Hercostomus Loew
|
publication ID |
https://doi.org/ 10.5281/zenodo.170753 |
|
publication LSID |
lsid:zoobank.org:pub:7BDC5C6A-D9C8-4DDB-964A-F37059FA2B3D |
|
DOI |
https://doi.org/10.5281/zenodo.6266938 |
|
persistent identifier |
https://treatment.plazi.org/id/D40A8783-FF83-2E3E-7350-FC60FBA9D833 |
|
treatment provided by |
Plazi |
|
scientific name |
Hercostomus Loew |
| status |
|
Genus Hercostomus Loew View in CoL View at ENA
( Figs. 14 View FIGURE 14 A–E, 15A–F, 16A–E) Hercostomus Loew, 1857: 9 . Type species: Sybistroma longiventris Loew [Palaearctic], by original designation.
Phalacrosoma Becker 1922b: 44 View in CoL . Type species: Phalacrosoma amoenum Becker [Oriental], designation by Dyte, 1975: 242. syn. nov.
Microhercostomus Stackelberg, 1949: 687 (as subgenus). Type species: Hercostomus (Microhercostomus) dilatitarsis Stackelberg View in CoL [Palaearctic], by original designation. Synonymized by Grichanov (1997) (see “Remarks”).
Steleopyga Grootaert & Meuffels, 2001: 208 . Type species: Steleopyga dactylocera Grootaert & Meuffels [Oriental], by original designation. syn nov.
Ahercostomus Yang & Saigusa, 2001c: 239 View in CoL (as subgenus). Type species: Hercostomus (Ahercostomus) jiangchenganus Yang & Saigusa View in CoL [Oriental], by original designation (see “Remarks”).
New Combinations. The following new combinations are hereby established: Hercostomus amoenus ( Becker, 1922b) View in CoL comb. nov. ( Phalacrosoma View in CoL ); Hercostomus argyreus ( Wei & Lui, 1996) comb. nov. ( Phalacrosoma View in CoL ); Hercostomus briarea ( Wei & Lui, 1996) comb. nov. ( Phalacrosoma View in CoL ); Hercostomus dactylocera ( Grootaert & Meuffels, 2001) View in CoL comb. nov. ( Steleopyga ); Hercostomus fulgidipes ( Becker, 1922b) View in CoL comb. nov. ( Phalacrosoma View in CoL ); Hercostomus hubeiensis ( Yang, 1998a) View in CoL comb. nov. ( Phalacrosoma View in CoL ); Hercostomus imperfectus ( Becker, 1922b) View in CoL comb. nov. ( Phalacrosoma View in CoL ); Hercostomus postiseta ( Yang & Saigusa, 2001b) View in CoL comb. nov. ( Phalacrosoma View in CoL ); Hercostomus zygolipes ( Grootaert & Meuffels, 2001) View in CoL comb. nov. ( Steleopyga ).
Recognition. Hercostomus sensu lato, as traditionally defined, is a polyphyletic assemblage of species with wing vein M straight or weakly bent anteriorly beyond crossvein dmcu, R4+5 and M parallel or convergent and lacking the defining features of the other dolichopodine genera. Hercostomus sensu lato can be recognized by the following combination of characters: vertical setae stronger than postverticals; clypeus of male not strongly bulging, lower margin usually straight and not reaching lower eye margin; scape and pedicel normally developed; arista simple with short to moderately developed pubescence, rarely with apical lamella; eyes separated at lower margin; proboscis and palps usually short, sometimes slightly elongated; thorax lacking distinct dark spot above notopleuron; usually with 6 dorsocentral setae; sutural and presutural setae present; pleural surface in front of posterior spiracle bare; fore tarsus usually simple, mid and hind femur usually with 1 preapical seta; hind basitarsus usually without dorsal setae; wing vein M beyond crossvein dmcu with weak sinuous anterior bend or straight; R4+5 and M convergent or parallel; male genitalia with sperm pump rounded or cylindrical, not folded back on itself, postgonite usually lacking medioventral projection, apicoventral epandrial lobe not greatly elongate and setose; female terminalia with T6, T7, S6, S7 undivided, T8 and S8 separate anterolaterally. Hercostomus in its strictest sense (i.e. the lineage including the type species H. longiventris ) can be recognized by the following combination of characters: vein M beyond crossvein dmcu with weak sinuous anterior bend, fore tarsus of male simple or modified, mid femur with 1 strong posterior preapical about even with anterior preapical, hypopygium with basiventral epandrial lobes and hypandrium forming a complex of entangled asymmetrical lobes.
Description (based on H. longiventris lineage). Head: Vertex not excavated, 1 pair of strong vertical setae, stronger than postverticals. Frons about 2.0–4.2 x wider than high, sides convergent anteriorly. Face narrow to broad in male, sides converging below, usually broader in female with sides weakly converging; clypeus flat to weakly produced in male, usually more strongly produced in female, lower margin usually straight and ending above lower eye margin, rarely strongly produced and beaklike in lateral view with lower margin angled outward and rounded below, projecting to or beyond lower eye margin (e.g., H. amoenus ). Palp usually small, ovoid, with fine setae on outer surface, 1 distinct to strong apical seta. Proboscis occasionally enlarged (e.g., H. amoenus ). Antenna: Scape short to slightly elongated, subconical, with weak to welldeveloped acute medioventral process; pedicel short, occasionally with medial margin strongly projecting into first flagellomere (e.g., H. dactylocera ); first flagellomere variable, subtriangular to subrectangular or ovoid, often shorter in female; arista dorsal, 2segmented, distal segment with short pubescence, arista rarely arising from preapical dorsal projection of first flagellomere (e.g., H. dactylocera ). Postvertical setae usually stronger than uppermost pair of postoculars, occasionally reduced in male and subequal to postverticals (e.g., H. amoenus ).
Thorax: Setae usually welldeveloped, occasionally reduced in male (e.g., H. amoenus ). Acrostichals biserial; 6 dorsocentrals, fifth pair aligned or weakly offset medially; 1 strong outer posthumeral, 1 weak inner posthumeral, sometimes indistinct; 2 notopleurals; 1 presutural; 1 sutural; 2 supraalars; 1 postalar. Upper and lower part of propleuron with fine, often sparse hairs; lower part propleuron with 1 strong prothoracic seta; pleural surface in front of posterior spiracle bare; metepisternum bare or with 1 or more fine hairs. Scutellum with 1 strong inner seta and 1 small outer seta on lateral margin, sometimes with a few marginal hairs.
Legs: Pulvilli developed normally on all legs. Foreleg: Tibia occasionally with anterodorsal comblike row of setae (e.g., H. fulvicaudis , H. tibialis ); femur sometimes with distinct posterior preapical; tarsus of male unmodified or modified with tarsomeres 1 and 2 slender, sometimes laterally flattened (e.g., H. chetifer ), tarsomere 2 occasionally with curved medial setae (e.g., H. enghoffi Grichanov ), tarsomeres 3–5 usually with a crest of dorsal setae, tarsomere 3 usually flattened and broad, tarsomeres 4 and 5 flattened and broad or slender, tarsomere 4 occasionally with dorsal projection (e.g., H. patellitarsis (Parent)) , tarsomere 5 occasionally elongate (e.g., H. enghoffi ). Midleg: Femur with 1 anterior or anterodorsal preapical seta, occasionally lost in male (e.g., H. amoenus ), 1 posterior preapical, about even with anterior preapical, in addition to terminal posteroventral preapical seta that is sometimes developed, occasionally with long setae ventrally (e.g., H. fulvicaudis , H. imperfectus ); tibia of male sometimes with specialized hairs or spines (e.g., H. amoenus ); tarsus of male occasionally modified, with long fine hairs (e.g., H. amoenus ). Hindleg: Coxa with strong lateral seta near or slightly above middle; femur usually with 1 strong anterodorsal preapical seta, occasionally with a preapical anteroventral row of 4 weaker setae (e.g., H. dactylocera , H. zygolipes ); tibia occasionally with dorsal comblike row of setae (e.g., H. fulvicaudis , H. tibialis ), male with distinct posteroapical projection, occasionally large (e.g., H. enghoffi , H. fulvicaudis , H. tibialis ); basitarsus shorter than second tarsomere, usually with 3–4 ventral setae, occasionally with distinct basiventral seta (e.g., H. krivosheinae Grichanov, H. dactylocera ), or tuft of short, flattened setae (e.g., male H. amoenus ), male with pointed or ridgelike process posterobasally, occasionally bifurcate (e.g., H. enghoffi ).
Wing: Grayish to brownish, sometimes with brown infuscation anteriorly in males (e.g., H. amoenus ). Costa occasionally thickened in male (e.g., H. amoenus ); R2+3 relatively straight, sometimes with weak anterior bend near apex; R4+5 straight or with weak to distinct posterior curve in apical portion; distal section of M beyond crossvein dmcu with weak sinuous anterior bend before middle, ending slightly before wing apex, bend occasionally stronger in male (e.g., H. amoenus ); R4+5 and M weakly to distinctly convergent distally, sometimes widely spaced in male (e.g., H. amoenus ); crossvein dmcu distinctly longer to distinctly shorter than distal section of CuA1, rarely absent (e.g., H. zygolipes ).
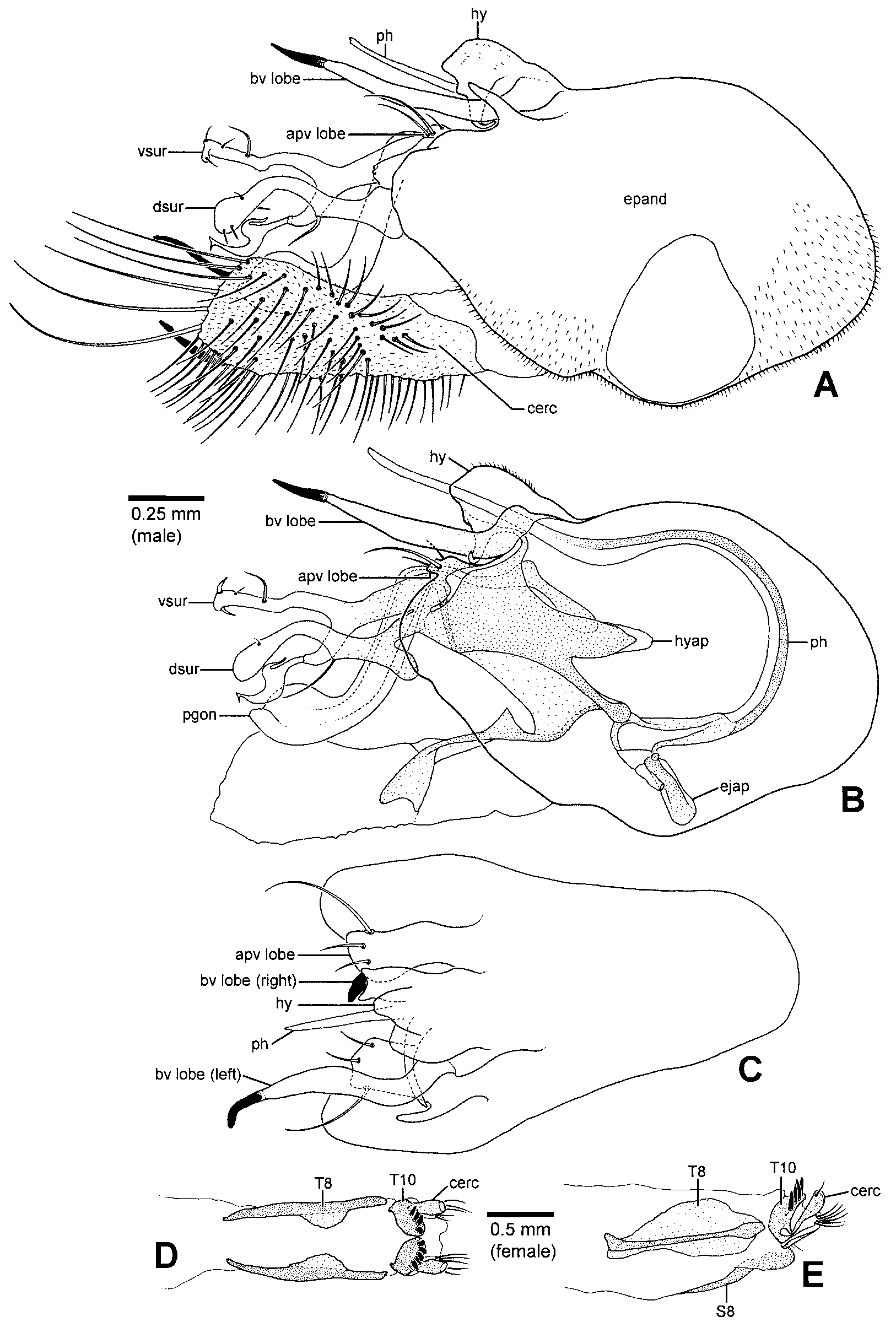
Abdomen: Usually subconical, cylindrical in males of some species (e.g., H. longiventris , H. amoenus ). Male: T6 bare; S2 unmodified to weakly sclerotized; S3 weakly sclerotized, often emarginate and membranous posteriorly; S4 usually weakly sclerotized, emarginate and membranous posteriorly or divided and membranous medially; S5 mainly membranous, sometimes with medial Yshaped sclerite, occasionally with a pair of internal glandular structures (e.g., H. longiventris ); S6 mainly membranous, sclerotized along anterior margin and sometimes along lateral margin; segment 7 forming welldeveloped peduncle, often elongate; S8 usually ovoid to teardrop or heartshaped, setose, occasionally pedunculate with a cluster of thick spines near base of left margin (e.g., H. dactylocera , H. zygolipes , Fig. 16 View FIGURE 16 D). Hypopygium ( Figs. 14 View FIGURE 14 A–C, 15A–D, 16A–C,E): Usually asymmetrical, occasionally somewhat twisted dextrally on longitudinal axis (e.g., H. dactylocera ). Epandrium about 1.4–2.3 x longer than high; foramen usually midlateral to anterolateral, usually wellseparated from base of cerci, sometimes dorsolateral and close to base of cerci (e.g., H. longiventris , Fig. 14 View FIGURE 14 A); basiventral epandrial lobes shifted ventrally and lying beside hypandrium, right and left lobes asymmetrical, apex of one or both lobes often with contrasting, pointed or knoblike tip (modified basiventral epandrial seta) ( Figs. 14 View FIGURE 14 A–C, 15A,C,D), sometimes absent or indistinct, left basiventral epandrial lobe sometimes bifurcate; apicoventral epandrial lobe variable, elongate to weakly developed, usually with 3 setae, sometimes dorsoventrally flattened (e.g., H. longiventris , Fig. 14 View FIGURE 14 B,C), epandrium occasionally with textured, saclike accessory lobe near base of apicoventral lobe and ventral surstylar lobe (e.g., H. chetifer , H. amoenus Fig. 15 View FIGURE 15 A,D). Surstylus bilobed. Ventral lobe variable in shape, often digitiform, usually with thickened or flattened mediodorsal seta, lobe occasionally bifurcate with flattened seta arising apically on dorsal fork (e.g., H. longiventris , Fig. 14 View FIGURE 14 B). Dorsal lobe variable in shape, with strong dorsal to dorsoapical seta, which is occasionally flattened. Postgonite with anteroventral portion weakly to moderately sclerotized, occasionally somewhat flattened laterally (e.g., H. longiventris ), sometimes absent; posterodorsal portion welldeveloped, often strongly upturned (e.g., H. longiventris , Fig. 14 View FIGURE 14 B), sometimes with wellsclerotized medioventral projection, projection often textured, occasionally bifurcate (e.g., H. amoenus , Fig. 15 View FIGURE 15 B). Proctiger brushes absent. Cerci usually arising near apex of epandrium, sometimes arising preapically (e.g., H. longiventris , Fig. 14 View FIGURE 14 A), variable in shape, often with welldeveloped basolateral lobe, occasionally with thick setae on apical margin ( Figs. 14 View FIGURE 14 A, 16A,B). Hypandrium variable in shape, often asymmetrical, laterally flanked by basiventral epandrial lobes and usually fused with lobes basally, forming an asymmetrical complex ( Figs. 14 View FIGURE 14 C, 15B,D, 16A,B,E); hypandrial apodeme present or absent, sometimes welldeveloped (e.g., H. longiventris , Fig. 14 View FIGURE 14 B); hypandrial arms usually connected to hypandrium, sometimes reduced to narrow bands, occasionally absent (e.g., H. chetifer , H. dactylocera , Fig. 16 View FIGURE 16 C). Sperm pump spherical, usually small; ejaculatory apodeme usually short and rodlike, sometimes flattened laterally (e.g., H. longiventris ), occasionally very reduced (e.g., H. dactylocera , Fig. 16 View FIGURE 16 C); basal sclerite of sperm pump usually weakly developed, straight or Vshaped in dorsal view, occasionally forming elongate rod (e.g., H. dactylocera , Fig. 16 View FIGURE 16 C). Phallus usually long and slender ( Figs. 14 View FIGURE 14 B, 15B), occasionally thick with broad flattened apex (e.g., H. dactylocera , Fig. 16 View FIGURE 16 C), sometimes with weak preapical dentiform projections (e.g., H. krivosheinae ). Female ( Figs. 14 View FIGURE 14 D,E, 15E,F): T6, T7, S6 and S7 undivided; T8 divided medially, S8 undivided, sometimes very weakly sclerotized, tergite and sternite not fused anterolaterally; T10 divided medially into hemitergites each bearing 4–5 spines, apex of spines pointed. Upper lobe of cercus with short apical seta.
Geographical Distribution. Hercostomus sensu lato is known from the Holarctic, Afrotropical, Oriental, Australasian ( New Caledonia, New Guinea, New Zealand) and Neotropical regions. Robinson (1970b) considered that the Neotropical species likely belong to other genera. Most Hercostomus species are known from the Palaearctic. Hercostomus sensu stricto (the H. longiventris lineage) occurs in the Holarctic, Afrotropical and Oriental regions.
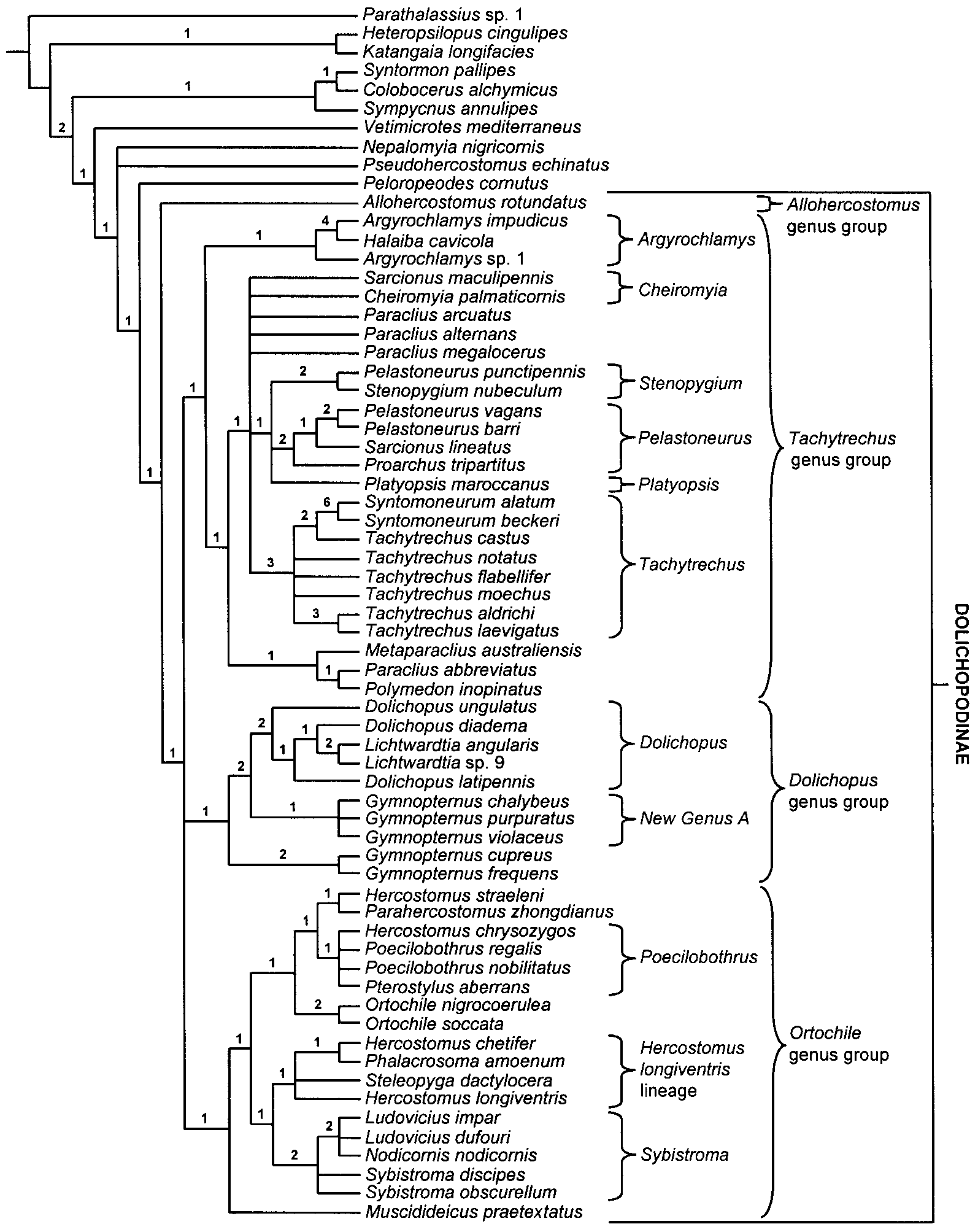
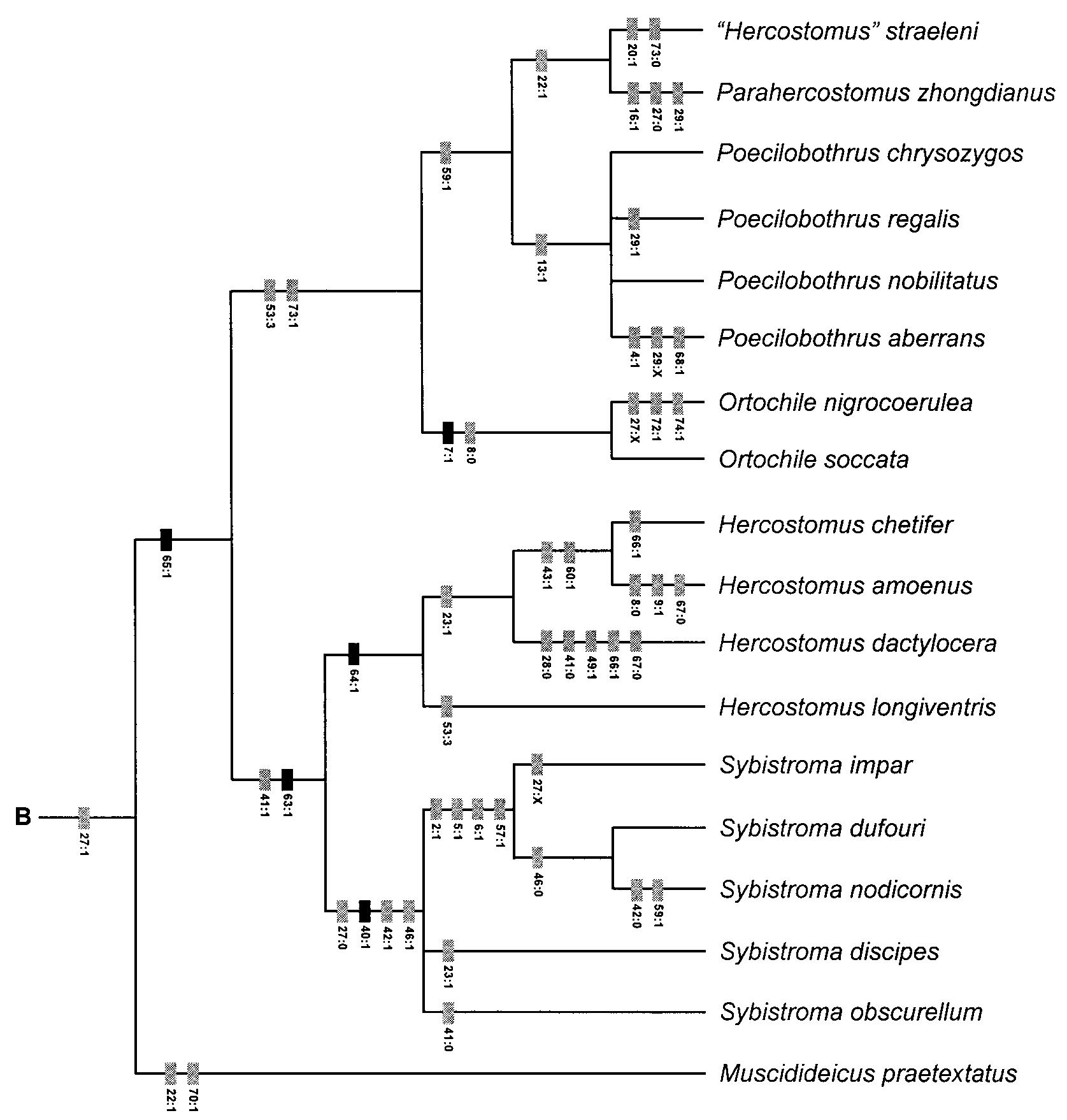
Phylogenetic Relationships. As demonstrated in the cladistic analysis ( Figs. 1 View FIGURE 1 , 4 View FIGURE 4 ) Hercostomus sensu lato is polyphyletic with species related to Parahercostomus , Phalacrosoma , Poecilobothrus and Steleopyga . The Hercostomus longiventris lineage is most closely related to Sybistroma .
Remarks. In addition to Grichanov’s (1999a) Afrotropical species group 2, H. chetifer , H. fulvicaudis , H. tibialis , and species formerly in Steleopyga and Phalacrosoma (except “ Phalacrosoma ” zhejiangense Yang and “ Phalacrosoma ” sichuanense Yang & Saigusa, see below), the following species also appear to be part of the Hercostomus longiventris lineage based on the examination of gentalic figures and descriptions from the literature: H. absimilis Yang & Grootaert , H. acutangulatus Yang & Saigusa, H. acutatus Yang & Yang , H. apicilaris Yang & Grootaert , H. apiciniger Yang & Grootaert , H. basiflavus Yang , H. beijingensis Yang , H. bigeminatus Yang & Grootaert , H. bispinifer Yang & Saigusa , H. calcaratus Stackelberg , H. concavus Yang & Saigusa , H. crassiseta Yang & Saigusa , H. curvispinosus Yang & Saigusa , H. curvispinus Yang & Saigusa , H. cuspidiger Yang & Saigusa , H. dissectus Yang & Saigusa , H. dissimilus Yang & Saigusa , H. emeiensis Yang , H. erectus Yang & Grootaert , H. exacutus Wei , H. flavicans Grootaert & Meuffels , H. flaviscapus Yang & Saigusa , H. flaviscutellum Yang , H. guizhouensis Wei, H. henanus Yang , H. jindinganus Yang and Saigusa , H. longifolius Yang & Saigusa , H. longisetus Yang & Grootaert , H. loushanguananus Yang & Saigusa , H. luoshanensis Yang & Grootaert , H. proctus Wei , H. projectus Yang & Saigusa , H. prolongatus Yang , H. proxilus Wei , H. quadratus Yang & Grootaert , H. serrulatus Yang & Grootaert, H. sichuanensis Yang , H. spiniger Yang , H. spinitarsis Yang & Saigusa , H. subnovus Yang & Yang , H. wudangshanus Yang , H. xanthodes Yang & Grootaert , H. xishuangbannensis Yang & Grootaert , H. yadonganus Yang , H. zunyianus Yang & Saigusa.
Phalacrosoma View in CoL was erected for a group of three autapomorphic species with silvery color, reduced setation, broad face and clypeus with the lower margin rounded and extending beyond the lower eye margin, and a modified fore tarsus in males. Becker (1922b) apparently did not notice the setae on the dorsal surface of the scape and placed the genus in the Hydrophorinae View in CoL . Following an examination of the types, Negrobov (1980) placed the genus in Dolichopodinae View in CoL and this classification has been followed by subsequent authors (e.g., Ulrich 1981; Yang et al. 2001). The male genitalia of the type species of Phalacrosoma View in CoL are remarkably similar to that of H. chetifer View in CoL and this genus is part of a species group within the H. longiventris View in CoL lineage that does not warrant generic status. Two of the species recently described in Phalacrosoma View in CoL , i.e. “ Phalacrosoma View in CoL ” zhejiangense and “ Phalacrosoma View in CoL ” sichuanense, do not appear to belong to the H. longiventris View in CoL lineage. “ Phalacrosoma View in CoL ” zhejiangense appears to be part of the Poecilobothrus View in CoL + Parahercostomus View in CoL + “ Hercostomus View in CoL ” straeleni View in CoL clade based on the genitalic figure in Yang (1997b). The placement of “ Phalacrosoma View in CoL ” sichuanense is currently unclear. Rather than transferring these two species to Hercostomus View in CoL , I have left them as unplaced until further phylogenetic studies can acertain their position.
Steleopyga was established for two Southeast Asian species known only from males. Although Grootaert & Meuffels (2001) described Steleopyga as a separate genus they indicated that it was part of the “ Hercostomus View in CoL complex”. The genus was based primarily on the possession of a cluster of spines on sternite 8 and a preapical anteroventral row of 4 setae on the hind femur. Like Phalacrosoma View in CoL , Steleopyga is an autapomorphic species group within the H. longiventris View in CoL lineage. The hypopygium of Hercostomus dactylocera View in CoL represents the most complex and extreme case of male genitalic asymmetry encountered in this study. This asymmetry is further complicated by the complete loss of the hypandrial arms that often serve as important landmark structures in homologizing the hypandrium. These two factors resulted in some uncertainty regarding the limits of the hypandrium and basiventral epandrial lobes (i.e. characters 63 and 65). Despite these uncertainties, it is apparent that these elements form a complex of entangled asymmetrical lobes (character 64: 1, Figs. 16 View FIGURE 16 A,B,E), which I interpret to be homologous with the condition observed in the other members of the Hercostomus longiventris View in CoL lineage.
The recently erected subgenus Ahercostomus ( Yang & Saigusa 2001c) and the recently synonymized subgenus Microhercostomus ( Grichanov 1997) are provisionally listed as synonyms of Hercostomus until a more extensive analysis can determine their phylogenetic position.
Dasyarthrus was synonymized with Hercostomus by Becker (1917–1918); however, the type species, Gymnopternus inornatus Loew , and the closely related species Hercostomus lorifer Mik , are clearly congeneric with Sybistroma based on the symmetrical, digitiform basiventral epandrial lobes and the elongate and setose apicoventral epandrial lobes. Accordingly, these species have been transferred to Sybistroma and Dasyarthrus is listed as a junior synonym of Sybistroma . Hercostomus caudatus (Loew) also appears to be referable to Sybistroma based on figures in Becker (1917–1918) and Parent (1938); however, I have not examined specimens of this species and cannot confirm this generic assignment at present.
Although this study confirms that Hercostomus is a polyphyletic assemblage and provides a phylogenetic framework for future studies, the analysis itself is not extensive enough to resolve all of the associated problems with this group. As such, Hercostomus will have to continue to serve as holding genus for many of the species listed below until more detailed phylogenetic work is done.
In addition to the species included in Grichanov’s Afrotropical Hercostomus group 1, the following examined species appear to be referable to the Poecilobothrus + Parahercostomus + “ Hercostomus ” straeleni clade: H. fuscipennis (Meigen) , H. germanus (Wiedemann) , H. nigripennis (Fallén) , H. gracilus (Stannius) , H. nigriplantis , H. sahlbergi (Zetterstedt) and H. vockerothi d’Assis Fonseca. The following species (not examined) appear to belong to this lineage based on genitalic figures in Parent (1938): Hercostomus argentifrons Oldenberg , Hercostomus conformis Loew , Hercostomus flavipes von Roder , Hercostomus laufferi Strobl , Hercostomus lichtwardti Villeneuve , Hercostomus pandellei Parent , Hercostomus rostellatus (Loew) . As noted above, “ Phalacrosoma ” zhejiangense also appears to be referable to this clade.
The following examined species may be more closely related to Sybistroma : H. cachae Harmston & Knowlton , H. nanus (Macquart) , H. orbicularis Harmston , H. parvilamellatus (Macquart) and H. truncatus Harmston & Knowlton. Hercostomus nanus and H. parvilamellatus seem close to Sybistroma nodicornis in the structure of the phallus and sperm pump, but the basiventral epandrial lobehypandrium complex is more extensively fused and not symmetrical. Several additional species currently placed in Hercostomus (not examined) also seem to be referable to Sybistroma based on genitalic figures in the literature (i.e. all apparently have elongate, symmetrical basiventral epandrial lobes): H. curvarmatus Yang & Saigusa , H. curvativus Yang & Saigusa , Hercostomus digitatus Yang , H. flavimarginatus Yang, H. incisus Yang & Saigusa , H. longus Yang & Saigusa , H. nudiusculus Yang , H. polleti Yang & Saigusa , H. shennongjiensis Yang & H. sublongus Yang & Saigusa. Many of the remaining examined species do not show obvious affinities to any of the clades in the analysis.
Material Examined. Hercostomus additus Parent , [PA]: 2ɗ syntypes, 6Ψ syntypes ( MNHN); Hercostomus amoenus Becker , [OR]: 2ɗ, 2Ψ (LEM); Hercostomus argentifacies Parent , [AU]: 2ɗ, 1Ψ ( CNC); 1ɗ, 1Ψ ( BMNH); Hercostomus argyropus par Parent, [AF]: 3ɗ, 1Ψ ( ISNB); Hercostomus aurifacies Parent , [AU]: 1ɗ, 1Ψ ( BMNH); Hercostomus aurifer (Thompson) , [NE]: 3ɗ, 2Ψ ( CAS); Hercostomus blagoderovi Grichanov , [AF]: 1ɗ ( CNC); Hercostomus cachae Harmston & Knowlton , [NE]: 1ɗ ( CAS); Hercostomus chetifer (Walker) , [NE, OR, PA]: 5ɗ, 1Ψ ( CNC); 4ɗ paratypes, 1Ψ paratype [of Hercostomus ornatus (Van Duzee) ] ( CAS); Hercostomus congoensis (Curran) , [AF]: 3ɗ, 1Ψ ( ISNB); 1ɗ ( CNC); Hercostomus curvativus Yang & Saigusa , [PA]: 2ɗ paratypes ( ISBN); Hercostomus dactylocera (Grootaert & Meuffels) , [OR]: 1ɗ paratype ( ISNB); Hercostomus dissectus Yang & Saigusa , [PA]: 1ɗ paratype ( ISNB); Hercostomus enghoffi Grichanov , [AF]: 1ɗ paratype ( ISNB); Hercostomus eronis Curran , [AF]: 1ɗ ( ISNB); Hercostomus fugax (Loew) , [PA]: 7ɗ, 4Ψ ( CNC); 2ɗ (LEM); Hercostomus fulvicaudis (Haliday) , [PA]: 1ɗ (LEM); Hercostomus fuscipennis (Meigen) , [PA]: 1ɗ, 1Ψ ( CAS); Hercostomus germanus (Wiedemann) , [PA]: 4ɗ, 2Ψ ( CNC); 1ɗ, 1Ψ (LEM); Hercostomus gracilus (Stannius) , [PA]: 3ɗ, 3Ψ ( CNC); Hercostomus krivosheinae Grichanov , [AF]: 2ɗ paratypes ( BMNH); Hercostomus longiventris (Loew) , [PA]: 2ɗ, 2Ψ ( CNC); Hercostomus nanus (Macquart) , [PA]: 3ɗ, 3Ψ (LEM); Hercostomus nigripennis (Fallén) , [PA]: 1ɗ, 1Ψ ( CNC); 1ɗ, 1Ψ (LEM); Hercostomus nigriplantis (Stannius) , [PA]: 2ɗ, 2Ψ ( CNC); 1ɗ, 1Ψ (LEM); Hercostomus occidentalis Cole , [NE]: 3ɗ, 2Ψ ( CAS); Hercostomus orbicularis Harmston , [NE]: 2ɗ ( CAS); Hercostomus ovchinnikovae Grichanov , [AF]: 4ɗ ( MRAC); Hercostomus parvilamellatus (Macquart) , [PA]: 7ɗ (LEM); Hercostomus regularis Becker , [OR]: 1ɗ, 1Ψ ( DEI); Hercostomus sahlbergi (Zetterstedt) , [PA]: 4ɗ, 2Ψ ( CNC); Hercostomus straeleni Vanschuytbroeck , [AF]: 2ɗ paratypes, 2Ψ ( ISNB); 1ɗ ( BMNH); Hercostomus strictilamellatus Parent , [AF]: 3ɗ paratypes, 2Ψ paratypes ( ISNB); Hercostomus syncolus Steyskal , [NE]: 4ɗ, 2Ψ ( CNC); Hercostomus tibialis (Van Duzee) , [NE]: 8ɗ, 10Ψ ( CAS); Hercostomus tobiasi Grichanov , [AF]: 2ɗ paratypes, 1Ψ paratype ( BMNH); Hercostomus truncatus Harmston & Knowlton , [NE]: 4ɗ, 2Ψ ( CAS); Hercostomus ultimus Parent , [AF]: 1ɗ, 1Ψ ( ISBN); 1ɗ, 1Ψ ( MRAC); Hercostomus unicolor Loew , [NE]: 9ɗ, 9Ψ ( CNC); Hercostomus utahensis Harmston & Knowlton , [NE]: 1ɗ ( CAS); Hercostomus vivax (Loew) , [PA]: 6ɗ, 3Ψ ( CNC); Hercostomus vockerothi d’Assis Fonseca , [PA]: 4ɗ, 3Ψ ( CNC); Hercostomus wasatchensis Harmston & Knowlton , [NE]: 1ɗ ( CAS); Hercostomus wittei Grichanov , [AF]: 2ɗ paratypes ( ISNB).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Hercostomus Loew
| SCOTT E. BROOKS 2005 |
Steleopyga
| Grootaert 2001: 208 |
Ahercostomus
| Yang 2001: 239 |
Phalacrosoma
| Dyte 1975: 242 |
| Becker 1922: 44 |