Tasmanicosa leuckartii ( Thorell, 1870 ) Thorell, 1870
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4213.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:9C76B987-3897-4666-87EF-62EB5BF5CF04 |
|
DOI |
https://doi.org/10.5281/zenodo.5676935 |
|
persistent identifier |
https://treatment.plazi.org/id/0B32B23C-7B07-9F5D-BEF8-3937FCF2F9EF |
|
treatment provided by |
Plazi |
|
scientific name |
Tasmanicosa leuckartii ( Thorell, 1870 ) |
| status |
comb. nov. |
Tasmanicosa leuckartii ( Thorell, 1870) View in CoL comb. nov.
Leuckart’s Wolf Spider, Hay Spider
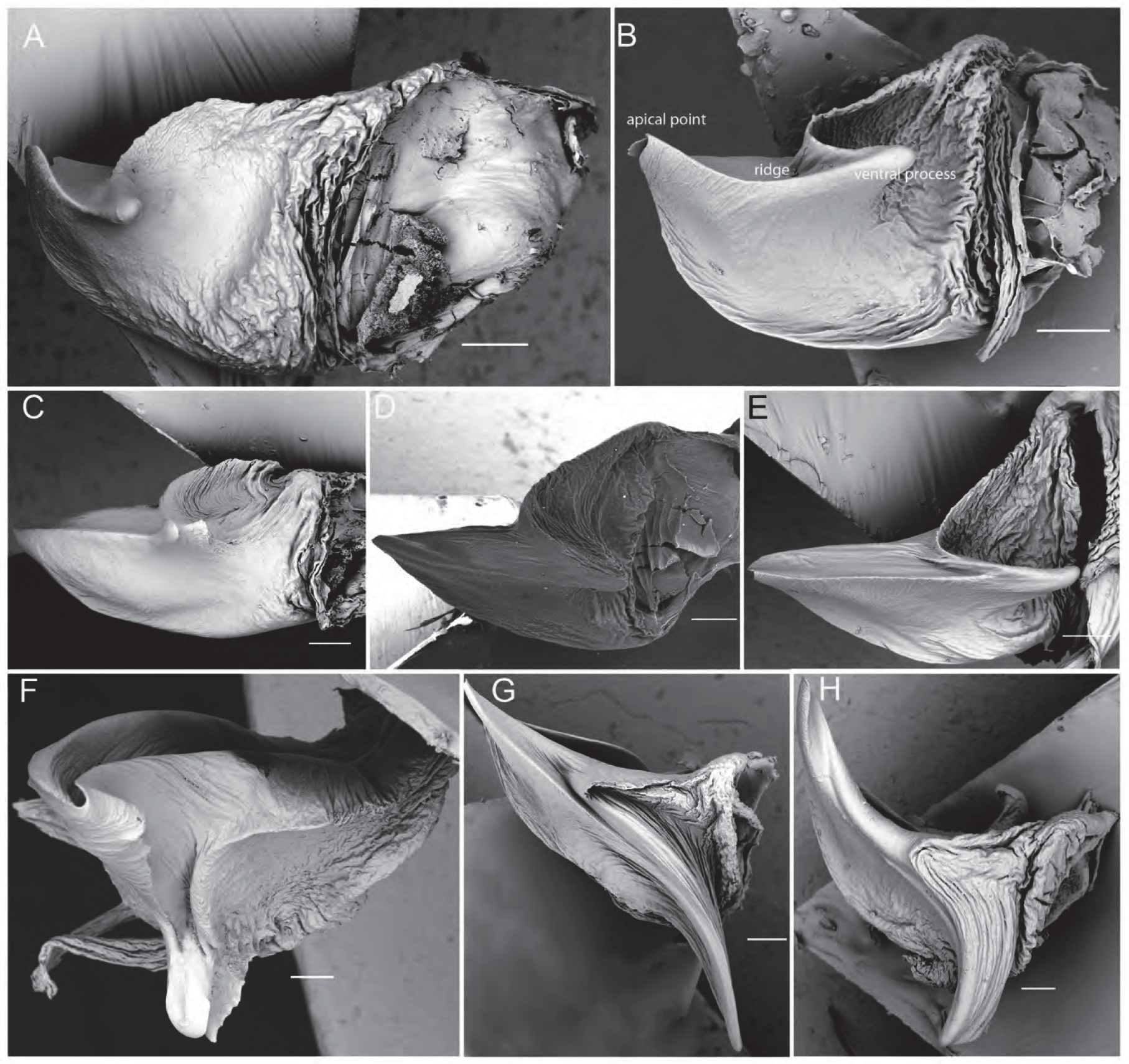
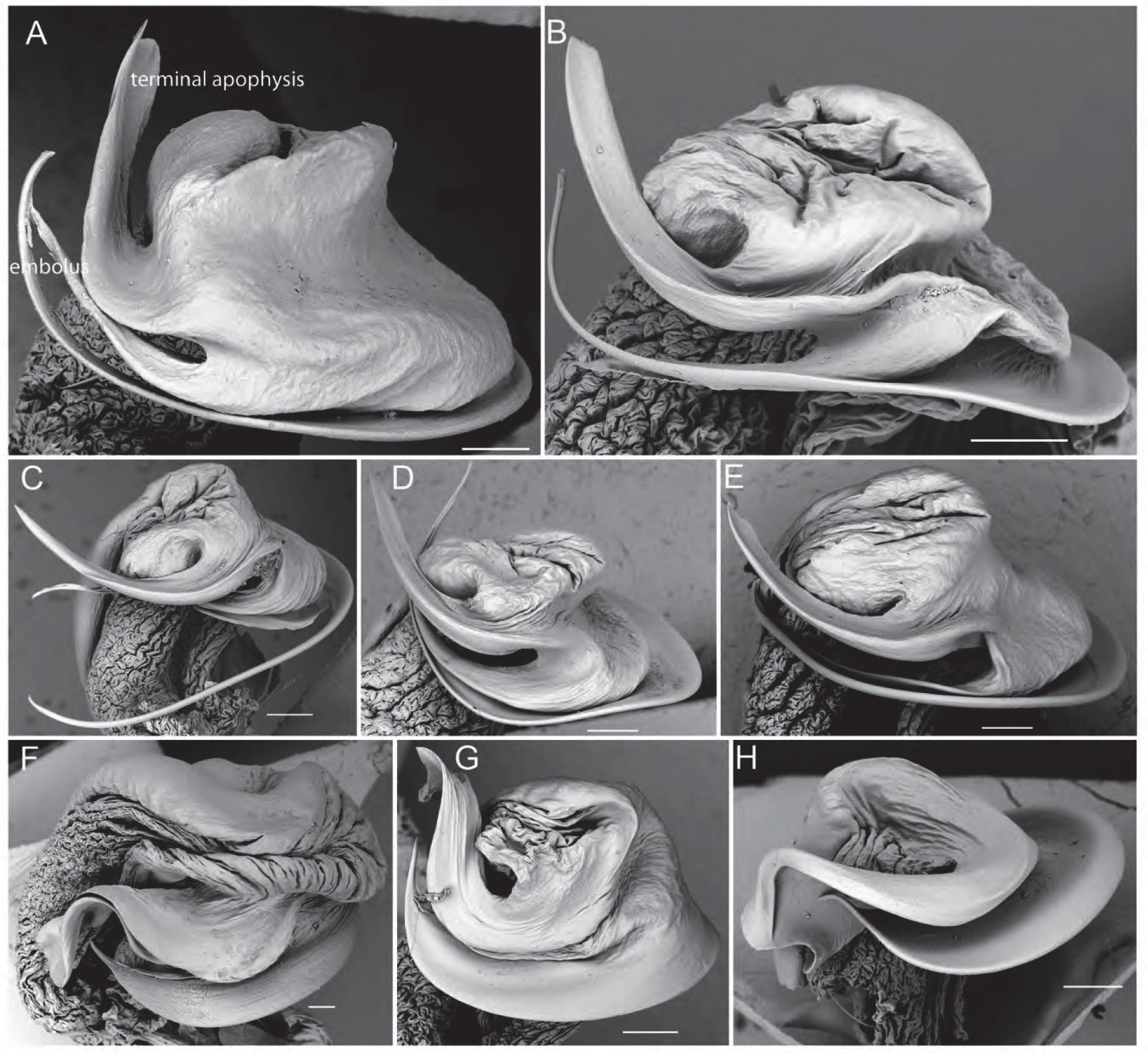
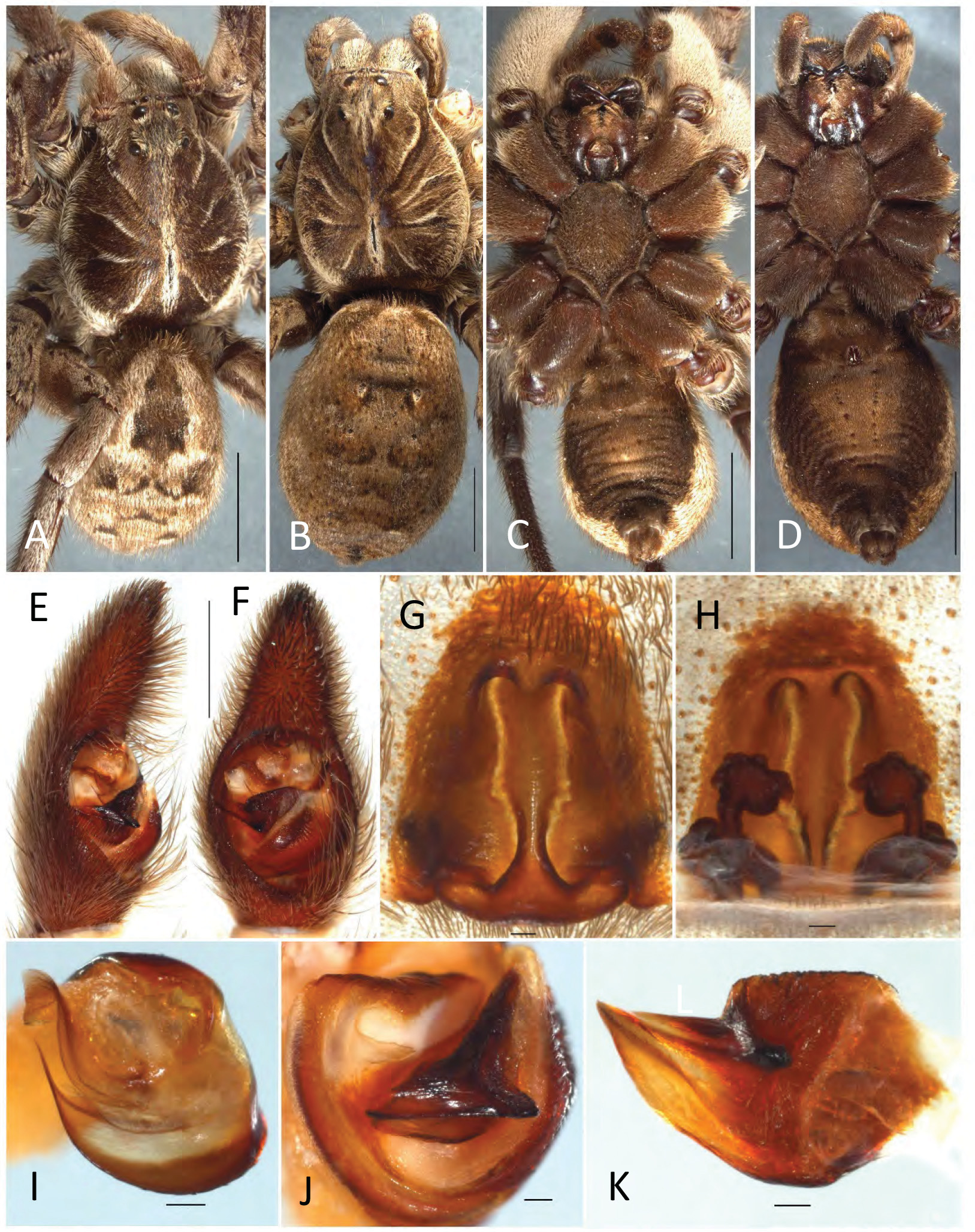
( Figs 1E–F View FIGURE 1 , 3A View FIGURE 3 , 6D View FIGURE 6 , 7G View FIGURE 7 , 16 View FIGURE 16 , 17A–K View FIGURE 17 )
Tarentula leuckartii Thorell 1870: 388 –389.
Lycosa leuckartii (Thorell) .— Koch 1877: 896 –899, pl. 77, figs 3, 3A; pl. 81, figs 1, 1A; Rainbow 1911: 269; Bonnet 1957: 2649; Main 1964: 120, figs E–F; McKay 1975: 320 –325, figs 1 A–E, 2 A–I, 3 E–F; McKay 1985: 79.
Lycosa molyneuxi Hogg 1905: 575 –577, figs 82, 82 A–B; Rainbow 1911: 270; Bonnet 1957: 2653; McKay 1973: 379; McKay 1975: 326 –328; McKay 1985: 80; Hirst 1988: 77; Platnick 1993: 488. New synonymy.
Lycosa leuckarti (Thorell) View in CoL .— Simon 1909: 182; Platnick 1993: 488.
Lycosa christopheri Simon 1909: 182 ; Rainbow 1911: 266; Bonnet 1957: 2638 (synonymy established in McKay, 1975).
Allocosa molyneuxi (Hogg) .— Roewer 1955: 206.
Avicosa christopheri (Simon) .— Roewer 1955: 236; Rack 1961: 37. Scaptocosa leuckartii (Thorell) .— Roewer 1955: 291.
Schizocosa christopheri (Simon) .— McKay 1973: 381.
Schizocosa leuckartii (Thorell) .— McKay 1973: 381.
Type data. Lectotype (designated here) of Tarentula leuckartii . Female, locality label fainted, possibly Echuka, =? Echuca, 36°08’S, 144°45’E (Victoria, AUSTRALIA), ‘ Leuckart ded.’, Collectio T. Thorell ( SMNH) (examined). GoogleMaps
Paralectotype of Tarentula leuckartii . Penultimate female, data as lectotype (examined).
Lectotype (designated here) of Lycosa christopheri . Female, Fremantle [32°03’S, 115°44’E, Western Australia, AUSTRALIA], W. Wölting leg. 1907, H. Christopher ded. 1.vii.1907 ( ZMH, Rack (1961) -catalogue 448) (examined). GoogleMaps
Paralectotype of Lycosa christopheri . Juvenile, same data as lectotype (ZMH, Rack (1961) -catalogue 448) (examined).
Holotype of Lycosa molyneuxi . Female, Gilbert River , Riverton [34°10’S, 138°45’E, South Australia, AUSTRALIA], A. Molineux ( SAM NN462) (examined). GoogleMaps
Other material examined. 1,768 males, 764 females (29 females with eggsac, 41 females with spiderlings) and 619 juveniles in 957 records (Appendix B).
Diagnosis. Tasmanicosa leuckartii is the only species in the genus which has a light patch of variable size in the centre of the venter ( Figs 17C–D View FIGURE 17 ). Genital morphology is similar to T. gilberta and T. fulgor . Males are most similar to T. gilberta , but can be distinguished by the shape of the terminal apophysis, which is broad and apically bent in T. gilberta ( Fig. 7H View FIGURE 7 ), but twisted in T. leuckartii ( Fig. 7G View FIGURE 7 ) (that of T. fulgor is broadly sickle-shaped without apical modifications ( Fig. 9J View FIGURE 9 )). Females are most similar to T. fulgor ( Fig. 4H View FIGURE 4 ), but the epigyne is much shallower and overall narrower in T. leuckartii ( Fig. 17G View FIGURE 17 ) (that of T. gilberta has a much narrower median septum ( Fig. 4G View FIGURE 4 )). Care should be taken and genitalia examined in detail for each specimens when large series of pitfall trap material of T. leuckartii are identified, as the species can be found sympatrically with T. godeffroyi , T. gilberta and T. fulgor , all of which have been found as singletons within large numbers of T. leuckartii in traps.
Description. Male (based on NMV K11541 View Materials ).
Total length 17.6.
Prosoma. Length 10.0, width 7.7; reddish-brown with genus-specific Union-Jack pattern and narrow median and lateral bands ( Fig. 17A View FIGURE 17 ); sternum very dark brown to black ( Fig. 17C View FIGURE 17 ).
Eyes. Diameter of AME 0.33, ALE 0.21, PME 0.81, PLE 0.73.
Chelicerae. Black, with an elongated patch of golden setae frontally.
Labium. Black with lighter anterior rim ( Fig. 17C View FIGURE 17 ).
Endites. Glabrous dark brown, anteriorly somewhat lighter ( Fig. 17C View FIGURE 17 ).
Legs. Greyish-brown, covered with silvery setae; venter of coxae dark brown ( Fig. 17C View FIGURE 17 ).
Opisthosoma. Length 8.7, width 6.4; dorsally with folium pattern that is bordered by light patches in particular anteriorly ( Fig. 17A View FIGURE 17 ); venter black with light discolouration centrally ( Fig. 17C View FIGURE 17 ).
Pedipalps. Cymbium dorsally with dense layer of silvery setae; tip with ca. 7–10 macrosetae ( Figs 17E–F View FIGURE 17 ); tegular apophysis with almost straight ridge which is almost as long as tegular apophysis width ( Figs 6D View FIGURE 6 , 17J–K View FIGURE 17 ). Embolus broad at base, narrowing apically into a sharp tip; terminal apophysis tip flat and broad with subapical twist ( Figs 7G View FIGURE 7 , 17I View FIGURE 17 ).
Female (based on NMV K11546 View Materials ).
Total length 17.3.
Prosoma. Length 10.0, width 7.7; colouration of carapace and sternum as male, but with less distinct radial pattern on carapace ( Figs 17B, D View FIGURE 17 ).
Eyes. Diameter of AME 0.15, ALE 0.14, PME 0.77, PLE 0.65.
Chelicerae, labium, endites, legs and opisthosoma. Opisthosoma length 12.0, width 8.2, otherwise as male, but light ventral spot larger and more distinct ( Fig. 17D View FIGURE 17 ).
Epigyne. Longer than wide; median septum inverted T-shaped with irregular edges ( Fig. 17G View FIGURE 17 ); spermathecal heads kidney-shaped, situated laterally at half of the length of the epigyne length; spermathecal stalks twisted and bent ( Fig. 17H View FIGURE 17 ).
Remarks. Thorell (1870) listed two adult females in the original species description of T. leuckartii ; however, only one of the two females in the syntype series is mature and here designated as lectotype to unequivocally fix the species-group name of the taxon. The female is redescribed here based on more recent material, as the lectotype is in poor condition.
McKay (1975) synonymised Lycosa christopheri Simon, 1909 with T. leuckartii based on the examination of the (p. 320) ‘Holotype female, Hamburg. Zool. Mus. Inst. No. 448’. In contrast, Rack (1961: 37) listed a (translated from German), ‘♀syntype (damaged)’ as her ZMH catalogue no. 448. Her syntype designation may be due to the presence of an immature spider in the same vial. Simon’s (1909) original description of L. christopheri shows that it was based on more than one specimen, as he gave a size range for the total length of the species, rather than a distinct size for a single specimen. We therefore support Rack’s (1961) assumption of a syntype series of the species consisting of a female and juvenile and here designate the female as lectotype to unequivocally fix the species-group name of this taxon.
McKay (1975) already pointed to the possibility that Lycosa molyneuxi and T. gilberta may represent junior synonyms of T. leuckartii . This is confirmed here for L. molyneuxi , of which the holotype female conforms to the species diagnosis of T. leuckartii as presented here. Lycosa molyneuxi is here considered a junior synonym of T. leuckartii . The original type locality for L. molyneuxi , ‘Gilbert River, Riverina , New South Wales’ was amended to a South Australian locality by Hirst (1988).
We consider the correct original spelling for the species-group name of Leuckart’s wolf spider to be T. leuckartii rather than T. leuckarti . This is in accordance with article 33.4 in the fourth edition of the International Code of Zoological Nomenclature ( ICZN 1999), which states the change from - ii to - i is a subsequent incorrect spelling.
Life history and habitat preferences. Habitat preferences of T. leuckartii are typical for those in the genus Tasmanicosa , with records from a variety of forest and bushland habitats (Yate, River Red Gum, Black Box, old growth mallee, Casuarina , Callitris, Acacia ) to semi-arid grasslands and paddocks. However, this species appears to be more common than other Tasmanicosa species in floodplains or near permanent to ephemeral waterbodies of varying salinity. Frequent habitat descriptions included samphire, saltbush country, river terrace, alluvial flats and clayey soil with crab holes. This is consistent with McKay’s (1975: 323) habitat description of ‘lateritic gravels, loam, or clay soils, especially on alluvial clay soils near swamps, streams, and on riverbanks’ and Main (1976) detailing ‘dry open woodland and forest with expanses of bare to lightly littered ground; invade dry pasture and crop.’
Burrows of T. leuckartii are shallow with depth ranging from approximately 60 to 110 mm in a study on South Australian floodplain populations. Burrow depth increased over summer apparently counteracting increasing temperatures, but there was no direct correlation with decreasing soil moisture ( Steggles 2001; Steggles et al. 2003). Burrow fidelity was low in this study, with spiders frequently vacating but also re-occupying empty burrows. Apparently, spiders take advantage of soil fissures in cracking clays that provide ample opportunity as day-time hideouts ( Steggles et al. 2003). Laboratory studies confirmed that an association with burrows was highest in reproductive females ( Steggles et al. 2003). The senior author of this study (VWF) has found T. leuckartii females building a thin, trapdoor-like lid on their burrow in Quorn (South Australia).
With a similar distribution pattern, the phenology of T. leuckartii appears to be very similar to that of T. godeffroyi . Females with eggsac were found predominantly between January and April, with single records from June and October. Consistent with this, most females carrying spiderlings were found in April, but also in January to March and May to June.
In some areas T. leuckartii is called the hay spider because of its abundance around stooks and bales of cut hay where it is a nuisance to farm workers ( Main 1976).
Recent experiments on the effect of T. leuckartii as natural control agents for the cotton bollworm, Helicoverpa armigera (Hübner, 1808) , indicated that these spiders can be effective predators of the cotton bollworm late instars and moths, but also suggested that, under some conditions, the presence of spiders could increase the damage to individual cotton bolls ( Rendon et al. 2016).
Distribution. Tasmanicosa leuckartii is very common south of approximately 25°S Latitude in New South Wales, South Australia and Western Australia ( Fig. 16 View FIGURE 16 ). Records in southern Victoria and Tasmania are rare and the species has only occasionally been found in Queensland.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Tasmanicosa leuckartii ( Thorell, 1870 )
| Framenau, Volker W. & Baehr, Barbara C. 2016 |
Schizocosa christopheri
| McKay 1973: 381 |
Schizocosa leuckartii
| McKay 1973: 381 |
Allocosa molyneuxi
| Roewer 1955: 206 |
Avicosa christopheri
| Rack 1961: 37 |
| Roewer 1955: 236 |
| Roewer 1955: 291 |
Lycosa leuckarti
| Platnick 1993: 488 |
| Simon 1909: 182 |
Lycosa christopheri
| Bonnet 1957: 2638 |
| Rainbow 1911: 266 |
| Simon 1909: 182 |
Lycosa molyneuxi
| Platnick 1993: 488 |
| Hirst 1988: 77 |
| McKay 1985: 80 |
| McKay 1975: 326 |
| McKay 1973: 379 |
| Bonnet 1957: 2653 |
| Rainbow 1911: 270 |
| Hogg 1905: 575 |
Lycosa leuckartii
| McKay 1985: 79 |
| McKay 1975: 320 |
| Main 1964: 120 |
| Bonnet 1957: 2649 |
| Rainbow 1911: 269 |
| Koch 1877: 896 |
Tarentula leuckartii
| Thorell 1870: 388 |