Synemon sophia ( White, 1841 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4895.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:C17AFF30-1035-4A81-8C4F-C33A430A7712 |
|
DOI |
https://doi.org/10.5281/zenodo.4332210 |
|
persistent identifier |
https://treatment.plazi.org/id/03FD87E7-140E-F17E-EAAD-DAD6FC3FB091 |
|
treatment provided by |
Plazi |
|
scientific name |
Synemon sophia ( White, 1841 ) |
| status |
|
Synemon sophia ( White, 1841) View in CoL
Flame Sun-moth
Figs 1 View FIGURES 1–2 , 3–6 View FIGURES 3–10 , 19, 20 View FIGURES 19, 20 , 35 View FIGURES 35–42
Hesperia sophia White, In Grey, G. (ed.). 1841: 474, fig. 7.
Literature: Doubleday 1846: 516, pl. 3, fig. 5; Klug 1850: 248, figs 1, 2 (part misidentification of S. parthenoides ); Walker 1854: 34; Walker 1856: 1583; Boisduval [1875]: 550; Stretch 1875: 13; Butler 1877: 6, pl. 3, fig. 6; Westwood 1877: 194, pl. 33, fig. 14a–h (part misidentification of S. cf. leucospila Meyrick, 1891 ); Kirby 1885: 169; Guest 1886: 62 (misidentification of S. parthenoides ); Tepper 1890: 16 (misidentification of S. parthenoides ); Kirby 1892: 10 (part misidentification of S. cf. leucospila ); Lower 1892: 10 (misidentification of S. petrophila sp. n.); Lower 1893: 13 (misidentification of S. parthenoides ); Aflalo 1896: 262 (misidentification of S. parthenoides ); Kirby 1897: 39; Froggatt 1907: 232 (misidentification of S. cf. leucospila ); Strand 1911a: 1 (part misidentification of S. parthenoides ); Strand 1911b: 139 (part misidentification of S. parthenoides ); Dalla Torre 1913: 20 (part misidentification of S. parthenoides and S. cf. leucospila ); Common 1970: 828 (misidentification of S. parthenoides ); Laithwaite, Watson & Whalley 1975: 284, pl. 45 (misidentification of S. cf. leucospila ); Common & Edwards 1981: 295; (misidentification of S. parthenoides ); Fletcher & Nye 1982: 157; McQuillan & Forrest 1985: 12, fig. C14 (misidentification of S. parthenoides ); Miller 1986: 489–494, figs 208, 210–211 (misidentification of S. parthenoides ); Edwards 1996: 138; Edwards 1997: 8–9; Williams et al. 2016: 101–102, figs 10, 11.
Type material examined. Lectotype: Ƌ ( Fig. 1 View FIGURES 1–2 , here designated), ‘1652a’ (and on reverse) ‘16 12 40 316’ (numbers in circle on round label, some of the numbers crossed out but the specimen, interpreted from the register, accessed on 16 December, 1840, accession number 316, Hesperia Pres. By Captain Grey ) ‘ Synemon sophia ’ ‘Specimen photog for CHECKLIST AUST LEP Film 109/13’ (antennae missing) ( NHMUK). Paralectotype: 1♀, ‘1652b’ (and on reverse) ’16 12 40 317’ (also partly crossed out, the register gives the same data) ‘ sophia ’ (abdomen missing) ( NHMUK).
Additional material examined. 1Ƌ ‘Zell[er]. Coll[ection] 1884’ ( NHMUK); 1Ƌ ‘Zell[er]. Coll[ection] 1884, Synemon selene Klug Mon. p. 249, f. 3, 4. Austral[ia] 1870’ ( NHMUK); 1♀ ‘ Synemon sophia White Australia’ ( MAMU); 2Ƌ ‘K[ing]. G[eorge]. S[ound]. K 7688’ ( AMS); 1♀ ‘K[ing]. G[eorge]. S[ound]., Synemon sophia White ssp. parthenoides Feld. ’ ( Fig. 20 View FIGURES 19, 20 , Castniidae slide No 3, AMS); 1Ƌ ‘Wilsons Inlet S.W. Australia 11 Nov. [19]12 [F.B.L. Whitlock], G.M. Goldfinch Collection’ ( Fig. 19a, d View FIGURES 19, 20 , Castniidae slide No 2, AMS); 1Ƌ, 1♀ ‘Victoria’ (mislabelled!) ( MAMU); 1Ƌ, Crystal Springs, 7 mi W Walpole, 14.xii.1970, G.A. Holloway & H. Hughes ( Fig. 19b, c View FIGURES 19, 20 , Castniidae slide No 1, AMS); 1Ƌ, 1♀, 34.58S 117.55E Hooper Rd, Albany, 26.xi.1996, 28.xi.1996, E.D. Edwards ( ANIC); 15Ƌ, 2♀, 3457’ 19.5 S View Materials 11636’ 15.9E, 2.5km N of Crystal Springs, 17.xi.2010 (2Ƌ, 1♀), 18.xi.2010 (9Ƌ, 2♀), 24.xi.2010 (4Ƌ, 1♀), A.A.E. Williams ( WAM, ANIC) ( Figs 3, 4 View FIGURES 3–10 ); 2Ƌ, 1♀, D’Entrecasteaux N.P., NE of Walpole, Junction South Western High-way and Railway Parade, 2–4.xi.2007, D.J. Hilton ( ANIC, CAK, CDH) ( Figs 5, 6 View FIGURES 3–10 ); 11Ƌ, 3♀, 2.1 km north of Crystal Springs, 3457’35.7” S 11635 View Materials ’59.7”E, 3.xii.2009, T. Gamblin (1Ƌ), 16.xi.2010, T. Gamblin (2Ƌ), 17.xi.2010, A.A.E. Williams (4Ƌ, 2♀), 19.xi.2010, T. Gamblin (2Ƌ, 1♀), 24.xi.2010, A.A.E. Williams (1Ƌ), 3457’34.4” S 11636 View Materials ’00.7”E, 10.xi.2017, A.A.E. Williams (1Ƌ) ( WAM); 6Ƌ, 3♀, 10 km S of Northcliffe, 3442’36.8”S, 11605’37.2”E, 9.xi.2017, A.A.E. Williams ( WAM); 11Ƌ, 2♀, D’Entrecasteaux N.P., 3442’55.2” S 11605 View Materials ’34.8”E, 9.xi.2017 (3Ƌ), 3442’56.8” S 11605 View Materials ’15.4”E, 9.xi.2017 (2Ƌ), 3444’43” S 11605 View Materials ’05”E, 9.xi.2017 (5Ƌ, 1♀), 13443’58.7” S 11605 View Materials ’09.0”E, 9.xi.2017 (1♀), 3443’25.5” S 11604 View Materials ’19.2”E, 9.xi.2017 (1Ƌ), A.A.E. Williams ( WAM); 1Ƌ, 1♀, D’Entrecasteaux N.P., Windy Harbour Road, 3443’58.7” S 11605 View Materials ’09.0”E, 23.xi.2017, A.A.E. Williams ( WAM); 1♀, D’Entrecasteau N.P., Gardner River, 3446’ 41.1 S View Materials 11610’43.2”E, 23.xi.2017, A.A.E.Williams ( WAM).
Synemon sophia is the type species of the genus. It was described by White (1841) who gave the type locality as ‘King George’s Sound’ and the collector as Captain George Grey. Captain Grey spent the latter part of 1839 and early 1840 at Albany ( Edwards 1997), a town in south-western Australia beside Princess Royal Harbour which opens into King George Sound. White did not indicate the number of specimens on which his description was based but the register of the NHMUK lists only these two specimens above accessed under ‘ Hesperia ’ from Captain Grey. As it is possible, however, that other syntypes exist that belong to different taxa, we here designated a lectotype to assure taxonomic stability.
Synemon sophia has had a confusing history. In almost all studies published after the original description, other species, most notably specimens close to S. leucospila Meyrick, 1891 and S. parthenoides , were misidentified as S. sophia . This was only rectified by Edwards (1996, 1997), who studied the type specimens of S. sophia and came to the conclusion that this name had been misused in most publications. Williams et al. (2016) correctly applied the name, figured S. sophia for the first time and applied the name Flame Sun-moth.
Redescription. Male ( Figs 3, 4 View FIGURES 3–10 ). Alar expanse 33–39 mm, forewing length 15–18 mm, body length 15–19 mm. Head, vertex dark grey with piliform and lamellar scales, frons with grey and white piliform scales, labial palpi porrect, projecting to about frons, grey above white beneath, haustellum well developed, antenna black annulated with white scales, distal few flagellar annuli white beneath, club black above, white beneath, nudum 21–23 dark brown, on anterior half of club, apiculus short, stout of single annulus. Thorax above dark grey of mixed piliform and broad lamellar scales, with a scattering of long pale grey hair scales when thoracic vestiture is intact, beneath white with some pale yellow, legs dark grey above, pale orange beneath, epiphysis clothed in short spines, tip reaching to foretibia. Abdomen black above, long orange hair scales on T2–T4 and some on T5, posterior to T4 with brown-orange scales, orange laterally, beneath pale grey.
Forewing costa slightly arched, apex very rounded, termen broadly rounded, inner margin almost straight. Upperside black, with markings of white and pale blue scales; subbasal area with scattered pale blue scales, submedian area with scattered bluish grey scales, sometimes sufficient to form a broad, very ill-defined submedian band in the discal area, a narrow white line at end of cell, proximal to this an area more intensely black, line sometimes straight, sometimes bent and followed distally by scattered pale blue and white scales to another less well defined white spot beyond cell, postmedian band represented by some white scales between M3 and CuA2, and a thicker scattering of pale blue scales, a subapical band of white spots, well developed, extending from R2 to CuA1, in an even arc, a narrow band of scattered pale blue scales well beyond subapical band, a narrow submarginal band of pale blue scales extending to 1A+2A, and a terminal area mostly black with scattered pale blue scales. Cilia grey. Underside black with white and deep orange markings; a broad deep orange median band from costa to CuA2, displaced distally at M3, a broad subapical band deep orange extending from R2 to M3, approaching median band near M3, a submarginal band of spots extending from apex to CuA2, those near apex white and merging together, the remainder deep orange with those between M1 and M3 merging with the subapical band, a narrow black terminal line. Cilia grey.
Hindwing termen evenly rounded, slightly flattened in tornal area. Upperside black with orange or deep orange spots; a scattering of orange scales towards base, a large deep orange spot at distal end of cell, a median band of deep orange spots extending from just beyond M1 to 1A+2A, a gap of ground colour between M3 and CuA1, spots between M1 and M3 and those between CuA1 and 1A+2A confluent, a submarginal band of deep orange discrete spots from Rs to 1A+2A, those anterior to M3 very small, sometimes absent or only some present, anal area orange merging into large deep orange tornal spot which is joined to the median band. Cilia grey, orange between Rs and M2 and at tornus. Underside black with white and deep orange spots; portion of wing anterior to median vein and M1 grey, posterior black, a deep orange spot at distal end of cell, a median band of deep orange spots from Sc+R1 to 1A+2A, spots between M1 and M3 confluent and centred with white, a gap to CuA1, spots between CuA1 and 1A+2A confluent, sometimes also centred with white, a submarginal row of spots from Sc+R1 to 1A+2A, those anterior to M3 small and some white or pale orange, a narrow black terminal line, anal area grey with orange scales merging to orange tornal area. Cilia grey, orange between Rs and M2 and at tornus.
Female ( Figs 5, 6 View FIGURES 3–10 ). Alar expanse 44–49 mm. Similar to male, larger, wings slightly longer, coloration similar to male but more extensively brown-orange on abdomen.
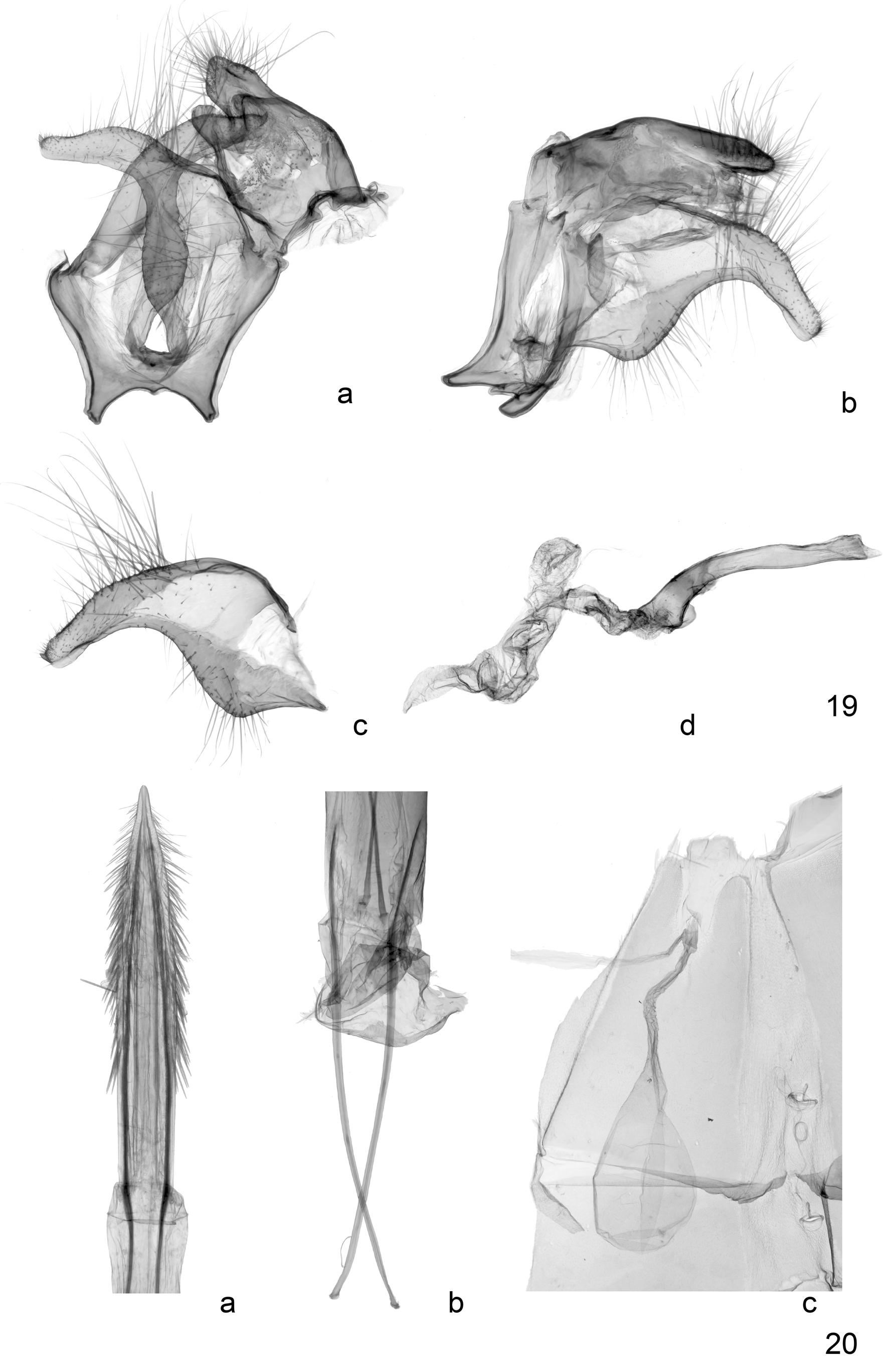
Male genitalia ( Fig. 19 View FIGURES 19, 20 ). Uncus wide, tapering towards the tip, with long setae; gnathos arms shorter than uncus; anal tube well sclerotized; tegumen broad, slightly curved; vinculum angled, evenly sclerotized; saccus with well-developed bifurcated arms, broad and well separated; juxta well developed; valva elongate, strongly curved ventrally, narrowing abruptly at about 2/3 its length, tip with upturned point, sacculus and costa with stout setae; phallus long, narrow, of even diameter, well sclerotized, tip oblique, barely curved; ductus ejaculatorius longer than phallus, with several coils.
Female genitalia ( Fig. 20 View FIGURES 19, 20 ). Papillae anales narrow, sharply pointed, short and heavily sclerotized; ovipositor long, narrow, extensible heavily sclerotized, with numerous stout dorsal and lateral spines near tip; apophyses long, well-sclerotized, apophyses anteriores less than half the length of apophyses posteriors which extend from the tip of the papillae; sinus vaginalis broad; ostium bursae in long unsclerotized window extending about one quarter of way down S7; ductus seminalis from close to ostium; ductus bursae long, not coiled, corpus bursae large, ovoid, without signum, extending beyond S7.
Diagnosis. Synemon sophia is similar to several species, including S. cf. leucospila ( Figs 7–10 View FIGURES 3–10 , see also notes below), a common species from Western Australia, S. parthenoides ( Figs 11–14 View FIGURES 11–18 ) from south-eastern Australia, and S. maja Strand, 1911 ( Figs 15–18 View FIGURES 11–18 ), also from Western Australia. Synemon sophia can be separated from all three species by the extensive orange marking of the hindwing upperside in both sexes (lighter orange to yellow and less extensive in the species compared, very pale in S. maja and female S. cf. leucospila ). Synemon sophia differs from S. cf. leucospila and S. parthenoides by the shorter and wider wings, the orange-yellow markings of the hindwing, which extend into the anal area and the apex of the hindwings in particular in females (not extending into anal area and apex of the hindwing in S. cf. leucospila , with only small marks in the apex of the hindwing in S. parthenoides ); the more extensive and regular scattering of grey scales across the forewing (less pronounced and less extensive in S. cf. leucospila and S. parthenoides ); the less distinct discal spots of the forewing; the more extensive orange markings on the ventral side of their wings, and the extensive white scales that cover a broad band along the costa, the outer orange spots and the apex of the hindwing (much reduced in in S. cf. leucospila , absent in S. parthenoides ). Due to the more extensive orange markings of the hindwing, the black anal area forms a distinct projection along vein 1A+2A in S. sophia , which is lacking in S. cf. leucospila and less pronounced in S. parthenoides ). Furthermore, males of S. sophia are characterised by a black area along anal margin of the forewing (lacking in the other three species). Importantly, S. sophia differs from S. cf. leucospila , the species with which it is most likely to be confused, by the different shape of the valva of the male genitalia ( Figs 19 View FIGURES 19, 20 , 21 View FIGURES 21, 22 ). Finally, S. sophia differs from S. maja by the coloration of the forewings (with wide somewhat diffuse light postmedial and subapical bands in S. maja , largely absent in S. sophia ).
Variation. Some specimens have orange, rather than deep orange, spots on the hindwing. The extent of the white centring in the deep orange spots of the median band on the underside of the hindwing varies, as does the number of spots anterior to M 3 in the submarginal band on the upperside of the hindwing.
Distribution. Limited to the high rainfall areas of the south-west corner of Western Australia.
Habitat and Biology ( Fig. 35 View FIGURES 35–42 ). Most specimens have been recorded between early and late November, exceptionally as late as early and mid-December. Near Crystal Springs, S. sophia favors low-lying winter-wet swampland alongside Railway Parade ( Fig. 35 View FIGURES 35–42 ), where the vegetation is dominated by tall dense sedges and stands of Melaleuca shrubs ( Myrtaceae ) on moist peaty soil. In this habitat, both male and female sun-moths were encountered along old overgrown vehicle tracks in boggy soil where regenerating Lyginia sp. ( Restionaceae ), the suspected food-plant, was common. Adult S. sophia also occupied slightly elevated sandy areas adjacent to the swampland. In these elevated areas, male sun-moths often followed regular flight pathways round visually prominent patches of trees and exposed granite sheeting.
At D’Entrecasteaux National Park, S. sophia is widespread in the extensive winter-wet sedge swamplands and adjacent marginally elevated sandy ridges, where the Lyginia sp. is plentiful. In November 2017, after a recent fire, the sun-moths were locally common and active in warm sunny conditions between 10:00 am and 3 pm. Adults flew close to the ground amongst sedges and along sandy tracks close to swampy areas. A female was observed ovipositing at the base of Lyginia species.
Remarks. Based on an unpublished molecular study by the authors, specimens of the ‘ Synemon sp. Perth species complex’ (Williams et al. 2016) cluster closely with S. leucospila and may indeed represent a yellow southern form of this species. Typical northern S. leucospila specimens from Western Australia have hindwings with very pale cream to almost white markings (see Williams et al. 2016). Specimens from South Australia that belong to this group were described as S. larissa Grund & Stolarski, 2012 . The taxonomy of this group needs to be clarified. The common name ‘Flame Sun-moth’ was coined by Williams et al. (2016).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.