Sylvicanthon obscurus ( Schmidt, 1920 )
|
publication ID |
https://doi.org/ 10.5852/ejt.2018.467 |
|
publication LSID |
lsid:zoobank.org:pub:8D27AAB8-B7F2-424C-B1A6-66FEFA66EDFF |
|
persistent identifier |
https://treatment.plazi.org/id/A72C87FB-FF06-FF25-0D36-098C0ACA95C7 |
|
treatment provided by |
Valdenar |
|
scientific name |
Sylvicanthon obscurus ( Schmidt, 1920 ) |
| status |
|
Sylvicanthon obscurus ( Schmidt, 1920) View in CoL
Figs 7B View Fig , 11D View Fig , 12B View Fig , 13B View Fig , 15M View Fig , 16C View Fig , 19B View Fig , 20 View Fig , 40B View Fig , 41–42 View Fig View Fig
Canthon obscurus Schmidt, 1920: 131–133 View in CoL .
Canthon obscurus View in CoL – Schmidt 1922: 64, 78. — Balthasar 1939: 187–188. — Halffter & Martínez 1977:
63. — Krajcik 2012: 64. Canthon obscurum – Blackwelder 1944: 200. Glaphyrocanthon (Glaphyrocanthon) obscurus – Pereira & Martínez 1956: 126, 128. — Pereira &
Martínez 1960: 45. — Martínez et al. 1964: 5, 8, 13. — Vulcano & Pereira 1964: 663. Sylvicanthon obscurum : Vaz-de-Mello 2000: 195. Sylvicanthon View in CoL sp. – Costa et al. 2009: 90; 2013: 330–331. — Silva et al. 2010: 362. — Iannuzzi et al.
2016: 201.
Etymology
From the Latin word ‘ obscurus ’, meaning ‘dark’. Probable reference to the dark green or dark blue elytra.
Material examined
Lectotype (here designated)
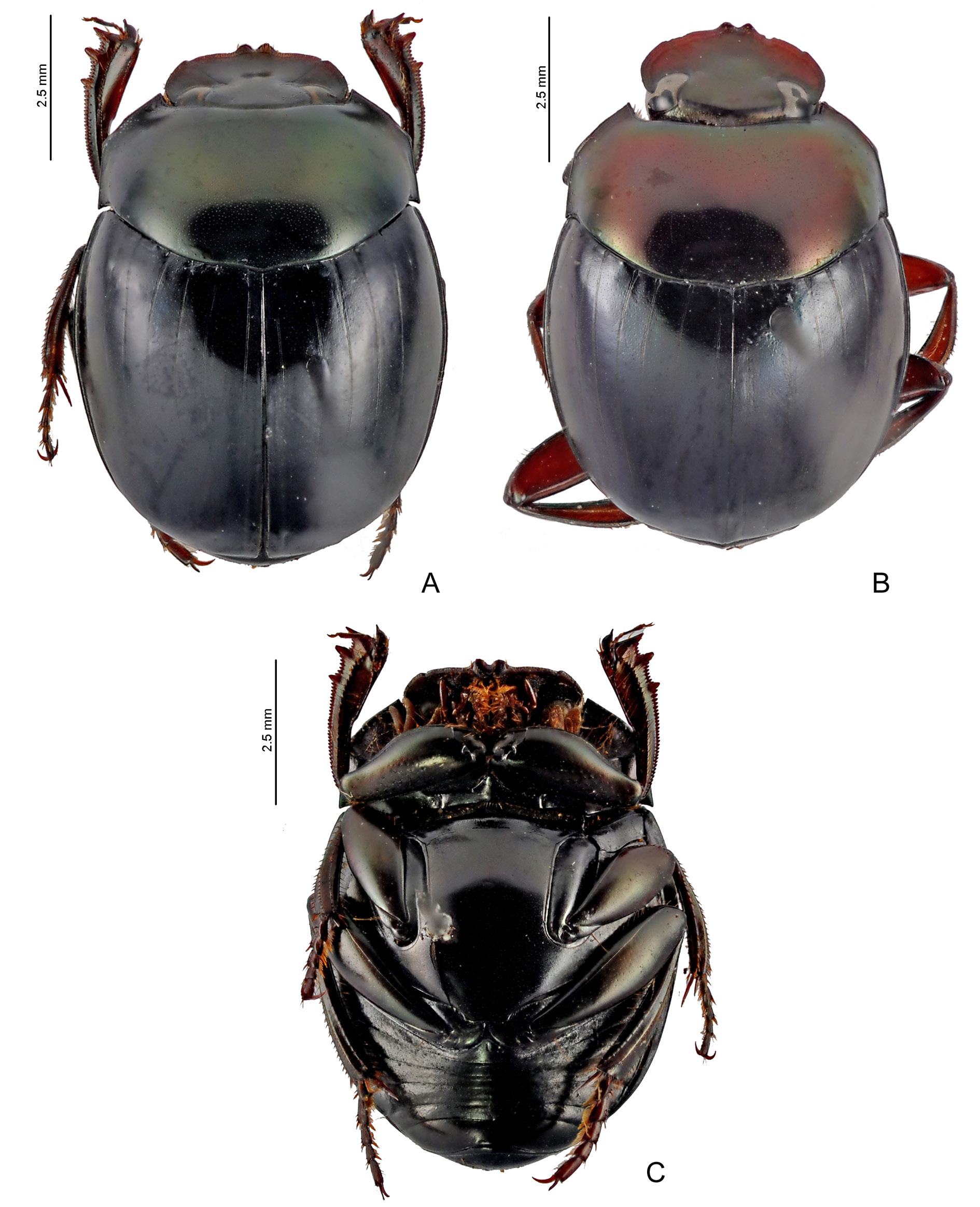
BRAZIL: ♂, Espírito Santo, (“ Esp. Santo ”, “Coll. C. Felsche / Kauf 20, 1918”, “Typus”, “ Canthon / obscurus / A. Schmidt ”, “ LECTOTYPE ♂ / Canthon / obscurus / Schmidt / des. F. Z. Vaz-de-Mello, 20 14 ”) ( SMTD) ( Fig. 40 View Fig Ba–b).
Paralectotypes (7 ♂♂, 4 ♀♀)
BRAZIL: 1 ♂, (“ LECTOTYPE ♂ / Canthon / obscurus / A. Schmidt / des. F. Z. Vaz-de-Mello, 20 14 ”, “ PARALECTOTYPE / ♂ / Canthon / obscurus Schm. / des. F. Z. Vaz-de-Mello, 20 13 ”, “ Glaphyrocanthon / obscurus / (Schm.) / P. Pereira det. 60 ”, “ obscurus / A. Schm.”, “9933 / E92 +”, “ obscurus ”, “Espir. / Santo”) ( NHRS) ( Fig. 40 View Fig Bc); 1 ♂, (“ PARALECTOTYPE / ♂ / Canthon / obscurus Schmidt / des. F. Z. Vaz-de-Mello, 20 13 ”, “ obscurus ”, “Espir. / Santo”, “9935 / E92 +”) ( NHRS); 1 ♀, (“ PARALECTOTYPE / ♀ / Canthon / obscurus Schmidt / des. F. Z. Vaz-de-Mello, 20 13 ”, “29 / 56 ”, “9936 / E92 +”, “ obscurus ”, “Esp. Santo”, “obscurus / Schmidt”) ( NHRS) ( Fig. 40 View Fig Bd); 1 ♀, (“ Glaphyrocanthon / obscurus / (Schm.) / P. Pereira et. 60 ”, “Espir. Santo”, “9934 E92 +”, “ PARALECTOTYPE / ♀ / Canthon / obscurus Schmidt / des. F. Z. Vaz-de-Mello, 20 13 ”) ( NHRS); 1 ♂, (“ PARALECTOTYPE / ♂ / Canthon / obscurus / Schmidt / des. F. Z. Vaz-de-Mello, 20 14 ”, “Coll. C. Felsche / Kauf 20, 1918”, “Coll. C. Feslche / Kauf 20, 1918”) ( SMTD); 1 ♂, (“ PARALECTOTYPE / ♂ / Canthon / obscurus / Schmidt / des. F. Z. Vaz-de- Mello, 20 14 ”, “Coll. C. Felsche / Kauf 20, 1918”, “Esp. Santo”), ( SMTD); 1 ♂, (“ PARALECTOTYPE / ♂ / Canthon / obscurus / Schmidt / des. F. Z. Vaz-de-Mello, 20 14 ”, “Coll. C. Felsche / Kauf 20, 1918”, “Esp. Santo”) ( SMTD); 1 ♂ (“ PARALECTOTYPE / ♂ / Canthon / obscurus / Schmidt / des. F. Z. Vaz-de- Mello, 20 14 ”, “Coll. C. Felsche / Kauf 20, 1918”, “Esp. Santo”) ( SMTD); 1 ♀, (“ PARALECTOTYPE / ♀ / Canthon / obscurus / Schmidt / des. F. Z. Vaz-de-Mello, 20 14 ”, “Coll. C. Felsche / Kauf 20, 1918”, “Esp. Santo”) ( SMTD) ( Fig. 40 View Fig Be); 1 ♀, (“ PARALECTOTYPE / ♀ / Canthon / obscurus / Schmidt / des. F. Z. Vaz-de-Mello, 20 14 ”, “Coll. C. Felsche / Kauf 20, 1918”, “Esp. Santo”) ( SMTD); 1 ♂, (“S. Amerika / W. Meier / Hamburg ”, “ Ypilissus spec? ”, “ Canthon / obscurus / A. Schmidt ”, “ SYNTYPUS / Canthon / obscurus Schmidt, 1920 / labelled by MNHUB 2014”) ( ZMHB).
Additional material (141 ♂♂, 126 ♀♀)
BRAZIL: 1 ♂, no further data ( NMPC, ex coll. Balthasar, B. Schwarzer and Lansberge). – Alagoas: 1 ♂, 2 ♀♀, Ibateguara, edge of Coimbra fragment, 6 Oct. 2011, B. Filgueiras leg. ( UFPE). – Bahia: 1 ♂, Encruzilhada, Nov. 1980, A. Martínez and M. Alvarenga leg. ( CMNC); 1 ♀, Santa Teresinha, Serra da Jiboia, 12º51,31′ S, 39º28,575′ W, 2 Feb. 2009, P.P. Lopes and L.R. M. Oliveira leg. ( MZFS). – Espírito Santo: 1 ♀, no further data ( BMNH); 1 ♂, no further data ( MNHN, van de Poll collection); 1 ♂, no further data ( ZMHB); 5 ♂♂, 3 ♀♀, Marechal Floriano, Jan. 2003, pitfall, L. Dias leg. ( CEMT); 1 ♂, Santa Teresa, Estação Biológica Augusto Ruschi, Trilha da Preguiça, 19º54′39″ S, 40º32′30″ W, 760 m, 29 Jan. 2015, pitfall with human faeces, T. Vargas leg. ( CEMT); 3 ♂♂, Santa Teresa, Estação Biológica Augusto Ruschi, Trilha da Preguiça, 19º54′37″ S, 40º32′31″ W, 761 m, 29 Jan. 2015, pitfall with human faeces, T. Vargas leg. ( CEMT); 1 ♂, Santa Teresa, Estação Biológica de Santa Lúcia, 19º58′25″ S, 40º31′45″ W, 648 m, 29 Jan. 2015, pitfall with human faeces, T. Vargas leg. ( CEMT); 1 ♂, Santa Teresa, Estação Biológica de Santa Lúcia, Trilha Bonita, 19º58′26″ S, 40º31′46″ W, 659 m, 29 Jan. 2015, pitfall with human faeces, T. Vargas leg. ( CEMT); 1 ♀, Santa Teresa, Estação Biológica de Santa Teresa, Trilha Bonita, 19º58′30″ S, 40º31′50″ W, 684 m, 29 Jan. 2015, pitfall with human faeces, T. Vargas leg. ( CEMT); 1 ♂, Santa Teresa, Estação Biológica de Santa Lúcia, Trilha do Rio, 19º58′22″ S, 40º31′45″ W, 649 m, 29 Jan. 2015, pitfall with human faeces, T. Vargas leg. ( CEMT); 1 ♂, Santa Teresa, Estação Biológica de Santa Lúcia, Trilha Indaia-Açu, 19º57′56″ S, 40º32′24″ W, 626 m, 28 Jan. 2015, pitfall with human faeces, T. Vargas leg. ( CEMT); 1 ♀, Santa Teresa, Estação Biológica de Santa Teresa, 19º57′57″ S, 40º32′23″ W, 631 m, 29 Jan. 2015, pitfall with human faeces, T. Vargas leg. ( CEMT); 1 ♂, Santa Teresa, Estação Biológica de Santa Teresa, Trilha Indaia-Açu, 19º57′57″ S, 40º32′21″ W, 661 m, 29 Jan. 2015, pitfall with human faeces, T. Vargas leg. ( CEMT); 3 ♂♂, 1 ♀, Santa Teresa, Estação Biológica de Santa Teresa, Trilha Indaia-Açu, 19º58′18″ S, 40º32′09″ W, 742 m, 29 Jan. 2015, pitfall with human faeces, T. Vargas leg. ( CEMT); 5 ♂♂, 1 ♀, Santa Teresa, Estação Biológica de Santa Lúcia, Trilha Indaia-Açu, 19º58′13″ S, 40º32′06″ W, 779 m, 29 Jan. 2015, pitfall with human faeces, T. Vargas leg. ( CEMT); 3 ♂♂, Santa Teresa, Estação Biológica de Santa Lúcia, Trilha Tapinoã, 19º58′07″ S, 40º31′55″ W, 679 m, 29 Jan. 2015, pitfall with human faeces, T. Vargas leg. ( CEMT); 7 ♂♂, 2 ♀♀, Santa Teresa, Estação Biológica de Santa Lúcia, Trilha Tapinoã, 19º58′10″ S, 40º31′48″ W, 692 m, 29 Jan. 2015, pitfall with human faeces, T. Vargas leg. ( CEMT); 1 ♂, 1 ♀, Venda Nova do Imigrante, Dec. 2000, F.Z. Vaz-de-Mello (“V-de-M”) leg. ( AMBC); 1 ♂, same collecting data as for preceding ( CEMT); 41 ♂♂, 42 ♀♀, Venda Nova do Imigrante, Lavrinhas, 20º12′29″ S, 41º07′23″ W, 850 m, 10–14 Jan. 2011, human faeces, F.Z. Vaz-de-Mello leg. ( CEMT); 31 ♂♂, 39 ♀♀, Venda Nova do Imigrante, Lavrinhas, 20º18′40″ S, 41º08′16″ W, Dec. 2012, L.F. Vaz-de-Mello leg. ( CEMT); 22 ♂♂, 20 ♀♀, Venda Nova do Imigrante, Lavrinhas, 20º12′29″ S, 41º07′23″ W, I.2013, L.F. Vaz-de-Mello leg. ( CEMT). – Minas Gerais: 4 ♂♂, 7 ♀♀, Berizal, Barreiros, Serra do Anastácio, 1375 m, 18–19 Dec. 2012, flight interception trap, P. Grossi leg. ( CEMT). – Pernambuco: 3 ♂♂, 2 ♀♀, Igarassu, Refúgio Ecológico Charles Darwin (“RECD”), 30 Oct. 2006, pitfall, Fernando A.B. Silva et al. leg. ( CEMT); 2 ♀♀, Sirinhaém, Usina Trapiche, 08º39′27″ S, 35º10′21″ W, fragmento Xanguazinho, 24 Jul. 2010, pitfall with human faeces, R.P. Salomão leg. ( UFPE).
No data: 1 ♀ ( CEMT).
Description
COLOURATION. Dorsum slightly shiny and lustrous. Head and pronotum with colouration ranging from light green with yellowish reflections to dark purple without any trace of yellowish or greenish sheen; between these two extremes, head and pronotum with mixture of greenish, yellowish, and coppery sheen varying in dominance of some of those tonalities. Elytra usually very dark green or blue, or, more rarely, with strong purplish-blue sheen. Pygidium and metaventrite dark green or blue, without metallic sheen or with reduced sheen. Legs reddish-brown.
HEAD. Tegument with diffuse sheen, with strong alveolar microsculpture obliterating micropunctation. Clypeus with two small apical teeth obtuse and contiguous at base; with single transverse row of very short setae covering base of both teeth. Genae with distinct tooth immediately after clypeal-genal juncture. Posterior edge of head unmargined between eyes.
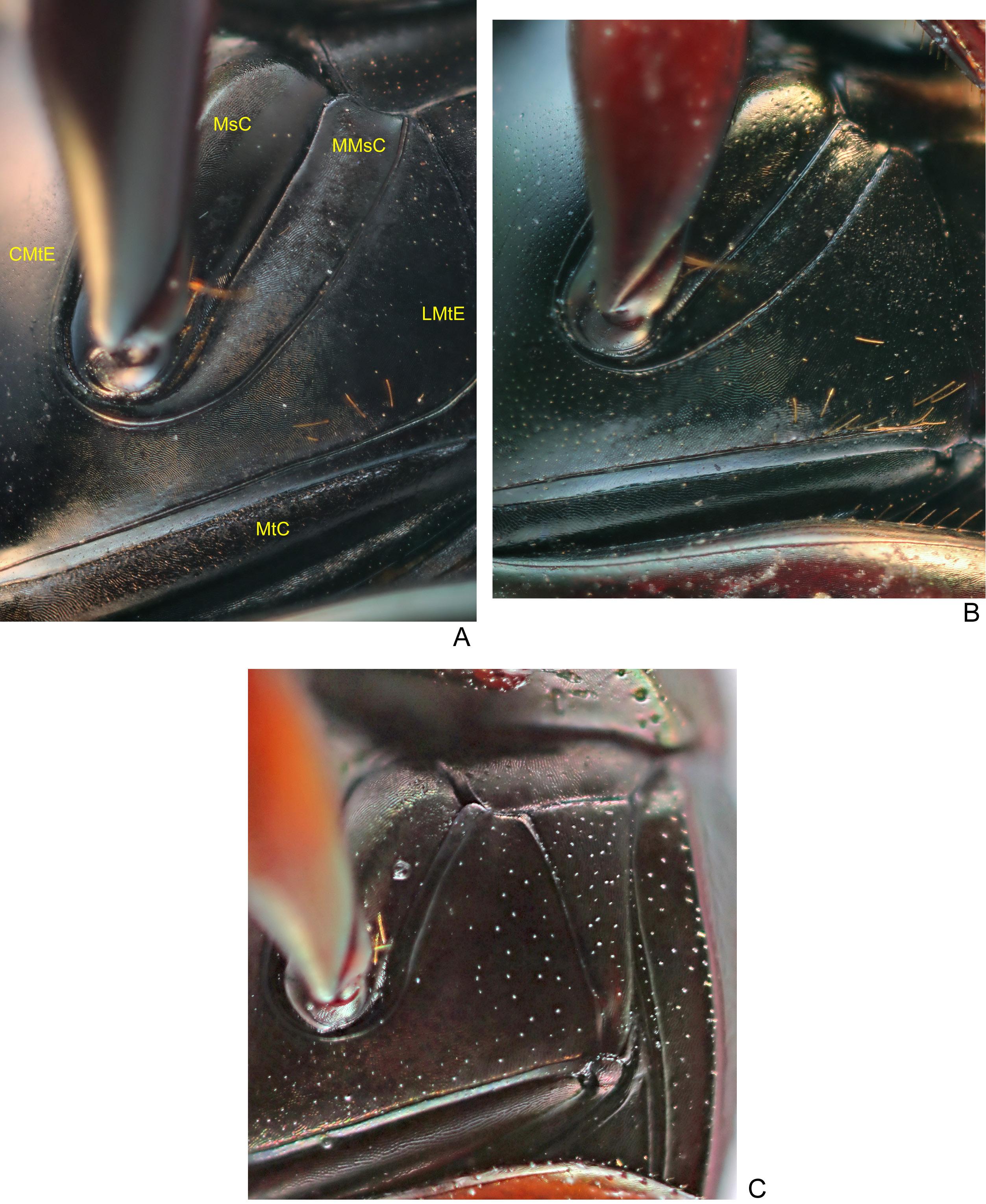
THORAX. Pronotum with shiny tegument, without microsculpture and with dense micropunctation at centre, punctures denser and with more marked than on head; on sides, micropunctation absent and tegument with diffuse shine due to alveolar microsculpture progressively more well marked towards outer edge. Posterior edge with fine transverse line at centre (usually extending only up to the second elytral stria). Hypomeral cavity with long yellowish setae; external edge with distinct tubercle. Metaventrite glabrous at centre; on the sides, with sparse setae close to anterior margin of metacoxae ( Fig. 7B View Fig ); anterior region of metaventrite with strong rivose microsculpture; centre and posterior region with dense micropunctation among strong alveolar microsculpture.
LEGS. Ventral surface of all femora and tibiae bright. Protibiae narrow and with distinct expansion at internal edge; at apical third, with three small acute teeth on external edge, two most apical ones subequal in length and larger than basal ( Fig. 11D View Fig ). Mesofemora margined anteriorly only at basal third or half; unmargined portion of anterior edge with row of setae; posterior margin absent; tegument with micropunctation almost imperceptible. Metafemora margined only anteriorly, posterior margin absent; apical third or half of anterior edge covered by row of setae; tegument with well-marked micropunctation, denser at base than at apex; without coarse elongate punctation at base ( Fig. 13B View Fig ). Metatarsomeres II and V subequal in length and larger than the others; metatarsomere IV shorter than the others.
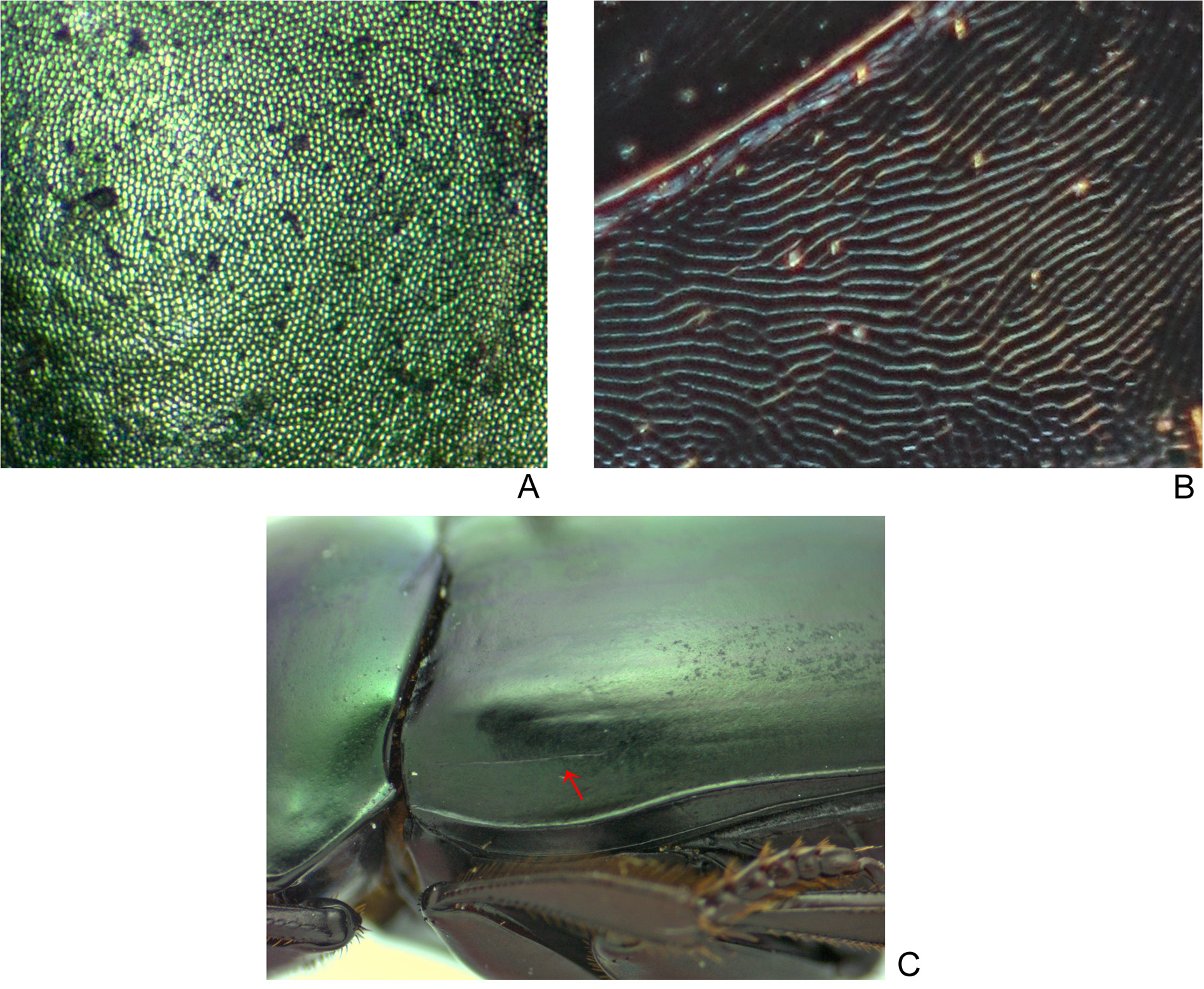
ELYTRA. With nine narrow striae: first six striae strongly marked, finely carinulate and widened at base; seventh stria present only after humerus and always very weak, without carinulae; eighth and ninth striae very tenuous, almost imperceptible; all striae lack carinulae before reaching apex of elytra; humeral carina present or absent. Interstriae with tegument at centre of disc bright, without microsculpture, and with dense micropunctation; on sides and apex, tegument with strong alveolar microsculpture and without distinct micropunctation.
ABDOMEN. Ventrite I–V with strong microsculpture throughout tegument; ventrite VI with microsculpture only slightly more diffuse than others. Pygidium covered by strong alveolar microsculpture obliterating indistinct micropunctation.
AEDEAGUS. Parameres almost as long as phallobase and symmetrical, with both faces flat. In lateral view, with apices widely bifurcate, with superior branch wider and more strongly projected than inferior one and bent upwards; inferior branch with acute apex and facing forwards; without ventral keel or notch ( Fig. 19B View Fig ).
SEXUAL DIMORPHISM. Males: Protibial spur broad and bifid, with long, straight and acute external projection, and internal projection shorter, bent, and wider ( Fig. 15M View Fig ). Abdomen glabrous and without lateral foveae. Ventrite VI strongly narrowed at centre. Pygidium very short (length between 1.5 and 1.2 mm); apical margin of pygidium much wider than lateral margin. Females: Protibial spur fine and short, spiniform. Abdomen with three pairs of transverse foveae on the sutures between ventrites I–II, II–III, and III–IV, respectively; foveae margined anteriorly by row of long yellowish setae; row of setae present also on ventrites IV and V ( Fig. 16C View Fig ). Ventrite VI broad at centre, only very slightly narrowed by medial expansion of ventrite V. Pygidium shorter (between 1.2 and 1 mm); apical margin of pygidium only slightly wider than lateral margin.
Measurements
Males (N = 26). TL: AV: 8.5 ± 0.49; MX: 9.5; MN: 7.7. EW: AV: 5.9 ± 0.33; MX: 6.5; MN: 5.3. PrL: AV: 2.6 ± 0.23; MX: 3; MN: 2.1. PrW: AV: 5.1 ± 0.28; MX: 5.7; MN: 4.7. PgL: AV: 1.3 ± 0.09; MX: 1.5; MN: 1.2. PgW: AV: 2.6 ± 0.17; MX: 2.9; MN: 2.3.
Females (N = 23). TL: AV: 8.2 ± 0.47; MX: 9; MN: 7.1. EW: AV: 5.9 ± 0.36; MX: 6.4; MN: 5.2. PrL: AV: 2.6 ± 0.19; MX: 2.9; MN: 2. PrW: AV: 5.1 ± 0.35; MX: 5.8; MN: 4.5. PgL: AV: 1.1 ± 0.07; MX: 1.2; MN: 1. PgW: AV: 2.5 ± 0.12; MX: 2.7; MN: 2.3.
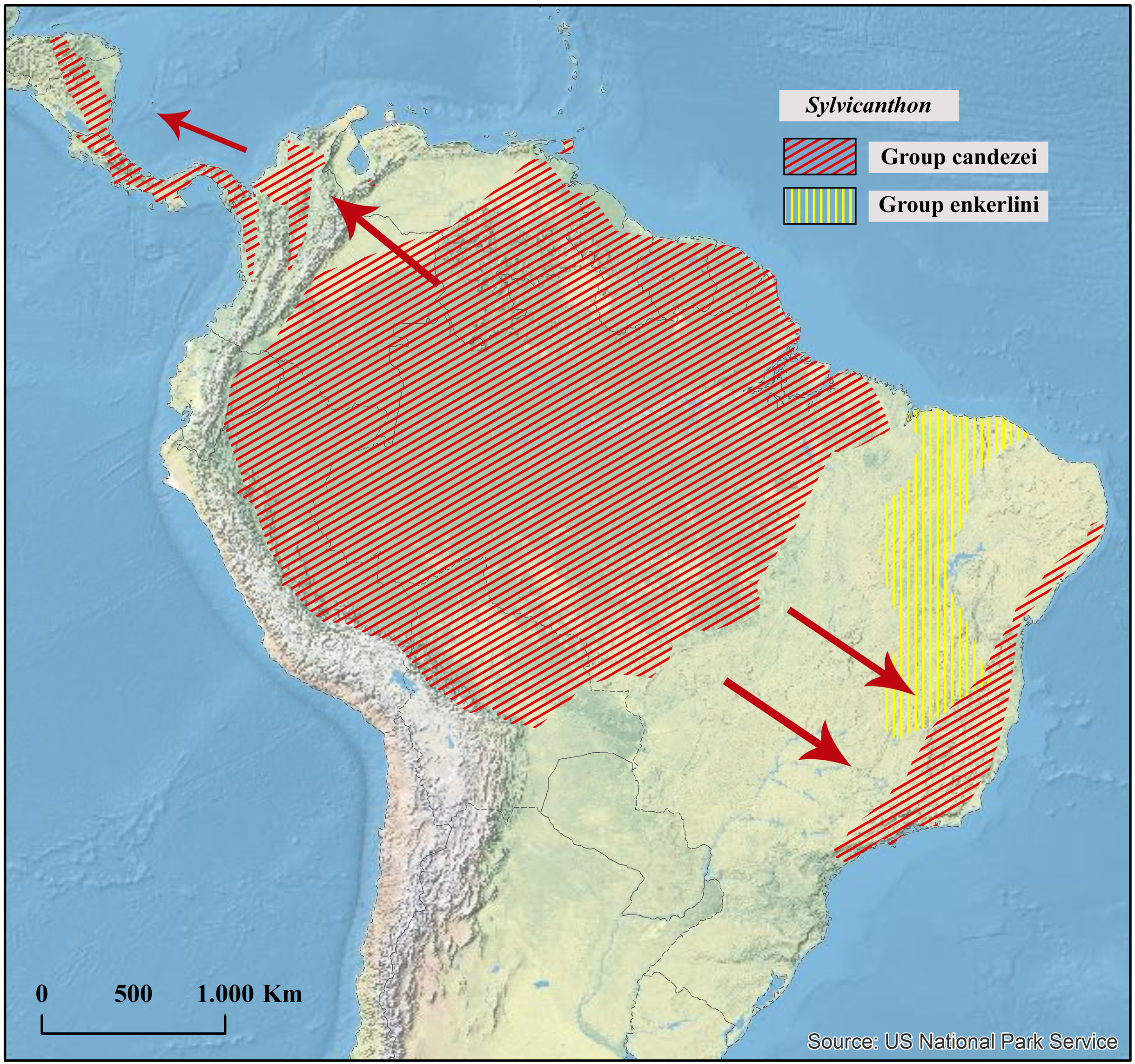
Geographical distribution
Northern Atlantic Forest from Alagoas to Espírito Santo ( Brazil).
Ecoregions
Pernambuco Coastal Forests, Bahia Coastal Forests, Bahia Interior Forests.
Collecting sites ( Fig. 41 View Fig )
BRAZIL. Pernambuco: Igarassu (Refúgio Ecológico Charles Darwin), Sirinhaém. Alagoas: Ibateguara. Bahia: Encruzilhada, Santa Teresinha. Minas Gerais: Berizal (Serra do Anastácio). Espírito Santo: Marechal Floriano, Santa Teresa (Estação Biológica Augusto Ruschi, Estação Biológica de Santa Lúcia), Venda Nova do Imigrante.
Intraspecific variation and taxonomic discussion
Sylvicanthon obscurus presents two important intraspecific variations, one intrapopulational and the other both geographical and intrapopulational. The former refers to the presence of a humeral carina at the eighth elytral stria ( Fig. 12C View Fig ): of the 267 specimens examined for this work, only 88 (46 ♂♂, 42 ♀♀) show some indication of this carina. Schmidt (1920, 1922) did not mention any variation on the presence of the humeral carina and considered it to be a characteristic differentiating S. obscurus from S. candezei , S. foveiventris , S. furvus and S. securus and that, at the same time, would approximate it to S. aequinoctialis , an opinion that was followed by Balthasar (1939), Pereira & Martínez (1956) and Martínez et al. (1964). Although it is a fact that the humeral carina is present only in S. obscurus , S. aequicnotialis and S. proseni , the first species distinguishes itself from the other two by being the only one in which this feature is not present in all the specimens examined. In S. proseni , and particularly in S. aequinoctialis , the carina may occasionally be only weakly marked, but it is never completely absent. Within a same population of S. obscurus , like the large series collected in Venda Nova do Imigrante (Espírito Santo, Brazil) (but also in other localities in the Brazilian northeast), there are specimens both with and without a humeral carina.
The second variation refers to colouration, of which two extreme forms can be observed: at one end of the spectrum, the head and pronotum colouration shows green and dark blue reflections ( Fig. 42B View Fig ); at the other end, head and pronotum are purple or dark purple, while the elytra are bright blue ( Fig. 42A View Fig ). Populations of S. obscurus in the Brazilian northeast (i.e., specimens collected in Alagoas, Pernambuco and Bahia) and in Minas Gerais have a colouration nearer to the latter extreme, with at most only some weak greenish or yellowish metallic reflections on the anterior region of the pronotum. The only specimen known from Bahia and those from Minas Gerais, in particular, show bright blue elytra; the other specimens from the Brazilian northeast have them as dark as in the green-yellowish specimens from Espírito Santo.
Differently, most of the individuals from Espírito Santo, including the lectotype ( Fig. 40B View Fig ), are more similar to the first end of the continuum described above. Nevertheless, there is a gradual intrapopulational variation in those specimens towards the other colour extreme, with individuals gradually showing a larger predominance of coppery sheen over greenish and yellowish tonalities up to a point where almost the totality of the pronotum and head have dark coppery or purple reflections. Therefore, while in the Brazilian northeast only the purple colouration is present, almost the entire variation spectrum is seen in Espírito Santo (although no specimens from this state are as close to the purple extreme as the darkest specimens from the northeast). The specimen from Encruzilhada (Bahia), which came from the former Antonio Martínez collection, bears a handwritten label “ Sp nov ”, probably making reference to its peculiar colouration among the other S. obscurus (all the other known specimens with purple colouration were only recently collected, after 2006). However, after seeing that this characteristic varies intrapopulationally, we consider specimens from the Brazilian northeast and Minas Gerais conspecific with those from Espírito Santo under the name S. obscurus .
Despite sharing several characteristics with other members of the furvus subgroup, such as female abdominal foveae, protibiae expanded on their internal margin and parameres apically bifurcate, S. obscurus is the most distinguishable species in the group. It differs from all the others by the absence of coarse punctation at the base of the metafemora ( Fig. 13B View Fig ), the absence of a fine membrane connecting both branches of the apical bifurcation of the parameres, besides both branches being acuminate ( Fig. 19B View Fig ), and the presence of a row of long setae on the anterior margin of the female abdominal foveae ( Fig. 16C View Fig ). Furthermore, the elytral microsculpture pattern seen in S. obscurus is unique to this species among members of the furvus subgroup and its geographical distribution is completely disjunct, with S. obscurus occurring only in the northern portion of the Atlantic Forest, while the other three species are Amazonian. See Table 5 for more information on the differences between S. obscurus and closely related species; for differences with S. foveiventris , a species with which S. obscurus can be found in sympatry in Espírito Santo (and, perhaps, in Bahia), see the discussion under that species.
Comments
We found 11 specimens that, thanks to Adolf Schmidt’s handwritten labels, we know are certainly part of the type series of S. obscurus : seven deposited in the SMTD (ex Bang-Hass collection) and four in the NHRS. Additionally, three other specimens deposited in the ZMHB bear modern labels indicating they would also be part of the original syntype series. According to Joachim Willers (personal communication to MC, 2015), curator at the ZMHB: “in our collection the species Canthon obscurus Schmidt, 1920 has a bottom label with an asterisc (*). This means that we should have type(s). Therefore I printed a syntype label for each specimen so that who is working on the species has an up-to-date information”. One of those specimens also has a label handwritten by Schmidt and, hence, should indeed be a syntype of S. obscurus (Vaz-de-Mello & Cupello in press). That specimen also bears a green label handwritten “ S. Amerika / W. Meier / Hamburg ”, information that probably makes reference to the collection of the German entomologist William Meier (1861–1940), from Hamburg, Germany (Joachim Willers, personal communication to MC, 2015; Weidner 1976). On the other hand, the other two specimens have labels indicating they were collected in Peru – therefore, different from the type locality cited by Schmidt (1920), the Brazilian state of Espírito Santo. Furthermore, after studying them, we could see that they are not actual S. obscurus , but rather two male S. bridarollii . It is, therefore, possible that someone other than Schmidt has positioned those two specimens bellow the label with the asterisk cited by Willers sometime after Schmidt’s study of the ZMHB specimens and, consequently, they would not be part of the type series of S. obscurus . In light of all the evidence to the contrary, we decided not to include those two specimens in the S. obscurus type material listed above and not to consider them as true syntypes of this name.
Natural history
Label information reports collecting in October, November, December, January and July, which perhaps shows an annual activity pattern similar to that of S. foveiventris , species whose adults are active mainly during the hottest and rainiest months of the year. All the specimens with collecting method information were caught in pitfall traps baited with human faeces. Silva et al. (2010) collected S. obscurus (cited by them as “ Sylvicanthon sp.”) in Pernambuco only in areas of closed forest, although they have also set up traps in open habitats.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Sylvicanthon obscurus ( Schmidt, 1920 )
| Cupello, Mario & Vaz-De, Fernando Z. 2018 |
Canthon obscurus
| Balthasar V. 1939: 187 |
| Schmidt A. 1922: 64 |
Canthon obscurus
| Schmidt A. 1920: 133 |
Canthon obscurum
| Blackwelder 1944: 200 |
Glaphyrocanthon (Glaphyrocanthon) obscurus
| Pereira & Martínez 1956: 126 |
| Pereira & |
Sylvicanthon obscurum
| Vaz-de-Mello 2000: 195 |
Sylvicanthon
| Costa et al. 2009: 90 |
| Silva et al. 2010: 362 |
| Iannuzzi et al. |