Xangoniscus santinhoi, Cardoso & Bastos-Pereira & Souza & Ferreira, 2020
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4819.1.4 |
|
publication LSID |
lsid:zoobank.org:pub:AB417537-CE48-465A-8C23-5AF0B7602ADC |
|
DOI |
https://doi.org/10.5281/zenodo.10500534 |
|
persistent identifier |
https://treatment.plazi.org/id/B406DFCB-C22B-4FCA-A6B5-38B9C5A8F52C |
|
taxon LSID |
lsid:zoobank.org:act:B406DFCB-C22B-4FCA-A6B5-38B9C5A8F52C |
|
treatment provided by |
Plazi |
|
scientific name |
Xangoniscus santinhoi |
| status |
sp. nov. |
Xangoniscus santinhoi View in CoL n. sp.
Figs. 15– 18 View FIGURE 15 View FIGURE 16 View FIGURE 17 View FIGURE 18 , 19E View FIGURE 19
Material examined. Holotype: Male ( ISLA 77517), Minas Gerais , Itacarambi , Lapa d’água do Zezé cave, 15.006745ºS, 44.117087ºW, 12 December 2014, leg. R. L. Ferreira GoogleMaps . Paratypes: 5 males, 19 females, same data as holotype ( ISLA 77518) GoogleMaps ; 3 males, 6 females, same locality as holotype, 25 January 2015, leg. L. M. Rabelo ( ISLA 77519) GoogleMaps ; 7 males, 6 females, same data as holotype, 15 July 2019, leg. R. L. Ferreira GoogleMaps ; 3 males, 19 females, Lapa d’ Água do João Ferreira, 25 January 2015, leg. L. M. Rabelo ( ISLA 77520) .
Etymology. The specific epithet is given in honor of Euripes Pedro dos Santos, known among his friends as “Santinho”. He is the owner of the farm where Lapa D’água do Zezé is located and was the person who lead the researchers to the cave.
Diagnosis. The species is characterized by antennula with three aesthetascs; antennal flagellum with six articles; pereopods 1–4 merus sternal margin with fringed scales; pereopods 5 and 6 merus sternal margin straight; pleopod 1 exopod trapezoidal with fine setae on distal margin; pleopod 2 exopod subtriangular; pleopod 3 exopod triangular, straight distal margin; uropod endopod and exopod subequal in length.
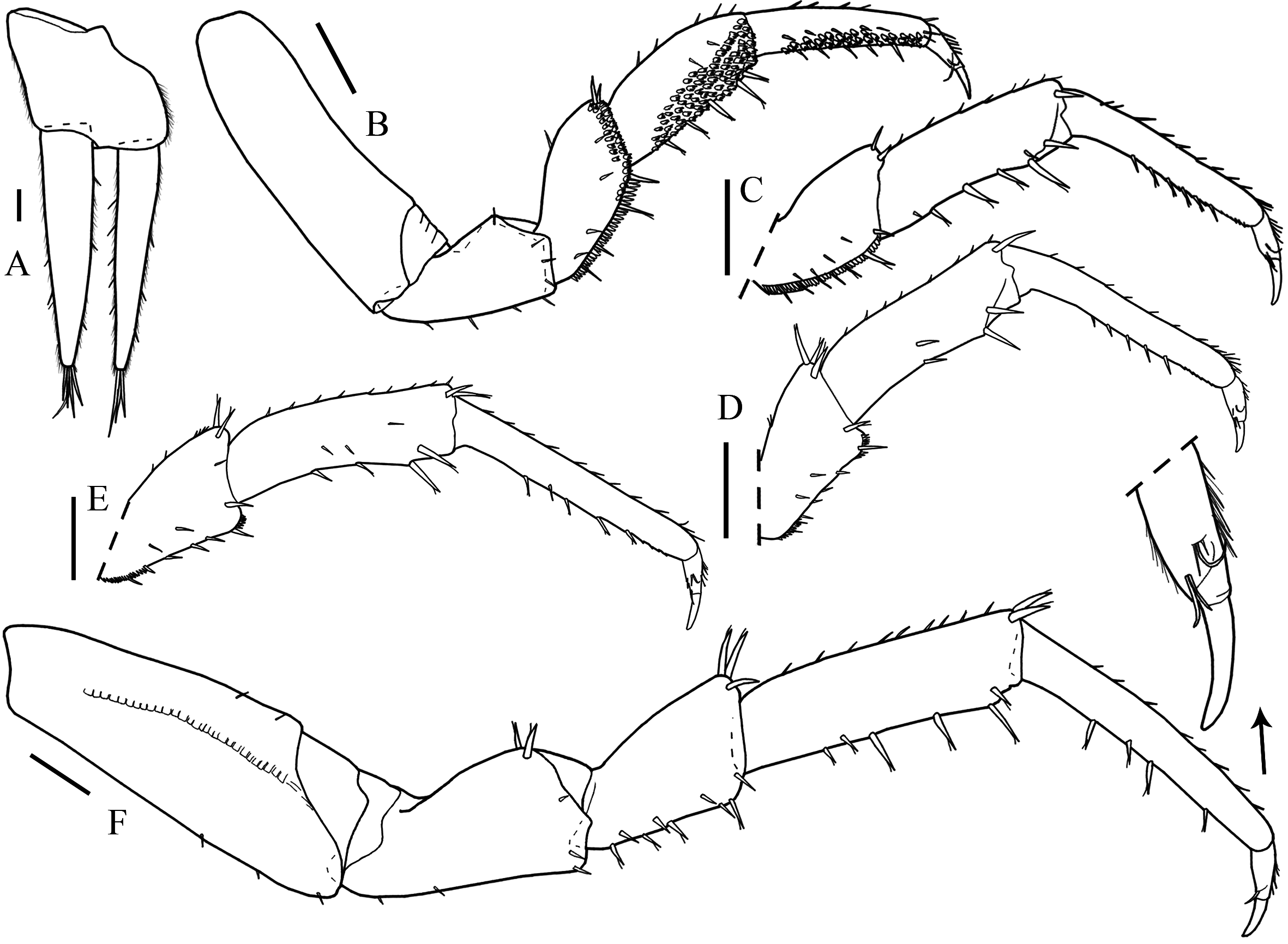
Description. Maximum length: male, 9 mm. Colorless, eyes absent, dorsal surface smooth with scattered simple and fringed scale-setae. Cephalon ( Fig. 15B View FIGURE 15 ) with antennary lobes; profrons with V-shaped suprantennal line. Posterior corners of pereonites progressively directed backwards, pereonite 7 surpassing distal margin of pleonite 2; pleonites 3–5 epimera posterior point developed; pleon narrower than pereon ( Figs. 15A View FIGURE 15 , 19E View FIGURE 19 ). Telson ( Fig. 15C View FIGURE 15 ) with concave sides, round apex.
Antennula ( Fig. 15D View FIGURE 15 ) with three articles, second article shortest, distal article with three aesthetascs, one subapical and two apical. Antenna ( Fig. 15E View FIGURE 15 ) reaches distal margin of pereonite 2 when extended backwards, fifth article of peduncle and flagellum subequal in length; flagellum with six articles. Left mandible with two penicils ( Fig. 1F View FIGURE 1 ), right mandible with one penicil, lacinia mobilis leaf-shaped ( Fig. 1G View FIGURE 1 ). Maxillula ( Fig. 1H View FIGURE 1 ) outer branch with 4 + 5 teeth, apically entire, and two thick plumose stalks; inner branch with three penicils. Maxilla ( Fig. 1I View FIGURE 1 ) with bilobate apex, inner lobe wider than outer lobe with several setae on distal margin. Maxilliped ( Fig. 1J View FIGURE 1 ) basis enlarged on distal portion; palp apex with four tufts of setae; endite rectangular, shorter than palp first tuft of setae, outer and medial margins setose, apex with one triangular penicil between two teeth.
Pereopod 1 antennal grooming brush composed by serrated scale setae longitudinally on propodus and on sternal margin of carpus. Uropod ( Fig. 16A View FIGURE 16 ) protopod longer than distal margin of telson; endopod and exopod subequal in length, exopod with proximal insertion.
Male. Pereopods 1–4 ( Fig. 16B, C View FIGURE 16 ) merus sternal margin concave with fringed scales; pereopods 2 and 3 sternal margin of propodus and distal margin of carpus with scale setae; pereopod 4 sternal margin carpus with scale setae. Pereopods 5 and 6 merus longer than wide, sternal margin straight proximal and distal margin setose ( Fig. 16D, E View FIGURE 16 ). Pereopod 7 ( Fig. 16F View FIGURE 16 ) basis with scales of water conduction system, with no distinct modifications. Genital papilla ( Fig. 17A View FIGURE 17 ) lanceolate. Pleopod 1 ( Fig. 17B View FIGURE 17 ) exopod trapezoidal with fine setae on distal margin, straight margin; endopod longer than exopod, with narrow basal article and flagelliform distal article; protopod distal margin shorter than exopod. Pleopod 2 ( Fig. 17C View FIGURE 17 ) exopod subtriangular, round distal margin, bearing three setae; endopod of two articles, basal article rectangular, shorter than exopod, distal article wrench-like, transverse pointed process on apex, directed outwards, with lateral membrane. Pleopod 3 exopod ( Fig. 17D View FIGURE 17 ) triangular, straight distal margin bearing setae. Pleopods 4 and 5 exopod ( Fig. 17E, F View FIGURE 17 ) rhomboidal wider than long, with distal margin rounded and bearing setae.
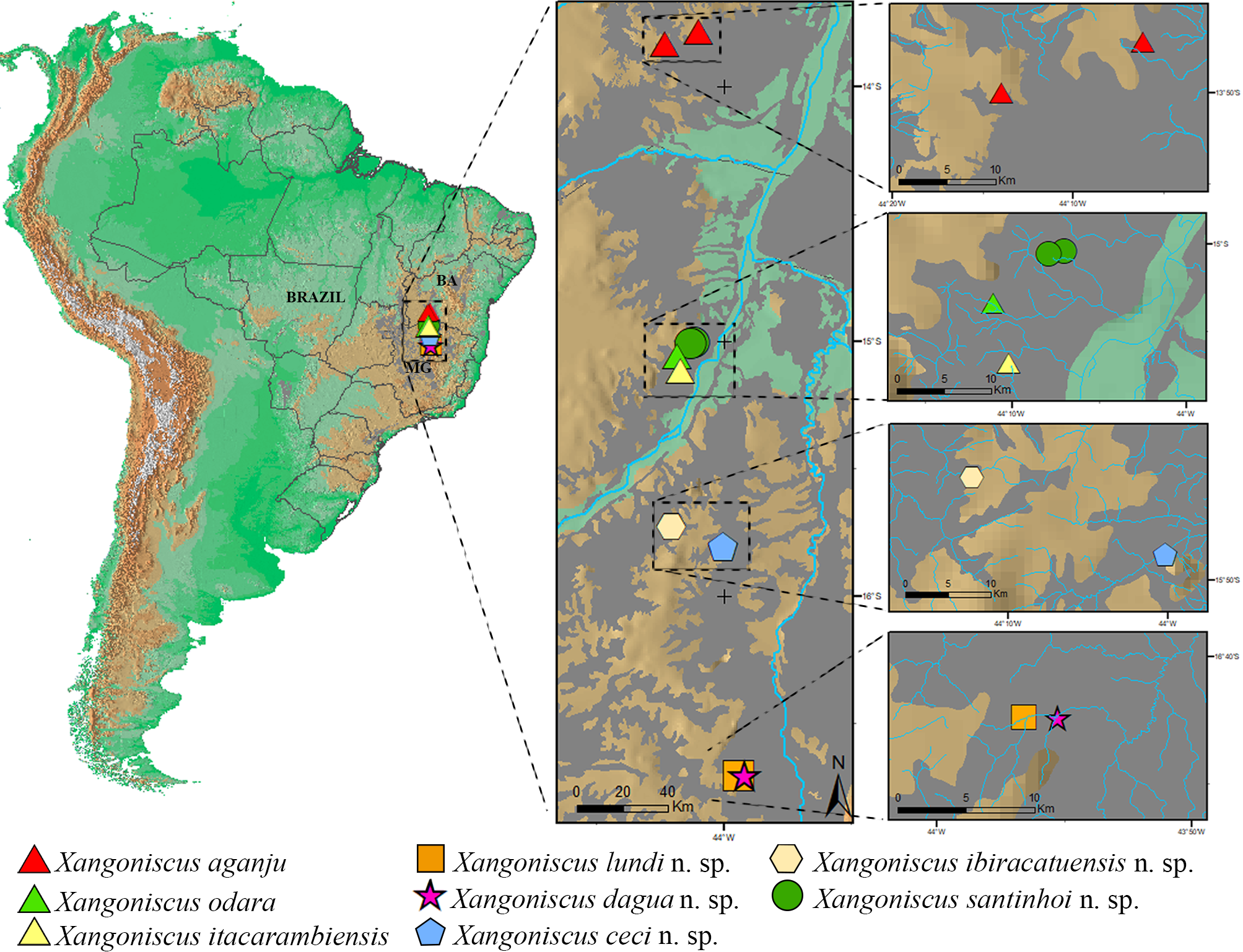
Remarks. Xangoniscus santinhoi n. sp. is distributed in two caves near the location of X. odara and X. itacarambiensis , however as can be seen in Figure 20 View FIGURE 20 each cave is located in a separate drainage system and currently are not connected. Xangoniscus santinhoi n. sp. is distinguished from X. odara and X. itacarambiensis by the antennula with three long aesthetascs, (versus two long in X. odara and six short in X. itacarambiensis ); by the pereopods 4–6 carpus sternal margin straight (versus carpus concave on distal half of sternal margin in X. odara and X. itacarambiensis , see Campos-Filho et al. 2014, 2016 for the illustrations); and by the shape of male pleopod 2 exopod subtriangular (versus triangular shape in X. odara and X. itacarambiensis ).
Habitat and threats. Lapa D’Água do Zezé and Lapa D’Água do João Ferreira caves are the only known habitats of X. santinhoi n. sp. until the present. Lapa D’Água do Zezé and Lapa D’Água do João Ferreira caves are inserted in an extensive limestone outcrop of the Bambuí formation (Neoproterozoic limestones) with a well-preserved deciduous forest on the top and immediate surroundings ( Fig. 18A View FIGURE 18 ), because most of this outcrop is inserted inside the limits of a National park (Parque Nacional Cavernas do Peruaçu). However, the original forest was severely altered around the outcrops and the landscape is mainly composed of pastures and crops. The area is located in the transition between two phytogeographic domains, Cerrado (Brazilian savannah) and Caatinga ( Velloso 2002), presenting several sub-types of vegetation in different degrees of conservation.
Lapa D’Água do Zezé cave is labyrinthine with one horizontal entrance (main entrance, Fig. 17B View FIGURE 17 ) and at least two vertical entrances. The farmers installed a gravitational pump inside the cave to drag water for consumption and irrigation. The cave presents two distinct conditions regarding the presence of water: the first is the only accessible part of the water table, corresponding to a narrow passage in the base of a diaclasis, close to a vertical entrance of the cave. In this place, a troglobitic amphipod, Spelaeogammarus uai Bastos-Pereira & Ferreira, 2017 ( Bastos-Pereira & Ferreira 2017) is easily observed, although no Xangoniscus were found during many visits to the cave. The second area comprises a very small drainage, which is apparently formed by the overflow of the water table. This drainage trespasses part of the cave and is partially altered by human activities. The farmers periodically remove the deposited sediment that could prevent the water to flow out, and some trunks were disposed in the floor to favor the water flow ( Fig. 18C, D View FIGURE 18 ). In this area, dozens of X. santinhoi n. sp. were found, and during several visits to the cave, only one amphipod was observed (they probably avoid this area due to the water flow). Although this small stream is altered by human activities, such interventions occur with low frequency (once in a year, according to the farmer), so it does not seem to have impacts to the cave communities, especially considering that a great part of the populations may be associated with inaccessible areas of the water table. Some physical and chemical parameters of the water were measured during one of the visits to the cave (January 2015): dissolved oxygen 3.46 mg /L, temperature 25.35ºC, pH 8.45, electrical conductivity 0.565 μS/cm, total dissolved solids 0.359 g /L.
The second cave where specimens of X. santinhoi n. sp. were found (Lapa D’Água do João Ferreira cave) presents a single conduit of around 150 meters of horizontal projection, which is trespassed by a perennial stream in its final portion. Although the cave entrance is dry ( Fig. 18E View FIGURE 18 ), the deeper portions of the cave presents high humidity. The farmers also drain the cave water gravitationally, which is used for consumption and for crop irrigation. However, since they do not use pumps, the impact of water drainage seems to be weak. Specimens of X. santinhoi n. sp. were observed in ponds formed by this drainage in deeper areas of the cave ( Fig. 18F, G View FIGURE 18 ). Water parameters were not measured in this cave.
Although other caves with water bodies have been visited in the same region, Xangoniscus species were only found in other two caves ( X. odara in Lapa do Cipó cave and X. itacarambiensis in Olhos d’água cave). Lapa D’Água do Zezé and Lapa D’Água do João Ferreira caves are not far from these caves. The distance between the Lapa D’Água do João Ferreira to those caves are 8.4 km and 12.2 km in straight line, respectively. However, the regional topography indicates some potential discontinuities in the limestone outcrops between the area of Lapa D’Água do João Ferreira and the other caves where X. aganju and X. itacarambiensis occur (with clear signs of drainages between both areas). Furthermore, the flow direction of underground streams in each area are opposite: the stream flow in the Olhos D’água cave is WSW-ENE and in the Lapa do Cipó cave is WNW-ESE, while that in the Lapa D’Água do João Ferreira cave flows ENE-WSW (i.e., in the opposite direction of the stream in Olhos D’água cave) ( Fig. 20 View FIGURE 20 ). This condition may indicate that such systems were formed by distinct drainages being supported by distinct flows. Accordingly, these conditions seem to prevent migrations among the systems, what led to speciation.
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |