Sylvicanthon edmondsi, Cupello & Vaz-De, 2018
|
publication ID |
https://doi.org/ 10.5852/ejt.2018.467 |
|
publication LSID |
lsid:zoobank.org:pub:8D27AAB8-B7F2-424C-B1A6-66FEFA66EDFF |
|
DOI |
https://doi.org/10.5281/zenodo.3846341 |
|
persistent identifier |
https://treatment.plazi.org/id/6C7F8A07-DAB4-4CB9-B0BA-D7CF986C19F5 |
|
taxon LSID |
lsid:zoobank.org:act:6C7F8A07-DAB4-4CB9-B0BA-D7CF986C19F5 |
|
treatment provided by |
Valdenar |
|
scientific name |
Sylvicanthon edmondsi |
| status |
sp. nov. |
Sylvicanthon edmondsi View in CoL sp. nov.
urn:lsid:zoobank.org:act:6C7F8A07-DAB4-4CB9-B0BA-D7CF986C19F5
Figs 11I View Fig , 13F View Fig , 15K View Fig , 18C View Fig , 20 View Fig , 34 View Fig , 38 View Fig A–B
Sylvicanthon View in CoL sp. 1 – Celi et al. 2004: 46.
Sylvicanthon cf. bridarolli [sic] – Noriega et al. 2008: 79 (tentative association).
Etymology
A tribute to the great American scarabaeidologist W.D. Edmonds, a student of the tribe Phanaeini and author of some of the major classics on the biology and morphology of Scarabaeinae . In recognition of his very kind support and continuous encouragement to MC since their very first contact. The holotype of S. edmondsi sp. nov. is deposited in the TAMU collection, the institution where the formerly private Edmonds collection is now housed ( Streit 2012). The specific name is a noun in the genitive case.
Material examined
Holotype
ECUADOR: ♂, Orellana, Parque Nacional Yasuní, Estación Científica Yasuní , 215 m (“ ECUADOR: Napo Prov. / Estación Cientifica Yasuní / IX-5-10- 1999, 215 m / Coll. E. G. Riley ”, “TAMU-ENTO / X0668859 / [código de barras]”) ( TAMU).
Paratypes (42 ♂♂, 43 ♀♀)
COLOMBIA: Amazonas: 1 ♂, 1 ♀, Leticia, Parque Nacional Natural Amacayacu, Dec. 1998, J. Noriega leg. ( CPJN). – Caquetá: 1 ♀, Parque Nacional Natural Sierra de Chiribiquete, 300 m, Feb. 2000, pitfall with human faeces, J. Noriega leg. ( CPJN).
ECUADOR: Morona Santiago: 5 ♂♂ (1 dissected), 2 ♀♀, Untsuants, sítio 3, 700 m, 13 Jan. 2002, pitfall with human faeces, J. Celi and M. Ortega leg. ( CMNC). – Orellana: 1 ♂, Parque Nacional Yasuní, Estación Científica Yasuní, 00º38′ S, 76º36′ W, 215 m, 27. Jul.–1 Aug. 1998, pitfall with human faeces, Ratcliffe, Jameson, Smith and Villatoro leg. ( CMNC); 29 ♂♂ (2 dissected), 32 ♀♀, Parque Nacional Yasuní, Estación Científica Yasuní, 215 m, 5–10 Sep. 1999, E.G. Riley leg. ( TAMU); 1 ♂, Parque Nacional Yasuní, Estación Científica Yasuní, 9–17 Sep. 1999, D.G. Marqua leg. ( TAMU); 1 ♂, Parque Nacional Yasuní, via Maxus km “Onkone Gare”, 220 m, 14 Nov. 2001, canopy fogging, P. Araujo leg. ( CEMT); 2 ♂♂, 1 ♀, Rodrigo Borja, IAMOE, 4 Jun. 2000, pitfall with human faeces, A. Dávalos leg. ( CEMT); 1 ♂, Tiputini Biodiversity Station, 0º38′ S, 76º09′ W, 220 m, sep. 2000, carrion trap, D. Inward leg. ( BMNH); 2 ♀♀, Tiputini Biodiversity Station, Río Tiputini, 0º40.5′ S, 76º24′ W, Jul. 1999, flight interception trap, A. Tishechkin leg. ( CEMT).
PERU: Junín: 1 ♂, Satipo, Oct.–Nov. 2002 ( CEMT). – Loreto: 3 ♀♀, Campamento San Jacinto, 02º18′44.85″ S, 75º51′46″ W, 175–215 m, 3–12 Jul. 1993, flight interception trap, R. Leschen leg. ( CMNC); 1 ♀, Río Pucacuro, 203 m, 21 Nov. 2007, dung pitfall, Cesar Moreno leg. ( CEMT).
Description
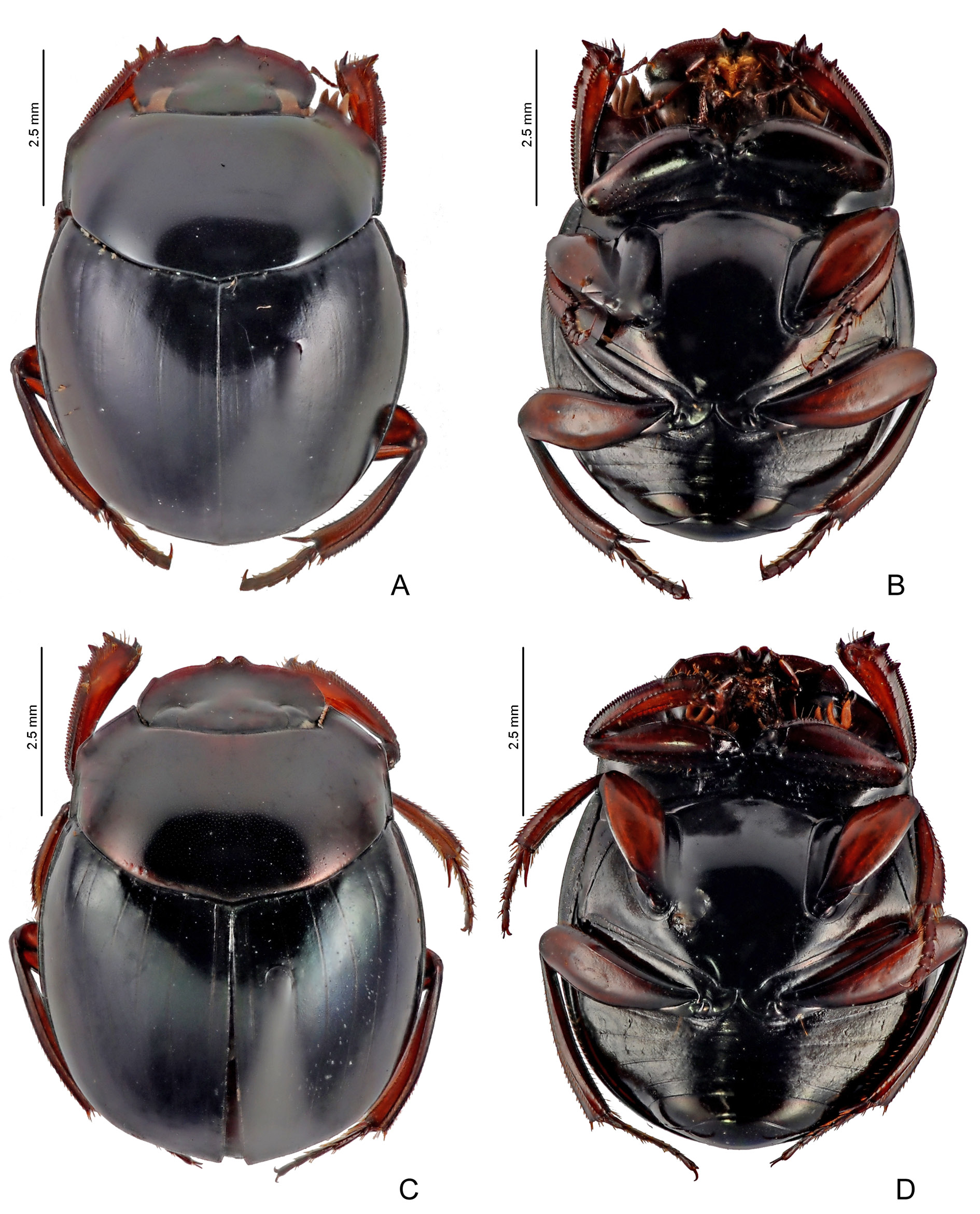
COLOURATION. Entire body with very dark tonalities. Head dark purple (in some specimens, frons with greenish reflections). Pronotum with strong greenish or bluish sheen at centre and purplish reflections on sides. Elytra usually dark blue or purple, occasionally with weak greenish reflections; striae with same colouration as the rest of tegument and not contrasting with it. Metaventrite black with very weak greenish or coppery shine. Meso- and metafemora orange-brown or yellowish, with base distinctly darker than at least apical two-thirds. Pygidium usually with predominant greenish shine and some coppery reflections, especially at base.
HEAD. Tegument little shiny, with strong alveolar microsculpture obliterating almost completely micropunctation, which is almost imperceptible or even absent throughout outer edge of head. Clypeus with two apical teeth obtuse and only slightly separated from one another; with single transverse row of setae covering base of both teeth. Genae with strong tooth immediately behind clypeal-genal juncture. Posterior edge of head completely unmargined.
THORAX. Pronotum with tegument slightly bright and lustrous, with very fine microsculpture (sometimes absent at centre), and dense, clearly marked central micropunctation. Posterior edge with fine transverse line at centre (usually extending only up to second elytral stria). Hypomeral cavity entirely glabrous or at most with very few short setae at centre; long setae, if present, restricted to anterior and posterior regions ( Fig. 35B View Fig ); external margin with weak tubercle. Metaventrite entirely glabrous; tegument with strong rivose microsculpture on anterior region and weaker microsculpture adjacent to internal margin of mesocoxae; at centre, alveolar microsculpture very fine and progressively more diffuse and undifferentiated towards posterior region; micropunctation very fine, but always evident.
LEGS. Ventral surface of all femora and tibiae bright. Profemora with tegument with strong rivose microsculpture and without micropunctation. Protibiae narrow and with internal edge straight and simple, without expansion; at apical third, with three acute teeth, two apical ones of subequal length and larger than basal ( Fig. 11I View Fig ). Mesofemora margined anteriorly only at their basal half; unmargined portion of anterior edge with row of very short setae; posterior margin absent; tegument with effaced rivose microsculpture. Metafemora margined anteriorly, posterior margin absent; apical half of anterior edge covered by row of setae; tegument covered by diffuse rivose microsculpture and without any trace of coarse punctation at base ( Fig. 13F View Fig ). Metatarsomeres II and V subequal in length and longer than the others; metatarsomere IV shorter than the others.
ELYTRA. With at most nine very narrow visible striae: in general, first two to four striae well marked, very finely carinulate, and without basal widening; remaining striae progressively more effaced and interrupted; eighth and ninth only seen in specimens with very well-marked striae and, in these cases, always very subtle; all striae lack carinulae before reaching apex of elytra, where they are completely indistinct; humeral carina absent. Tegument of interstriae with diffuse shine and lustrous, with alveolar microsculpture throughout elytra surface; micropunctation, in general, clearly visible at 20x magnification.
ABDOMEN. Tegument of ventrites I–V with rivose microsculpture, in general, diffuse at centre; ventrite VI with rivose microsculpture very diffuse and micropunctation very subtle; both sexes without lateral foveae. Pygidium with shiny tegument and covered by alveolar microsculpture; micropunctation subtle, but always evident among microsculpture.
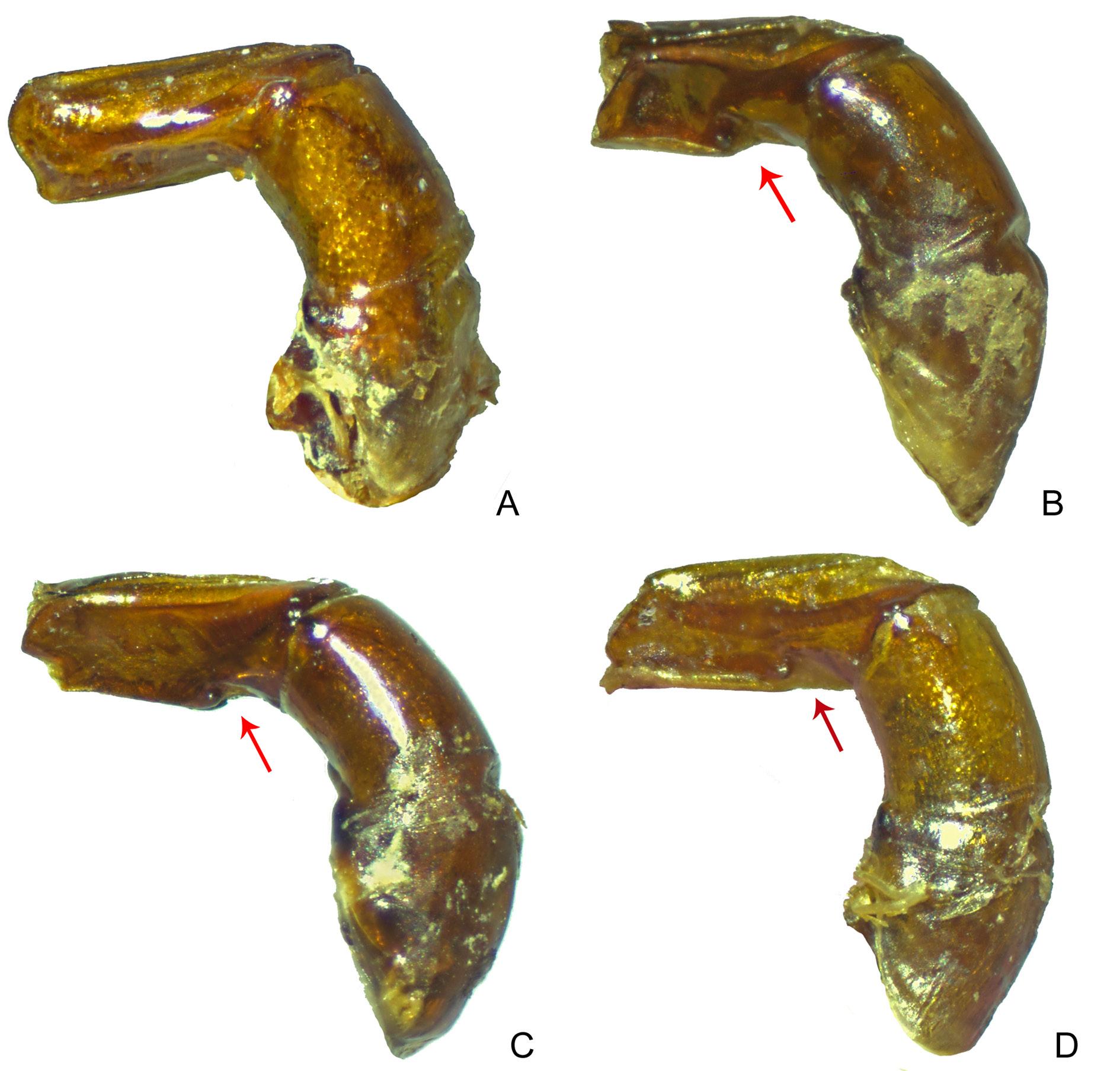
AEDEAGUS. Parameres at least half as long as phallobase and asymmetrical: external face of right paramere flat, external face of left paramere concave, strongly excavated. In lateral view, parameres with ventral keel ( Fig. 18C View Fig ).
SEXUAL DIMORPHISM. Males: Protibial spur narrow and bifid at apex, with spiniform projections, the external projection much longer than the internal one ( Fig. 15K View Fig ). Ventrite VI with posterior edge strongly narrowed at centre; anterior edge covered only very slightly by weak medial flange of ventrite V. Females: Protibial spur simple, spiniform. Ventrite VI very broad at centre; anterior edge covered by weak medial flange of posterior edge of ventrite V.
Measurements
Males (N =10). TL: AV: 7.0 ± 0.70; MX: 8.0; MN: 6.1. EW: AV: 5.3 ± 0.42; MX: 5.7; MN: 4.3. PrL: AV: 2.3 ± 0.10; MX: 2.5; MN: 1.8. PrW: AV: 4.5 ± 0.36; MX: 4.9; MN: 3.7. PgL: AV: 1.4 ± 0.12; MX: 1.5; MN: 1.1. PgW: AV: 2.2 ± 0.17; MX: 2.3; MN: 1.8.
Females (N = 12). TL: AV: 7.2 ± 0.49; MX: 8.0; MN: 6.4. EW: AV: 5.2 ± 0.30; MX: 5.7; MN: 4.7. PrL: AV: 2.3 ± 0.19; MX: 2.6; MN: 2.0. PrW: AV: 4.5 ± 0.26; MX: 4.9; MN: 4.1. PgL: AV: 1.3 ± 0.10; MX: 1.4; MN: 1.1. PgW: AV: 2.2 ± 0.17; MX: 2.5; MN: 1.9.
Geographical distribution
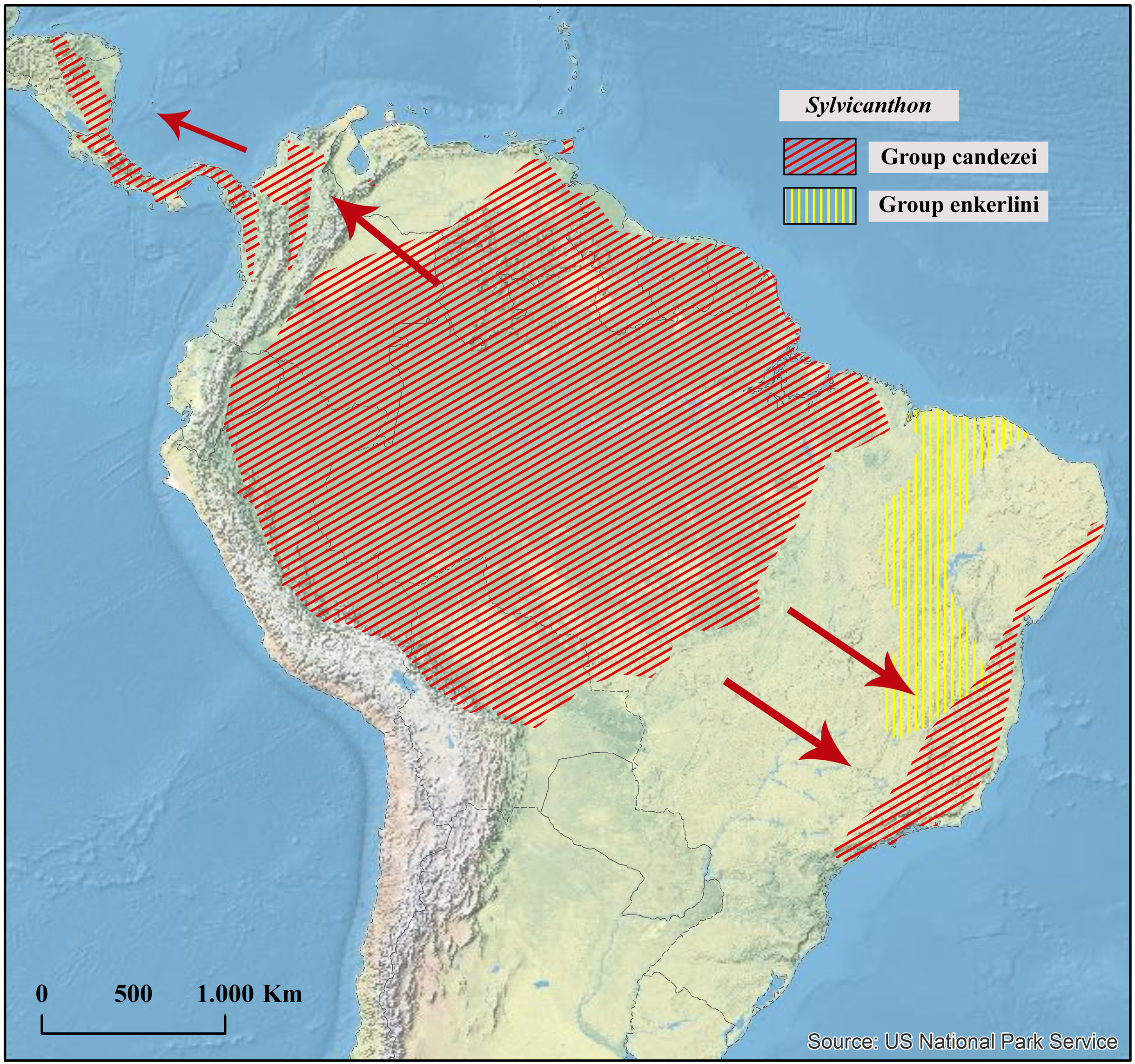
Northwestern Amazonia, mainly in Sub-Andean areas in Colombia, Ecuador, and Peru.
Ecoregions
Napo Moist Forests , Cordillera Oriental Montane Forests, Peruvian Yungas.
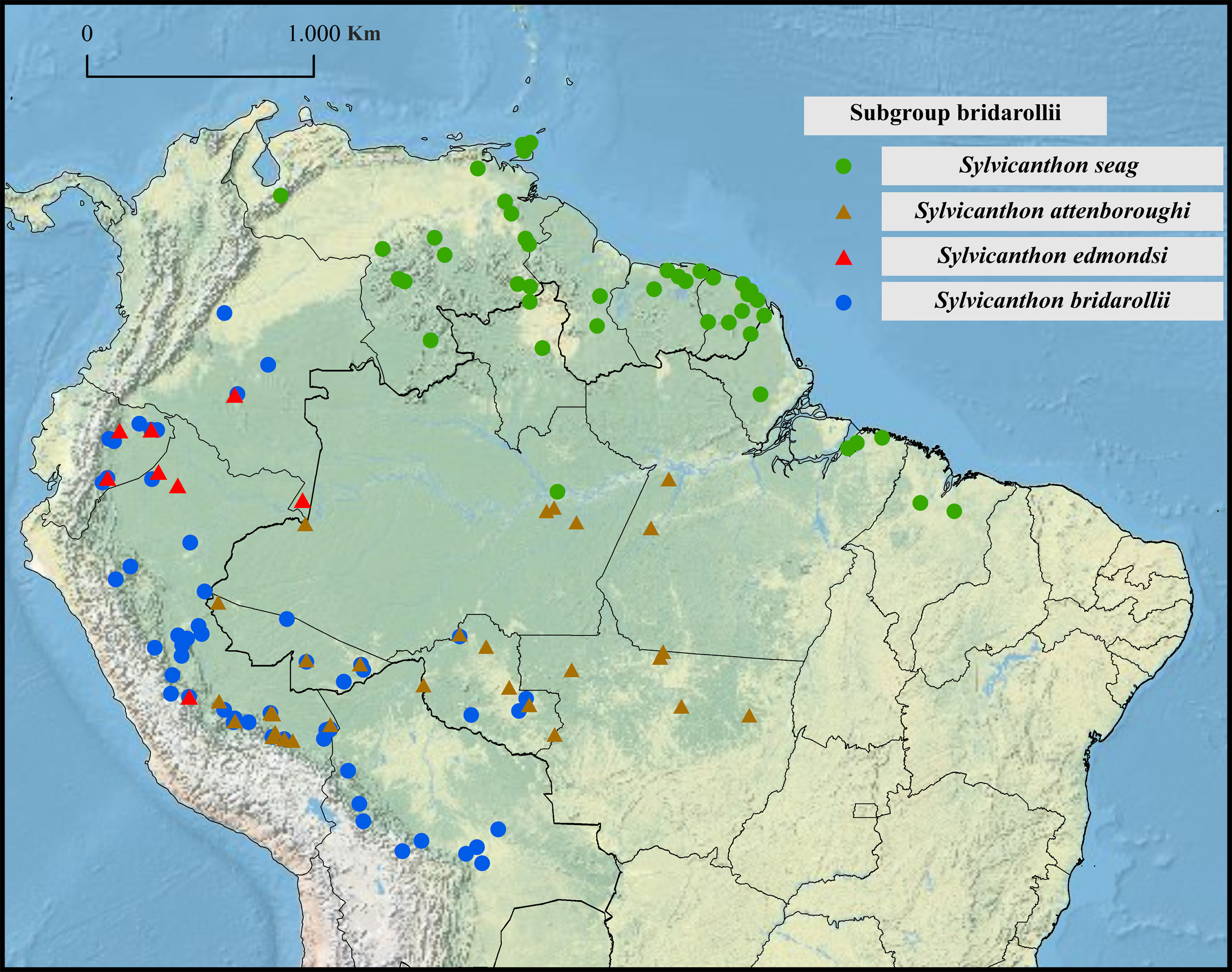
Collecting sites ( Fig. 34 View Fig )
COLOMBIA. Caquetá: Parque Nacional Natural Sierra de Chiribiquete. Amazonas: Leticia (Parque Nacional Natural Amacayacu).
ECUADOR. Orellana: Parque Nacional Yasuní, Tiputini Biodiversity Station. Morona Santiago: Untsuants.
PERU. Loreto: Campamento San Jacinto. Junín: Satipo.
Intraspecific variation and taxonomic discussion
Sylvicanthon edmondsi sp. nov. is an interesting case of a species easily recognizable at first glance by its darker colouration ( Fig. 38A View Fig ), its very subtle elytral striae, and its smaller size, but that does not have any exclusive morphological character, as the shape of the parameres or a specific micropunctation or microsculpture pattern, that could differentiate it from the other species in a more objective way. Along with S. seag sp. nov. and S. attenboroughi sp. nov., S. edmondsi sp. nov. is distinguished very easily from S. bridarollii by the shape of the parameres: in lateral view, a strong ventral keel is seen in S. edmondsi sp. nov. ( Fig. 18C View Fig ), while the parameres are simple in the latter species ( Fig. 18A View Fig ); besides, the parameres’ external faces are asymmetrical in S. edmondsi sp. nov. (left paramere with external face excavated and right paramere flat), while they are symmetric (both faces flat) in S. bridarollii . Furthermore, S. edmondsi sp. nov. distinguishes itself from S. bridarollii by the tegument at the centre of the pronotum, which shows a fine, sometimes smoothed microsculpture, and a very dense, clearly marked micropunctation; by the absence of long setae at the centre of the hypomeral cavity ( Fig. 35 View Fig A– B); metaventrite with a very fine microsculpture at the centre; and the shape of the protibiae ( Fig. 11I View Fig ). From S. attenboroughi sp. nov. and S. seag sp. nov., S. edmondsi sp. nov. differs simultaneously by head with micropunctation almost imperceptible and hypomeral cavity not as strongly excavated as in the first two species; from S. seag sp. nov., in particular, S. edmondsi sp. nov. is different mostly because of the shape of the protibial spur ( Fig. 15K View Fig ), the shape of the anterior margin of the female ventrite VI, and the shape of the parameres ( Fig. 18C View Fig ). See Table 4 for a detailed comparison between S. edmondsi sp. nov. and the other species of the bridarollii subgroup.
The distribution of S. edmondsi sp. nov. is the most limited in the bridarollii subgroup: this species is present in the humid forests on the slopes of the Andes, in altitudes between 200 and 1110 m, from Colombia in the north to Peru in the south ( Fig. 34 View Fig ). We have not seen any geographical variation among the studied populations.
Comments
It was possible to verify that the morphotype named “ Sylvicanthon sp. 1” by Celi et al. (2004) is, in fact, S. edmondsi sp. nov. because we examined some of the specimens collected by them (which are now housed at the CMNC) and found the following results: six specimens of S. edmondsi sp. nov. from site (“ Sítio ”) 3 (700 m); three specimens of S. bridarollii from site 1 (700 m), one from site 3 (700 m), four from site 5 (600 m) and seven from site 6 (600 m); and a male S. genieri sp. nov. from site 4 (1100 m) and three others from site 7 (900 m). Table 3 of Celi et al. (2004) shows that what they called S. bridarollii was collected between 500 and 900 m, “ Sylvicanthon sp. 1”, between 600 and 1110, and “ Sylvicanthon sp. 2”, between 600 and 1300 m. Putting all these data together, we conclude that the specimens of S. bridarollii were correctly identified by Celi et al. (2004), while morphotypes “ Sylvicanthon sp. 1” and “ Sylvicanthon sp. 2” refer, respectively, to S. edmondsi sp. nov. and S. genieri sp. nov.
Natural history
Label information tell us that adults of S. edmondsi sp. nov. are active at least between June and January (during that period, no records only from October and December). Specimens were collected mostly in pitfall traps baited with human faeces, but a male from the Tiputini Biodiversity Station (Morona Santiago, Ecuador) was attracted to carrion. Another male was collected at the Parque Nacional Yasuní (Orellana, Ecuador) by the canopy fogging method, but there are no data as to the height of the trees. As for the altitudinal amplitude, studied specimens were collected between 200 and 700 m; in Morona Santiago, Celi et al. (2004) collected 116 specimens between 600 and 1110 (cited as “ Sylvicanthon sp. 1”; see the discussion of S. bridarollii for details on the sympatry between that species and S. edmondsi sp. nov.).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Sylvicanthon edmondsi
| Cupello, Mario & Vaz-De, Fernando Z. 2018 |
Sylvicanthon cf. bridarolli
| Noriega J. A. & Cubillos A. M. & Castaneda C. & Sanchez A. M. 2008: 79 |
Sylvicanthon
| Celi J. & Terneus E. & Torres J. & Ortega M. 2004: 46 |