Thelocarpon andicola Flakus & Kukwa, 2014
|
publication ID |
https://doi.org/ 10.11646/phytotaxa.175.5.7 |
|
persistent identifier |
https://treatment.plazi.org/id/A57AE700-AB02-FFBB-E3DA-FE168AF7F844 |
|
treatment provided by |
Felipe |
|
scientific name |
Thelocarpon andicola Flakus & Kukwa |
| status |
sp. nov. |
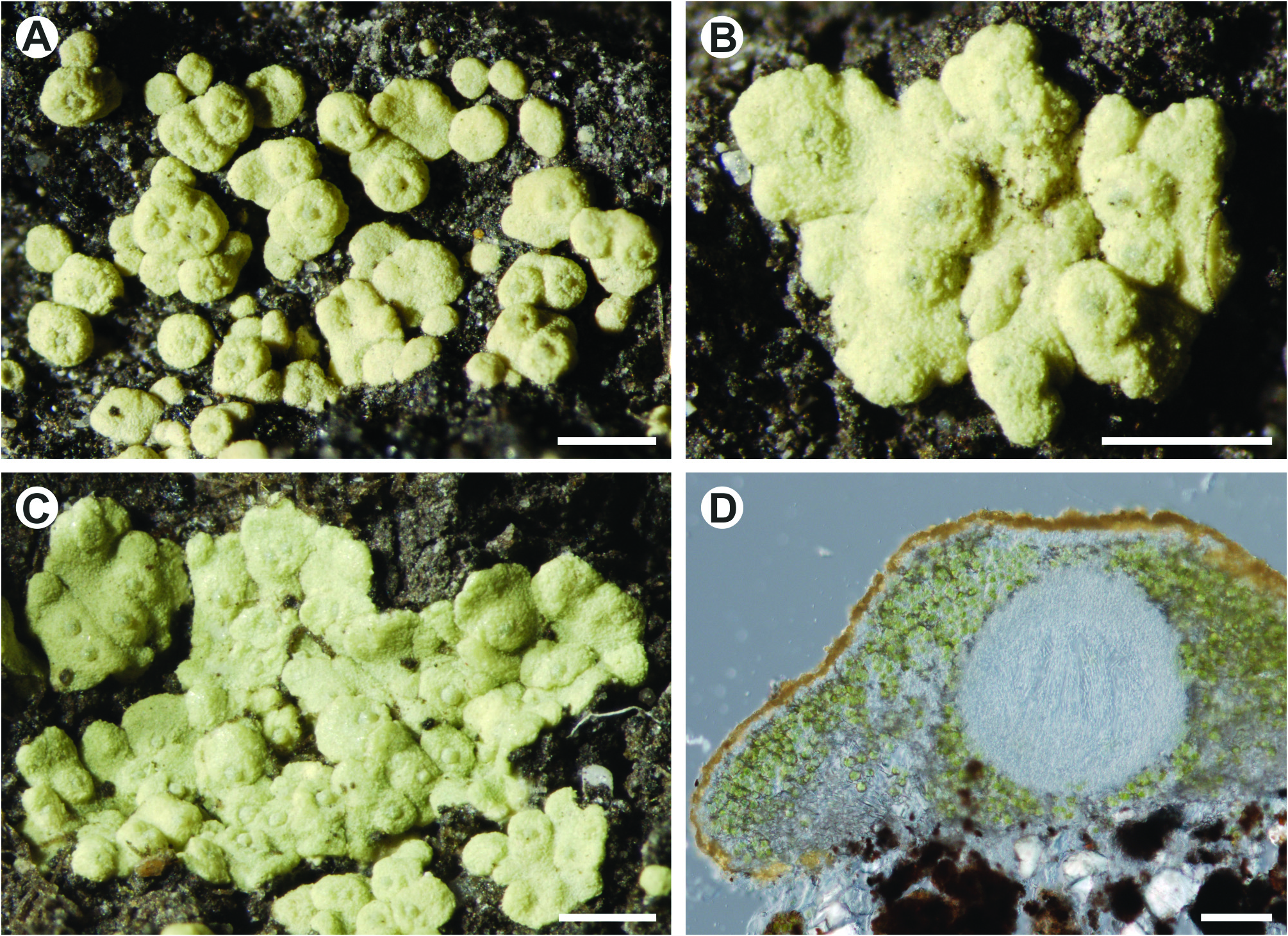
Thelocarpon andicola Flakus & Kukwa View in CoL sp. nov. ( Figs. 1A–D View FIGURE 1 , 2A–G View FIGURE 2 ) Mycobank MB 809675
Diagnosis: Differs from other Thelocarpon species by the following combination of characters: distinctly squamulose to placodioid and yellow pruinose thallus with numerous ascomata, non-amyloid hyemnial gel, branched paraphyses, amyloid asci without mass axial and broadly ellipsoid ascospores.
Type: — BOLIVIA. DEPT. COCHABAMBA: Prov. Carrasco, Parque Nacional Carrasco, Koricaza , 17°33’21”S, 65°16’29”W, 2950 m, Páramo Yungueño, on soil rich in organic humus, 18 Aug. 2012, A. Flakus 24628 (holotype KRAM!; isotypes LPB!, UGDA!, herb. Flakus!) GoogleMaps .
Thallus lichenized, 0.4–6.0 mm in diam., 0.1–0.3 mm thick, yellow pruinose, distinctly squamulose to placodioid, faintly lobed at the margins (lobes 0.2–0.5 × 0.2–0.3 mm) with (1–)6–30 (or more) immersed perithecia when mature, in young stage only surrounding the ascomata and forming a hemispherical verrucae (0.2–0.4 mm in diam.); upper cortical layer 10–15 µm wide, composed of several layers of cells (3–4 µm in diam.), with surface covered by yellow crystals; photobiont layer continuous, 40–60 µm thick; medulla often present, white, 40–100 µm thick; lower cortical layer usually absent, 5–10 µm wide, composed of several layers of strongly gelatinized hyphae; photobiont chlorococcoid, globose, cells 7–12 um in diam.; ascomata perithecioid, globose to slightly flattened at the top, 200–400 µm wide and 200–350 µm high; exciple prosoplectenchymatous, hyaline, 10–20 µm wide; outer wall (thalline layer) 70–100 µm wide, composed of loosely arranged hyphae (2–3 µm thick) mixed with photobiont cells (7–12 µm in diam.) and covered by cortical layer; surface pruinose by yellow crystals; hamathecium with non-amyloid gel (I–, K/I–), 110–160 µm high, composed of sparsely branched and anastomosing, 1–1.5 µm wide paraphyses, and c. 15 µm long, septate, strongly branched and anastomosing periphysoids; asci flask-shaped, (70–)110–160 × (10–)25–35 µm, apex nonamyloid and without visible mass axial, walls I+ pale blue, K/I+ dark blue, endospore I+ orange, multispored (>100 ascospores); ascospores simple, hyaline, broadly ellipsoidal, 4–5.5 × 2.5–3 µm, with 1(–2) oil droplets; conidiomata pycnidial, immersed in thallus, pyriform, 80–140 µm tall, 70–120 µm wide; pycnidial wall indistinct, 4–6 µm thick, hyaline, composed of loosely arranged hyphae (textura intricata); conidiophores regularly branched with one main axis and several lateral branches; conidiogenous cells terminal, simple, elongated, 12–15 × 1.5–2 µm; conidia acrogenous, hyaline, simple, broadly ellipsoid to oblong and slightly attenuated at the base (lacriform), rarely constricted in the middle, 4–5(–6.5) × 2–3 µm, with 1(–2) oil droplets.
Chemistry: Vulpinic acid (major) and pulvinic acid (trace).
Notes: Thelocarpon andicola clearly differs from all known species of the genus by its yellow pruinose, distinctly squamulose to placodioid, thick thallus with numerous ascomata (usually 6–30). The new species most resembles T. laureri ( Flotow 1847: 65) Nylander (1855: 191) by having yellow pruinose lichenized thallus and branched paraphysoids. The later however differs in thallus composed only of verrucae containing a single ascomata ( Salisbury 1966; Kocourková-Horáková 1998; Orange et al. 2009).
Thelocarpon albidum Nylander (1853: 317) View in CoL and T. robustum View in CoL auct. brit. non Eitner (1901: 13) ( Vondrák et al. 2010: 25–26) are two other species developing thalli of crowded or plane verrucae with irregular margins, however they never form squamulose to placodioid thalli. Furthermore, the first one differs in its epruinose thallus, simple paraphyses and larger ascospores (11–17 × 5.5–9 µm), and the latter by its greyish thallus with yellow pruina only present at the apex of ascomata, and narrowly ellipsoid ascospores (2.5–5 × 1.2–2 µm) ( Orange et al. 2009; Vondrák et al. 2010).
Five further species, i.e. T. olivaceum Bouly de Lesdain (1914: 149) View in CoL , T. magnussonii Salisbury (1953: 74) View in CoL , T. nigrum Aptroot & K.H. Moon View in CoL in Moon & Aptroot (2009: 309), T. pallidum Salisbury (1953: 75) View in CoL and T. palniense Awasthi & Singh (1975: 39) View in CoL , are also known as distinctly lichenized, but differ from T. andicola View in CoL in several respects. Thelocarpon olivaceum View in CoL and T. pallidum View in CoL lack paraphyses and additionally T. pallidum View in CoL is only sometimes pruinose at the ascomatal apex ( Salisbury 1966). Thelocarpon magnussonii View in CoL , T. nigrum View in CoL and T. palniense View in CoL have black or almost black (at least when dry) and epruinose fertile verrucae. Thelocarpon magnussoni lacks paraphyses and has narrowly oblong ascospores (3.5–5 × 1.2–1.8 µm), T. nigrum View in CoL produces larger ascospores (9–12 × 5–6 µm) and the ascospores of T. palniense View in CoL are broadly ellipsoidal to subglobose (3.8 × 2.8–3.8 µm) ( Salisbury 1966; Awasthi & Singh 1975; Awasthi 1991; Moon & Aptroot 2009).
Lichenization is one of the highly successful nutritional strategies in fungi and many major evolutionary lineages of Ascomycota are derived from lichens ( Lutzoni et al. 2001). We could assume that also non-lichenized species of Thelocarpon have secondarily lost the ability to form lichen symbiosis. Thus, T. andicola , the typically lichen-forming fungus due to its fully-developed heteromerous and suqamulose thallus, can possibly bear the most ancestral features within the genus. At present, however, there is little information to support this hypothesis which certainly requires further study using molecular framework.
Distribution and habitat: So far the new species is known only from the type locality in Bolivian Andes. Its population grows in open places in the biological soil crusts where it is a dominant lichen growing together with Arthrorhaphis alpina , Baeomyces rufus and Placynthiella icmalea . We have noticed two different strategies to colonize the substrate by Thelocarpon andicola . In the first strategy, initially single, dispersed verrucae of young ascomata start to form squamules , later individual ascomata surrounded by squamules are joined and fused together leading to establish large mature thalli. In, the second observed strategy, Thelocarpon andicola produces sterile thalli, which develop numerous immersed ascomata during growth, later that fertile thalli could also fuse together to form large mature thalli.
Additional specimens examined (paratypes). BOLIVIA. DEPT. COCHABAMBA: Prov. Carrasco, Parque Nacional Carrasco, Koricaza , 17°33’21”S, 65°16’29”W, 2950 m, Páramo Yungueño, on soil rich in organic humus, 18 August 2012, M. Kukwa 11698 & 11717 ( LPB, UGDA) GoogleMaps .
| LPB |
Herbario Nacional de Bolivia, Universidad Mayor de San Andrés |
| UGDA |
Gdansk University |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Thelocarpon andicola Flakus & Kukwa
| Flakus, Adam & Kukwa, Martin 2014 |
Thelocarpon albidum
| Vondrak, J. & Palice, Z. & Khodosovtsev, A. & Postoyolkin, S. 2010: 25 |
| Eitner, E. 1901: 13 |
| Nylander, W. 1853: ) |