neck is being bitten. The yell is a loud, abrupt high-pitched call associated with defensive threat. A growl/grunt is low-pitched, breathless and throaty, and is given while muzzle-wrestling. All but the growl appear to indicate submission or appeasement in social contexts of varying intensity.
Breeding. Brown Hyenas are polyestrous, non-seasonal breeders. Litters range from 1-4 cubs with a modallitter size of three. Estrus lasts approximately one week but mating in captivity occurs over a 15day period. Based on six observed mating bouts in the southern Kalahari, mating associations consist of multiple copulation attempts over a 5-90 minute period, and may be preceded by extended courtship, during which both animals may show aggression and there are mutual approaches and retreats. Gestation in captivity was 96 days. Interbirth intervals appear to range widely. In the southern Kalahari they were as short as a year and as long as 41 months apart, although lost litters in the interim could not be ruled out. Cubs are born with their eyes closed and their ears bent forward. Their fur is similar in color to that of adults. Their eyes begin to open at eight days and are completely open at 14 days. Unlike Spotted Hyenas, Brown Hyena cubs are born without teeth. As in the other bone-cracking hyaenids, den dependenceis long and weaning occurs late. Cubs from 0-3 months of age rarely leave the den hole except when their mother or another adult is present. During this period, mothers attend the den frequently, often at sunrise and sunset and cubs rely completely on their mother’s milk. At four months, visits by the mother become less frequent, with mothers visiting about once a night, but suckling periods are longer. At this time, mothers and other group members begin bringing food to the den for the cubs. Weaning normally occurs at 12-16 months of age, yet weaning conflicts have been observed at ten months. Starting at ten months, cubs begin extensive, and very often solitary, foraging movements away from the den. Length of den residence is variable however, ranging from 8-15 months. Regardless of their dependence on the den, by 16 months weaning has occurred and full adult dentition is present. As they mature, subadults themselves begin to bring food back to the den for younger cubs. This has been observed in subadults as young as 22 months. Adult size is reached at 30 months. The earliest breeding recorded in the wild is 35 months; breeding continues until at least ten years of age. Cubs are raised in underground, sometimes extensive, tunnels, always small enough to prevent adults and potential predators from entering. They are easily distinguished by accumulations of bones, hair, feathers, horns, pieces of hide, and hyena feces. In the southern Kalahari Brown Hyena dens appear to be used only rarely by multiple females at once, and cubs are typically raised in the same den in which they are born. However, communal denning appears to be common in the central Kalahari, with cubs of multiple females, and of different ages, raised together at a single den location. In this system, cubs are born in a solitary den and transported to the communal den sometime before they are four months of age. Throughout their development, den moves are common and cubs may reside at as many as seven different dens, though distances between dens are typically not large. In the central Kalahari, where a social rank system is evident, dominant females enjoy greater reproductive success in terms of number of surviving offspring, yet the number oflitters does not vary based on rank. There appears to be variation, both regionally and temporally, in the mating system of Brown Hyenas. In one system, females breed only with nomadic males that range over wide areas without defended territories or family groups. In the other, females breed not only with nomadic males but with resident immigrant males as well. These residents are members of the clan and assist in territory defense, yet their tenure, at least in the central Kalahari, is relatively short (less than three years). In this area, where both nomadic males and resident immigrant males are present, dominant clan males were observed to copulate with resident females more frequently than nomadic males. In the southern Kalahari, the only mating observed involved nomadic males, and no resident males, either immigrant or natal, were observed to mate. Although males known to be natal showed little sexual interest at all in any resident females, researchers were unable to observe immigrant males long enough to ascertain sexual interest in resident females. The source of the variation in the mating system of Brown Hyenas is unclear, although it may be related to dispersion of food. In the central Kalahari, where both systems were observed over time, mating with nomadic males was restricted to the dry season, when resident clan males would likely have difficulty maintaining contact with clan females (some were separated by 22 km). Because territories are very large in the southern Kalahari and only breeding with nomadic malesis seen, the food dispersion theory is further supported. Individual reproductive patterns in males are also likely to be influenced by individual status and the behavior of other males in the population. In either case, natal males are never observed to mate with females in their natal clan. Communal care of cubs is better developed among Brown Hyenas than in any of the other three hyaenid species. Non-parental aid in cub rearing includes communal suckling (although preference in nursing one’s own cubs has been shown), food provisioning, den maintenance, defense against predators, play, and adoption of orphans. Subadult and adult females ofall social ranks and reproductive states bring food items to the den for cubs. However, the extent of involvement in provisioning by adult males seems to vary by region. In the central Kalahari, subadult males provision cubs, but to a lesser degree than females, and they only bring food to closely related cubs. Neither immigrant nor natal adult males were seen provisioning cubs. It has been suggested thatthis is because males do not benefit from an increased group size, as they are likely to emigrate from their natal clan. In the southern Kalahari, however, group-living males and females, both adult and subadult, were observed to provision cubs regularly. Average distance in this population from which food was carried back to the den was 6-4 km.
Status and Conservation. Listed as Near Threatened on The IUCN Red List. In 1994 the species was down-listed from Appendix I status, which was afforded the species in 1975, to Appendix II by the IUCN and it has since been deleted from CITES listing altogether. [tis generally considered to be widespread yet rare. The total population is estimated to be 5000 to 8000, but this may be an underestimate due to the secretive nature and nocturnal habits of this animal. It is estimated that areas in excess of 1000 km?” are required to maintain a viable population of Brown Hyenas. These populations currently exist in the Kalahari Gemsbok National Park, South Africa and the adjacent Gemsbok National Park, Botswana, the Central Kalahari Game Reserve, Botswana, and the coastal regions of the southern Namib Desert. These are also the sites of the primary research projects that provide much of what we know about this species in the wild. Much of the habitat where Brown Hyenas occur outside protected areas is used for livestock ranching, and the hyenas are heavily persecuted (shot, poisoned, trapped, and hunted with dogs) in these areas because they are assumed to be livestock predators. This persecution, and habitat loss and fragmentation, are the primary threats to persistence of Brown Hyena. Because they are scavengers, many livestock carcasses where they are seen feeding are likely not to have been killed by Brown Hyenas. Although the species can be involved in depredation,this is usually restricted to a few individuals. Regardless, management of Brown Hyenas on ranchlands must address livestock losses. Typically, removal of individual problem hyenas ends the depredation. Because there is evidence that Brown Hyenas may be limited by the presence of Spotted Hyenas and perhaps other large predators, which are often absent from ranches, these ranchlands have the potential to be developed as Brown Hyena conservation areas, given proper management and conservation education efforts. Brown Hyenas are uncommon in captivity and traditionally do not breed well in confinement. Due to difficulties in captive breeding, the international studbook was discontinued in 1993 and as of 1995 there were only 16 specimens in nine collections. There is no known illegal trade in the species.
Bibliography. Eaton (1976), Gorman & Mills (1984), Maddock (1993), Mills (1982a, 1982b, 1982¢c, 1983a, 1983b, 1984a, 1990), Mills & Hofer (1998), Mills & Mills (1978, 1982), Mills et al. (1980), Owens, D.D. & Owens (1979a, 1979b, 1984, 1996), Owens, M.J. & Owens (1978), Schultz (1966), Shoemaker (1983), Siegfried (1984), Skinner (1976), Skinner & llani (1979), Skinner & Van Aarde (1981), Skinner et al. (1995), Stuart & Shaughnessy (1984), Wiesel (2006).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
1 (by conny, 2021-10-06 23:57:12)
2 (by ExternalLinkService, 2022-03-13 12:32:57)
3 (by ExternalLinkService, 2022-03-13 12:58:11)
4 (by diego, 2022-05-16 14:27:48)
5 (by ExternalLinkService, 2022-05-16 14:43:24)
6 (by diego, 2022-07-15 14:59:56)
7 (by plazi, 2023-11-08 04:18:17)
8 (by ExternalLinkService, 2023-11-08 15:51:54)
9 (by plazi, 2023-11-18 04:02:53)